167:
160:
140:
106:
36:) to a substrate. Many organic compounds contain vinyl groups, so the process has attracted significant interest, especially since the reaction scope includes substituted vinyl groups. The reactions can be classified according to the source of the vinyl group.
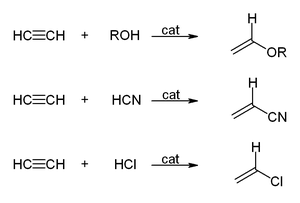
126:, acetylene participates in a variety of metal- or base-catalyzed reaction to afford vinyl derivatives. Alcohols, thiols, and secondary amines add to acetylene to give the
552:
Takashi Ohara; Takahisa Sato; Noboru
Shimizu; Günter Prescher; Helmut Schwind; Otto Weiberg; Klaus Marten; Helmut Greim (2003). "Acrylic Acid and Derivatives".
368:
602:
569:
186:, which is available on an industrial scale, can be used to produce other vinyl esters. The process is sometimes referred to as
66:
Vinylsiloxane and vinylboranes have also been used as sources of vinyl anion equivalents.These types of reactions require
321:
Donal F. O'Shea (2012). "Discussion
Addendum for: Suzuki-Miyaura Cross-Coupling: Preparation of 2'-Vinylacetanilide".
656:
290:"Vinylation with Inexpensive Silicon-Based Reagents: Preparation of 3-Vinylquinoline and 4-Vinylbenzophenone"
67:
661:
166:
159:
202:
378:
99:
139:
598:
565:
534:
493:
425:
364:
257:"Iridium-Catalyzed Enantioselective Allylic Vinylation with Potassium Alkenyltrifluoroborates"
17:
407:; Cheprakov, A. V. (2000). "The Heck Reaction as a Sharpening Stone of Palladium Catalysis".
631:
590:
557:
524:
483:
452:
417:
404:
356:
330:
301:
268:
95:
390:
348:
231:
147:
182:
typically requires indirect methods because vinyl alcohol is not a suitable reagent.
650:
206:
191:
183:
131:
83:
44:
513:"Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited"
472:"Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited"
443:
Reppe, Walter; Kutepow, N; Magin, A (1969). "Cyclization of
Acetylenic Compounds".
151:
123:
561:
360:
179:
25:
409:
127:
636:
620:"Iridium-catalyzed Synthesis of Vinyl Ethers from Alcohols and Vinyl Acetate"
619:
594:
334:
306:
289:
273:
256:
71:
538:
511:
Trotuş, Ioan-Teodor; Zimmermann, Tobias; Schüth, Ferdi (14 November 2013).
497:
470:
Trotuş, Ioan-Teodor; Zimmermann, Tobias; Schüth, Ferdi (14 November 2013).
456:
429:
105:
235:
110:
60:
198:
529:
512:
488:
471:
421:
154:
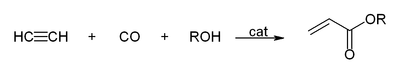
or acrylic esters. The net reaction is vinylation of carbon monoxide.
190:. Higher esters of vinyl acetate have been used in the synthesis of
91:
87:
56:
104:
618:
Tomotaka
Hirabayashi; Satoshi Sakaguchi; Yasutaka Ishii (2005).
351:(1982). "Palladium-catalyzed vinylation of organic halides".
197:
Alternatively, vinyl ethers can be prepared from alcohols by
102:
are required. This reaction is a way to substitute alkenes.
255:
Hamilton, James; Sarlah, David; Carreira, Erick M. (2015).
445:Angewandte Chemie International Edition in English
205:of vinyl esters, especially the widely available
288:Scott E. Denmark, Christopher R. Butler (2009).
587:Encyclopedia of Reagents for Organic Synthesis
554:Ullmann's Encyclopedia of Industrial Chemistry
8:
585:Manchand, Percy S. (2001). "Vinyl Acetate".
47:and vinyl magnesium bromide are sources of "
635:
528:
487:
305:
272:
50:
31:
247:
109:The Heck reaction in the production of
386:
376:
7:
146:In the presence of metal catalysts,
238:adds "across" an alkene double bond
355:. Vol. 27. pp. 345–390.
134:, and vinyl amines, respectively.
14:
165:
158:
138:
24:is the process of attaching a
1:
562:10.1002/14356007.a01_161.pub2
150:and acetylene react to give
361:10.1002/0471264180.or027.02
122:As originally developed by
40:Nucleophilic vinyl reagents
678:
118:Vinylation with acetylene
637:10.15227/orgsyn.082.0055
595:10.1002/047084289X.rv008
335:10.15227/orgsyn.089.0202
307:10.15227/orgsyn.086.0274
274:10.15227/orgsyn.092.0001
86:couples an unsaturated
78:Vinylation with alkenes
70:such as those based on
457:10.1002/anie.196907271
114:
108:
203:transesterification
178:The preparation of
115:
100:palladium catalyst
530:10.1021/cr400357r
489:10.1021/cr400357r
422:10.1021/cr9903048
405:Beletskaya, I. P.
370:978-0-471-26418-7
353:Organic Reactions
323:Organic Syntheses
294:Organic Syntheses
261:Organic Syntheses
18:organic chemistry
669:
642:
641:
639:
615:
609:
608:
582:
576:
575:
549:
543:
542:
532:
523:(3): 1761–1782.
517:Chemical Reviews
508:
502:
501:
491:
482:(3): 1761–1782.
476:Chemical Reviews
467:
461:
460:
440:
434:
433:
416:(8): 3009–3066.
401:
395:
394:
388:
384:
382:
374:
345:
339:
338:
318:
312:
311:
309:
285:
279:
278:
276:
252:
217:=CHOAc → ROCH=CH
169:
162:
142:
55:", which add to
54:
35:
677:
676:
672:
671:
670:
668:
667:
666:
657:Vinyl compounds
647:
646:
645:
617:
616:
612:
605:
584:
583:
579:
572:
551:
550:
546:
510:
509:
505:
469:
468:
464:
451:(10): 727–733.
442:
441:
437:
403:
402:
398:
385:
375:
371:
347:
346:
342:
320:
319:
315:
287:
286:
282:
254:
253:
249:
245:
232:Hydrovinylation
228:
220:
216:
188:transvinylation
176:
148:carbon monoxide
120:
80:
52:
48:
42:
33:
29:
12:
11:
5:
675:
673:
665:
664:
659:
649:
648:
644:
643:
610:
603:
577:
570:
544:
503:
462:
435:
396:
387:|journal=
369:
340:
313:
280:
246:
244:
241:
240:
239:
227:
224:
223:
222:
218:
214:
175:
172:
171:
170:
163:
144:
143:
132:vinyl sulfides
119:
116:
79:
76:
41:
38:
13:
10:
9:
6:
4:
3:
2:
674:
663:
660:
658:
655:
654:
652:
638:
633:
629:
625:
621:
614:
611:
606:
604:0-471-93623-5
600:
596:
592:
588:
581:
578:
573:
571:3-527-30673-0
567:
563:
559:
555:
548:
545:
540:
536:
531:
526:
522:
518:
514:
507:
504:
499:
495:
490:
485:
481:
477:
473:
466:
463:
458:
454:
450:
446:
439:
436:
431:
427:
423:
419:
415:
412:
411:
406:
400:
397:
392:
380:
372:
366:
362:
358:
354:
350:
344:
341:
336:
332:
328:
324:
317:
314:
308:
303:
299:
295:
291:
284:
281:
275:
270:
266:
262:
258:
251:
248:
242:
237:
233:
230:
229:
225:
212:
211:
210:
208:
207:vinyl acetate
204:
200:
195:
193:
192:vinyl formate
189:
185:
184:Vinyl acetate
181:
174:Vinyl acetate
173:
168:
164:
161:
157:
156:
155:
153:
149:
141:
137:
136:
135:
133:
129:
125:
117:
112:
107:
103:
101:
97:
93:
89:
85:
84:Heck reaction
77:
75:
73:
69:
64:
62:
58:
46:
45:Vinyl lithium
39:
37:
27:
23:
19:
662:Vinyl esters
627:
623:
613:
586:
580:
553:
547:
520:
516:
506:
479:
475:
465:
448:
444:
438:
413:
408:
399:
352:
343:
326:
322:
316:
297:
293:
283:
264:
260:
250:
196:
187:
180:vinyl esters
177:
152:acrylic acid
145:
128:vinyl ethers
124:Walter Reppe
121:
81:
65:
43:
21:
15:
349:Heck, R. F.
201:-catalyzed
26:vinyl group
651:Categories
624:Org. Synth
410:Chem. Rev.
243:References
22:vinylation
389:ignored (
379:cite book
72:palladium
68:catalysts
61:aldehydes
539:24228942
498:24228942
430:11749313
267:: 1–12.
236:ethylene
226:See also
213:ROH + CH
111:Naproxen
90:with an
329:: 202.
300:: 274.
199:iridium
57:ketones
630:: 55.
601:
568:
537:
496:
428:
367:
221:+ HOAc
98:and a
92:alkene
88:halide
556:: 7.
599:ISBN
566:ISBN
535:PMID
494:PMID
426:PMID
391:help
365:ISBN
96:Base
82:The
59:and
34:=CH−
632:doi
591:doi
558:doi
525:doi
521:114
484:doi
480:114
453:doi
418:doi
414:100
357:doi
331:doi
302:doi
269:doi
94:.
63:.
53:=CH
16:In
653::
628:82
626:.
622:.
597:.
589:.
564:.
533:.
519:.
515:.
492:.
478:.
474:.
447:.
424:.
383::
381:}}
377:{{
363:.
327:89
325:.
298:86
296:.
292:.
265:92
263:.
259:.
234:,
209::
194:.
130:,
74:.
49:CH
30:CH
20:,
640:.
634::
607:.
593::
574:.
560::
541:.
527::
500:.
486::
459:.
455::
449:8
432:.
420::
393:)
373:.
359::
337:.
333::
310:.
304::
277:.
271::
219:2
215:2
113:.
51:2
32:2
28:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.