1097:
666:
643:
51:
42:
3991:
1165:
949:
Although this increase was not statistically significant, there was a trend showing increased bleeding in the rivaroxaban with moderate CYP3A4 and P-glycoprotein inhibitors group. Therefore, it is important to monitor for bleeding when concurrently on rivaroxaban and moderate CYP3A4 and P-glycoprotein inhibitors.
948:
inhibitors because this results in significantly higher plasma concentrations of rivaroxaban. A small retrospective cohort study reported that the use of moderate CYP3A4 and P-glycoprotein inhibitors such as amiodarone or verapamil, increased the risk of bleeding when administered with rivaroxaban.
1087:
is dose-dependent. Doses of rivaroxaban under 10 mg can be taken with or without food, as it displayed high bioavailability independent of whether food was consumed or not. If rivaroxaban is given at oral doses of 15 mg or 20 mg, it needs to be taken with food to aid in drug absorption and achieve
1246:
On March 25, 2019, over 25,000 lawsuits over rivaroxaban in the US were settled for $ 775 million to get paid out to those affected. Plaintiffs accused the drugmakers of not warning about the bleeding risks, claiming their injuries could have been prevented had doctors and patients been provided
936:
When undergoing surgeries, due to the concern over managing bleeding, rivaroxaban can be discontinued 24 hours prior to low-bleeding risk surgery and 48-72 hours prior to high-bleeding risk surgeries. Once the surgery is over, it can be recommenced after 1 to 3 days with doctor consultation.
2352:
Roehrig S, Straub A, Pohlmann J, Lampe T, Pernerstorfer J, Schlemmer KH, et al. (September 2005). "Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- -1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor".
2784:
1264:, then Commissioner of the FDA, found rivaroxaban to be more effective than warfarin in reducing the likelihood of ischemic strokes in patients with atrial fibrillation. The validity of the study was called into question in 2014, when pharmaceutical sponsors
972:
showed liver toxicity, and further studies are needed to quantify this risk. In 2015, rivaroxaban accounted for the highest number of reported cases of serious injury among regularly monitored medications to the FDA's
Adverse Events Reporting System (AERS).
2840:
1259:
have been accused of withholding clinical data used to evaluate rivaroxaban. Duke tested rivaroxaban in a clinical trial known as the ROCKET AF trial. The clinical trial, published 2011 in the New
England Journal of Medicine and headed by
2792:
2330:
2696:
228:
2012:
Hanigan S, Das J, Pogue K, Barnes GD, Dorsch MP (May 2020). "The real world use of combined P-glycoprotein and moderate CYP3A4 inhibitors with rivaroxaban or apixaban increases bleeding".
4051:
1276:
used were not functioning properly, A subsequent analysis by the Duke team published in
February 2016 found that this had no significant effect on efficacy and safety in the trial.
921:
183:
799:
1181:
Co, the largest U.S. pharmacy benefits manager. As of 2016, Bayer claimed that the drug was licensed in 130 countries and that more than 23 million patients had been treated.
2703:
1830:
741:
4036:
4031:
81:
755:
1776:"Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups"
1124:, which is a known complication of long-term linezolid use. Studies found that neither rivaroxaban nor its metabolites have any antibiotic effect against
3831:
3069:
783:
InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
2118:"Liver injury with novel oral anticoagulants: assessing post-marketing reports in the US Food and Drug Administration adverse event reporting system"
3161:
2871:
1595:
2302:
881:
Rivaroxaban was patented in 2007 and approved for medical use in the United States in 2011. In the United States, it will not be available as a
1620:
1212:
also granted marketing authorization of rivaroxaban to prevent venous thromboembolism in adults undergoing elective hip and knee replacement.
3945:
2405:
886:
2785:"FDA Approves Xarelto (rivaroxaban tablets) to Help Prevent Deep Vein Thrombosis in Patients Undergoing Knee or Hip Replacement Surgery"
2970:
1352:
4046:
3316:
1554:
775:
989:
as an antidote for Factor Xa inhibitors with few adverse effects, and started Phase III trials. Andexanet alfa was approved by the
2532:
Stampfuss J, Kubitza D, Becka M, Mueck W (July 2013). "The effect of food on the absorption and pharmacokinetics of rivaroxaban".
2259:
Mo Y, Yam FK (February 2015). "Recent advances in the development of specific antidotes for target-specific oral anticoagulants".
3112:
1256:
1120:-derived core structure. Accordingly, rivaroxaban was studied for any possible antimicrobial effects and for the possibility of
1408:
3926:
3334:
3307:
3047:
2900:
2490:"A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement"
1826:"Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism"
384:
213:
113:
1960:"Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects"
4041:
3261:
1678:
1467:"Rivaroxaban (xarelto) for the prevention of thromboembolic disease: an inside look at the oral direct factor xa inhibitor"
4011:
3062:
3981:
2433:
1017:
with an onset of action of 2.5 to 4 hours. Inhibition of Factor Xa interrupts the intrinsic and extrinsic pathway of the
3545:
1231:
1216:
1041:
990:
514:
1859:"Comparison of temporary interruption with continuation of direct oral anticoagulants for low bleeding risk procedures"
132:
3962:
3636:
3356:
2331:"U.S. FDA Approves Portola Pharmaceuticals' Andexxa, First and Only Antidote for the Reversal of Factor Xa Inhibitors"
622:
1025:
formation and development of thrombi. Rivaroxaban does not inhibit thrombin (activated Factor II), and no effects on
2234:
2166:
1153:
1080:
1014:
969:
2754:
3718:
3692:
3684:
2072:
1642:
1441:
3055:
1096:
824:
1727:"Interventions for Preventing Thromboembolic Events in Patients With Atrial Fibrillation: A Systematic Review"
463:
3028:
2728:
2208:
3950:
2841:"Bayer, Johnson & Johnson settle more than 25,000 lawsuits over blood thinner Xarelto for $ 775 million"
1725:
Lowenstern A, Al-Khatib SM, Sharan L, Chatterjee R, Allen LaPointe NM, Shah B, et al. (December 2018).
1409:"Xarelto- rivaroxaban tablet, film coated Xarelto- rivaroxaban tablet, film coated Xarelto- rivaroxaban kit"
1178:
1145:
982:
169:
2814:
914:
661:
4016:
3299:
3223:
1194:
1125:
1121:
925:
2702:. Bayer AG Communications, Government Relations & Corporate Brand. September 29, 2016. Archived from
1325:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
940:
Dosing recommendations do not recommend administering rivaroxaban with drugs known to be strong combined
583:
4026:
3827:
2788:
454:
2571:
3889:
3468:
3423:
1911:"Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant"
1324:
1269:
1220:
1149:
1065:
889:. In 2021, it was the 86th most commonly prescribed medication in the United States, with more than 8
832:
322:
4021:
3279:
2845:
1909:
Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, et al. (November 2019).
1273:
1235:
1209:
902:
844:
840:
638:
409:
176:
2165:
Russmann S, Niedrig DF, Budmiger M, Schmidt C, Stieger B, Hürlimann S, et al. (August 2014).
3570:
2678:
2618:
2411:
2284:
1224:
1109:
143:
1774:
Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E (2013).
3732:
3135:
3130:
2047:
604:
3814:
3585:
3575:
3565:
3560:
3555:
3530:
3103:
3090:
3082:
3033:
2952:
2670:
2549:
2511:
2470:
2401:
2370:
2276:
2189:
2147:
2029:
1989:
1940:
1888:
1807:
1756:
1550:
1488:
1033:
and dose adjustments and routine coagulation monitoring; dietary restrictions are not needed.
962:
594:
563:
252:
240:
63:
2488:
Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. (November 2006).
2094:
1544:
503:
3788:
3619:
3580:
3550:
3023:
3013:
2978:
2942:
2934:
2815:"FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm"
2662:
2608:
2541:
2501:
2460:
2393:
2362:
2268:
2181:
2137:
2129:
2021:
1979:
1971:
1930:
1922:
1878:
1870:
1797:
1787:
1746:
1738:
1650:
1478:
1436:
1202:
1076:
913:
and embolic events in patients who are classified as moderate-to-high risk, as defined by a
871:
836:
678:
418:
287:
523:
3995:
3592:
1570:
1198:
1084:
1030:
851:
305:
295:
2921:
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. (September 2011).
2653:
Fassiadis N (December 2009). "Rivaroxaban, the first oral, direct factor Xa inhibitor".
2116:
Raschi E, Poluzzi E, Koci A, Salvo F, Pariente A, Biselli M, et al. (August 2015).
665:
642:
3956:
3483:
3169:
3078:
2947:
2922:
2397:
2142:
2117:
1984:
1959:
1935:
1910:
1883:
1858:
1802:
1751:
1726:
1483:
1466:
1010:
986:
945:
2506:
2489:
50:
4005:
3913:
3883:
3473:
3458:
3453:
3414:
3086:
2622:
1857:
Sheikh MA, Kong X, Haymart B, Kaatz S, Krol G, Kozlowski J, et al. (July 2021).
1377:
1261:
1190:
1117:
1061:
1057:
863:
654:
265:
105:
2682:
2415:
2288:
1926:
443:
3903:
3873:
3851:
3846:
3819:
3768:
3747:
3597:
3536:
3492:
3369:
3312:
3251:
3246:
3227:
3125:
3094:
1874:
1700:
1517:
1299:
1049:
1045:
882:
196:
191:
91:
2697:"Bayer comments on article in The British Medical Journal (BMJ) regarding Xarelto"
1670:
3773:
3701:
3602:
3503:
3497:
3487:
3390:
3184:
3174:
1378:"Xarelto 2.5 mg film-coated tablets - Summary of Product Characteristics (SmPC)"
1018:
855:
99:
2465:
2448:
2185:
2025:
41:
3878:
3795:
3778:
3763:
3742:
3727:
3722:
3665:
3650:
3624:
3614:
3609:
3463:
3432:
3364:
3344:
3322:
3211:
3195:
3191:
3140:
2666:
1596:"Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations"
1079:
across a wide spectrum of patients (age, gender, weight, race) and has a flat
1048:
also inhibit the activity of factor Xa, indirectly, by binding to circulating
717:
494:
2639:
2613:
2596:
2595:
Singh AK, Noronha V, Gupta A, Singh D, Singh P, Singh A, et al. (2020).
2235:"Possible Antidote Could Help Blood Thinner Patients In Bleeding Emergencies"
1647:
World Health
Organization model list of essential medicines: 22nd list (2021)
1279:
Under-representation of racial minorities in clinical trials has been noted.
850:
Common side effects include bleeding. Other serious side effects may include
3866:
3861:
3841:
3836:
3783:
3737:
3711:
3706:
3655:
3513:
3436:
3400:
3374:
3339:
3327:
3289:
3284:
3274:
3236:
3206:
3201:
3179:
3145:
3120:
1113:
1101:
1026:
1006:
875:
859:
335:
85:
33:
3037:
2956:
2674:
2553:
2515:
2474:
2374:
2280:
2193:
2151:
2033:
1993:
1944:
1892:
1811:
1760:
1492:
2938:
1792:
1775:
3660:
3645:
3478:
3449:
3440:
3428:
3395:
3241:
3018:
3001:
1177:
Using rivaroxaban rather than warfarin costs 70 times more, according to
1069:
1053:
1022:
958:
906:
867:
828:
474:
127:
1825:
1655:
483:
3931:
3921:
3898:
3696:
3525:
3269:
2762:
1234:
approved rivaroxaban for stroke prevention in people with non-valvular
1037:
429:
17:
2366:
2272:
2133:
1975:
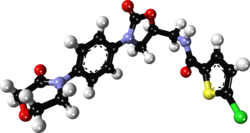
1104:(top) and rivaroxaban (bottom). The shared structure is shown in blue.
3893:
2545:
1742:
941:
910:
574:
314:
310:
1164:
543:
3155:
2758:
2732:
2388:
Ansell J (January 2019). "Outpatient Oral
Anticoagulant Therapy".
1265:
1163:
1141:
1095:
740:
731:
610:
554:
2755:"Bayer's Novel Anticoagulant Xarelto now also approved in the EU"
2434:"New blood thinner 'antidote' to help doctors move past warfarin"
3800:
2923:"Rivaroxaban versus warfarin in nonvalvular atrial fibrillation"
2894:
2892:
1621:"Bayer, J&J Win Ruling That Upholds Patent for Xarelto Drug"
534:
274:
246:
3051:
2534:
International
Journal of Clinical Pharmacology and Therapeutics
259:
122:
1332:
2971:"Meet Robert M. Califf, M.D., Commissioner of Food and Drugs"
2209:"ISMP Ranks Xarelto Most Dangerous Drug in the United States"
1227:(PE), in adults undergoing hip and knee replacement surgery.
1068:(VKA), decreasing a number of coagulation factors, including
2872:"Document Claims Drug Makers Deceived a Top Medical Journal"
1546:
874:. It works by blocking the activity of the clotting protein
627:
1193:
granted marketing authorization for rivaroxaban to prevent
222:
154:
3002:"Point-of-Care Warfarin Monitoring in the ROCKET AF Trial"
2333:(Press release). Portola Pharmaceuticals Inc. May 4, 2018
1052:(AT III) and must be injected, whereas the orally active
1132:
studies published before 2008 found the risk to be low.
1083:
across an eightfold dose range (5–40 mg). The oral
235:
2865:
2863:
2634:
2632:
887:
806:
2572:"CHP Assessment Report for Xarelto (EMEA/543519/2008)"
3979:
1294:
1292:
922:
National
Institute for Health and Clinical Excellence
3912:
3813:
3756:
3683:
3635:
3524:
3512:
3422:
3413:
3383:
3355:
3298:
3260:
3222:
3154:
3111:
3102:
1403:
1401:
1399:
729:
716:
677:
672:
653:
621:
593:
573:
553:
533:
513:
493:
473:
462:
453:
428:
408:
375:
334:
321:
304:
294:
286:
212:
207:
182:
168:
142:
112:
98:
80:
72:
62:
57:
1197:(VTE) in people who have undergone elective total
3029:20.500.11820/69b742f0-5b6d-4f54-93a5-98a1da909653
2901:"Duke clinical trial under scrutiny in drug case"
2527:
2525:
1831:National Institute for Health and Care Excellence
400:-{oxazolidin-5-yl]methyl} thiophene-2-carboxamide
2449:"New oral anticoagulants in atrial fibrillation"
2167:"Rivaroxaban postmarketing risk of liver injury"
1904:
1902:
915:score of a number of specific medical conditions
763:O=C1COCCN1c2ccc(cc2)N3C(OC3=O)CNC(=O)c4ccc(s4)Cl
442:
3000:Patel MR, Hellkamp AS, Fox KA (February 2016).
2392:(Fourth ed.). Elsevier. pp. 747–777.
2007:
2005:
2003:
1524:. American Society of Health-System Pharmacists
843:and following hip or knee surgery. It is taken
417:
1958:Mueck W, Kubitza D, Becka M (September 2013).
1852:
1850:
1465:Abdulsattar Y, Bhambri R, Nogid A (May 2009).
1431:
1429:
1372:
1370:
1368:
1366:
1364:
1362:
1360:
1219:(FDA) approved rivaroxaban for prophylaxis of
1029:have been demonstrated. It allows predictable
4052:World Health Organization essential medicines
3063:
2916:
2914:
985:completed Phase I and II clinical trials for
924:recommended rivaroxaban to prevent and treat
8:
905:, rivaroxaban appears to be as effective as
131:
32:
330:5–9 hours in healthy subjects aged 20 to 45
3521:
3419:
3108:
3070:
3056:
3048:
2427:
2425:
1512:
1510:
1508:
1506:
1504:
1502:
1144:. In the United States, it is marketed by
827:(blood thinner) used to treat and prevent
664:
641:
502:
3027:
3017:
2946:
2612:
2505:
2464:
2141:
1983:
1934:
1882:
1801:
1791:
1750:
1654:
1518:"Rivaroxaban Monograph for Professionals"
1482:
1005:Rivaroxaban inhibits both free and bound
522:
4037:Drugs developed by Johnson & Johnson
2122:British Journal of Clinical Pharmacology
1964:British Journal of Clinical Pharmacology
3986:
2565:
2563:
1288:
1140:Rivaroxaban was initially developed by
780:
760:
637:
482:
389:
104:
2390:Consultative Hemostasis and Thrombosis
2014:Journal of Thrombosis and Thrombolysis
655:
300:80–100%; Cmax = 2–4 hours (10 mg oral)
31:
1701:"Rivaroxaban - Drug Usage Statistics"
1681:from the original on January 15, 2024
1649:. Geneva: World Health Organization.
1088:appropriate bioavailability (≥ 80%).
603:
582:
562:
90:
7:
4032:3-(4-methoxyphenyl)-2-oxazolidinones
2729:"Bayer's Xarelto Approved in Canada"
609:
195:
3006:The New England Journal of Medicine
2927:The New England Journal of Medicine
2761:. February 10, 2008. Archived from
2053:. U.S. Food and Drug Administration
957:The most serious adverse effect is
872:interactions with other medications
831:. Specifically it is used to treat
542:
433:
2907:. Duke Student Publishing Company.
2570:European Medicines Agency (2008).
2398:10.1016/B978-0-323-46202-0.00037-6
2303:"Accelerated Approval for AndexXa"
1300:"Rivaroxaban Use During Pregnancy"
1128:. As for mitochondrial toxicity,
993:in May 2018, under the trade name
25:
2975:U.S. Food and Drug Administration
2655:Expert Opinion on Pharmacotherapy
2507:10.1161/CIRCULATIONAHA.106.642074
991:U.S. Food and Drug Administration
3989:
3685:Direct thrombin (IIa) inhibitors
3113:Glycoprotein IIb/IIIa inhibitors
1274:INRatio blood monitoring devices
1257:Duke Clinical Research Institute
698:
695:
689:
49:
40:
3308:Thromboxane synthase inhibitors
2432:Berkrot B (December 23, 2015).
1927:10.1001/jamainternmed.2019.2431
788:Key:KGFYHTZWPPHNLQ-AWEZNQCLSA-N
2791:. July 1, 2011. Archived from
2640:"Xarelto FDA Approval History"
2355:Journal of Medicinal Chemistry
1875:10.1016/j.thromres.2021.04.006
1571:"Generic Xarelto Availability"
1152:). It was the first available
710:
704:
683:
317:and CYP-independent mechanisms
1:
2095:"Xarelto Side Effects Center"
1353:Xarelto (Bayer Australia Ltd)
1112:similarity to the antibiotic
1108:Rivaroxaban bears a striking
3546:Low-molecular-weight heparin
3357:Phosphodiesterase inhibitors
3270:Acetylsalicylic acid/Aspirin
2819:US Food and Drug Association
2048:"Medication Guide – Xarelto"
1217:Food and Drug Administration
1116:: both drugs share the same
1075:Rivaroxaban has predictable
1042:low molecular weight heparin
819:, sold under the brand name
1731:Annals of Internal Medicine
901:In those with non-valvular
839:and prevent blood clots in
4068:
2870:Thomas K (March 1, 2016).
2597:"Rivaroxaban: Drug review"
2447:Turpie AG (January 2008).
2341:– via GlobeNewswire.
2186:10.1016/j.jhep.2014.03.026
2026:10.1007/s11239-020-02037-3
1659:. WHO/MHP/HPS/EML/2021.02.
1154:direct factor Xa inhibitor
1015:direct factor Xa inhibitor
970:post-marketing assessments
858:. It is unclear if use in
673:Chemical and physical data
3940:
3518:(with some II inhibition)
2667:10.1517/14656560903413559
1643:World Health Organization
1442:European Medicines Agency
1230:In November 2011, the US
1223:(DVT), which may lead to
1156:which is taken by mouth.
1019:blood coagulation cascade
885:until 2024. It is on the
796:
771:
751:
380:
355:metabolized in liver and
48:
39:
4047:Drugs developed by Bayer
2614:10.4103/CRST.CRST_122_19
2466:10.1093/eurheartj/ehm575
1549:. Springer. p. 11.
893:million prescriptions.
825:anticoagulant medication
1208:In the same month, the
1179:Express Scripts Holding
1146:Janssen Pharmaceuticals
1100:Chemical structures of
983:Portola Pharmaceuticals
920:In July 2012, the UK's
3828:Plasminogen activators
3300:Thromboxane inhibitors
3224:Prostaglandin analogue
2453:European Heart Journal
2073:"Xarelto Side Effects"
1915:JAMA Internal Medicine
1600:www.accessdata.fda.gov
1247:adequate information.
1195:venous thromboembolism
1169:
1126:Gram-positive bacteria
1122:mitochondrial toxicity
1105:
1011:prothrombinase complex
926:venous thromboembolism
4042:Janssen Pharmaceutica
3890:serine endopeptidases
3424:Vitamin K antagonists
2939:10.1056/NEJMoa1009638
2789:Janssen Pharmaceutica
2601:Cancer Res Stat Treat
2174:Journal of Hepatology
1671:"The Top 300 of 2021"
1270:Johnson & Johnson
1215:In July 2011, the US
1167:
1150:Johnson & Johnson
1099:
1066:vitamin K antagonists
866:is safe. Compared to
4012:Direct Xa inhibitors
3637:Direct Xa inhibitors
3469:Ethyl biscoumacetate
3335:Receptor antagonists
3019:10.1056/NEJMc1515842
2735:. September 16, 2008
2642:. September 7, 2020.
1446:. September 17, 2018
1221:deep vein thrombosis
1168:Rivaroxaban capsules
1013:. It is a selective
833:deep vein thrombosis
823:among others, is an
371:eliminated unchanged
3280:Carbasalate calcium
2846:The Washington Post
2795:on November 5, 2011
2765:on October 22, 2008
2709:on January 31, 2017
1863:Thrombosis Research
1793:10.1155/2013/640723
1255:Researchers at the
1236:atrial fibrillation
1210:European Commission
1189:In September 2008,
1160:Society and culture
1001:Mechanism of action
961:, including severe
903:atrial fibrillation
841:atrial fibrillation
255:(Prescription only)
231:(Prescription only)
36:
3967:Never to phase III
3815:Thrombolytic drugs
3531:glycosaminoglycans
3104:Antiplatelet drugs
3091:antiplatelet drugs
2876:The New York Times
2821:. November 4, 2011
1272:revealed that the
1225:pulmonary embolism
1170:
1106:
1021:, inhibiting both
883:generic medication
27:Anticoagulant drug
3977:
3976:
3809:
3808:
3679:
3678:
3409:
3408:
2787:(Press release).
2757:(Press release).
2731:(Press release).
2661:(18): 2945–2946.
2500:(22): 2374–2381.
2407:978-0-323-46202-0
2367:10.1021/jm050101d
2273:10.1002/phar.1532
2134:10.1111/bcp.12611
1976:10.1111/bcp.12075
1921:(11): 1469–1478.
981:In October 2014,
963:internal bleeding
932:Contraindications
814:
813:
742:Interactive image
623:CompTox Dashboard
278:
263:
250:
238:
226:
158:
125:
16:(Redirected from
4059:
3994:
3993:
3992:
3985:
3789:Drotrecogin alfa
3769:Antithrombin III
3620:Dermatan sulfate
3593:Oligosaccharides
3522:
3420:
3156:ADP receptor/P2Y
3109:
3072:
3065:
3058:
3049:
3042:
3041:
3031:
3021:
2997:
2991:
2990:
2988:
2986:
2977:. Archived from
2967:
2961:
2960:
2950:
2918:
2909:
2908:
2896:
2887:
2886:
2884:
2882:
2867:
2858:
2857:
2855:
2853:
2837:
2831:
2830:
2828:
2826:
2811:
2805:
2804:
2802:
2800:
2781:
2775:
2774:
2772:
2770:
2751:
2745:
2744:
2742:
2740:
2725:
2719:
2718:
2716:
2714:
2708:
2701:
2693:
2687:
2686:
2650:
2644:
2643:
2636:
2627:
2626:
2616:
2592:
2586:
2585:
2583:
2581:
2576:
2567:
2558:
2557:
2546:10.5414/CP201812
2529:
2520:
2519:
2509:
2485:
2479:
2478:
2468:
2444:
2438:
2437:
2429:
2420:
2419:
2385:
2379:
2378:
2349:
2343:
2342:
2340:
2338:
2327:
2321:
2320:
2318:
2316:
2307:
2299:
2293:
2292:
2256:
2250:
2249:
2247:
2245:
2230:
2224:
2223:
2221:
2219:
2204:
2198:
2197:
2171:
2162:
2156:
2155:
2145:
2113:
2107:
2106:
2104:
2102:
2091:
2085:
2084:
2082:
2080:
2069:
2063:
2062:
2060:
2058:
2052:
2044:
2038:
2037:
2009:
1998:
1997:
1987:
1955:
1949:
1948:
1938:
1906:
1897:
1896:
1886:
1854:
1845:
1844:
1842:
1840:
1822:
1816:
1815:
1805:
1795:
1771:
1765:
1764:
1754:
1743:10.7326/M18-1523
1722:
1716:
1715:
1713:
1711:
1697:
1691:
1690:
1688:
1686:
1667:
1661:
1660:
1658:
1639:
1633:
1632:
1630:
1628:
1623:. April 22, 2019
1617:
1611:
1610:
1608:
1606:
1592:
1586:
1585:
1583:
1581:
1567:
1561:
1560:
1543:Kiser K (2017).
1540:
1534:
1533:
1531:
1529:
1514:
1497:
1496:
1486:
1462:
1456:
1455:
1453:
1451:
1433:
1424:
1423:
1421:
1419:
1405:
1394:
1393:
1391:
1389:
1384:. August 9, 2022
1374:
1355:
1350:
1344:
1343:
1341:
1339:
1329:nctr-crs.fda.gov
1321:
1315:
1314:
1312:
1310:
1296:
1203:knee replacement
1077:pharmacokinetics
892:
837:pulmonary emboli
810:
809:
802:
744:
724:
712:
706:
700:
697:
691:
685:
668:
657:
646:
645:
631:
629:
613:
607:
586:
566:
546:
526:
506:
486:
466:
446:
436:
435:
421:
370:
368:
367:
364:
361:
354:
352:
351:
348:
345:
326:
276:
273:
268:
261:
258:
248:
245:
237:
234:
224:
221:
199:
156:
153:
135:
124:
121:
108:
94:
53:
44:
37:
35:
21:
4067:
4066:
4062:
4061:
4060:
4058:
4057:
4056:
4002:
4001:
4000:
3990:
3988:
3980:
3978:
3973:
3972:
3957:Clinical trials
3936:
3908:
3818:
3805:
3752:
3675:
3631:
3534:
3529:
3517:
3508:
3484:1,3-Indandiones
3426:
3405:
3379:
3351:
3294:
3256:
3218:
3170:Thienopyridines
3159:
3150:
3098:
3079:Antithrombotics
3076:
3046:
3045:
2999:
2998:
2994:
2984:
2982:
2981:on May 15, 2016
2969:
2968:
2964:
2933:(10): 883–891.
2920:
2919:
2912:
2898:
2897:
2890:
2880:
2878:
2869:
2868:
2861:
2851:
2849:
2839:
2838:
2834:
2824:
2822:
2813:
2812:
2808:
2798:
2796:
2783:
2782:
2778:
2768:
2766:
2753:
2752:
2748:
2738:
2736:
2727:
2726:
2722:
2712:
2710:
2706:
2699:
2695:
2694:
2690:
2652:
2651:
2647:
2638:
2637:
2630:
2594:
2593:
2589:
2579:
2577:
2574:
2569:
2568:
2561:
2531:
2530:
2523:
2487:
2486:
2482:
2446:
2445:
2441:
2431:
2430:
2423:
2408:
2387:
2386:
2382:
2351:
2350:
2346:
2336:
2334:
2329:
2328:
2324:
2314:
2312:
2305:
2301:
2300:
2296:
2261:Pharmacotherapy
2258:
2257:
2253:
2243:
2241:
2232:
2231:
2227:
2217:
2215:
2206:
2205:
2201:
2169:
2164:
2163:
2159:
2115:
2114:
2110:
2100:
2098:
2093:
2092:
2088:
2078:
2076:
2071:
2070:
2066:
2056:
2054:
2050:
2046:
2045:
2041:
2011:
2010:
2001:
1957:
1956:
1952:
1908:
1907:
1900:
1856:
1855:
1848:
1838:
1836:
1835:. July 25, 2012
1824:
1823:
1819:
1773:
1772:
1768:
1737:(11): 774–787.
1724:
1723:
1719:
1709:
1707:
1699:
1698:
1694:
1684:
1682:
1669:
1668:
1664:
1641:
1640:
1636:
1626:
1624:
1619:
1618:
1614:
1604:
1602:
1594:
1593:
1589:
1579:
1577:
1569:
1568:
1564:
1557:
1542:
1541:
1537:
1527:
1525:
1516:
1515:
1500:
1464:
1463:
1459:
1449:
1447:
1435:
1434:
1427:
1417:
1415:
1407:
1406:
1397:
1387:
1385:
1376:
1375:
1358:
1351:
1347:
1337:
1335:
1323:
1322:
1318:
1308:
1306:
1298:
1297:
1290:
1285:
1253:
1244:
1199:hip replacement
1187:
1175:
1162:
1138:
1094:
1085:bioavailability
1036:Unfractionated
1031:anticoagulation
1003:
979:
955:
953:Adverse effects
934:
899:
890:
852:spinal hematoma
805:
803:
800:(what is this?)
797:
792:
789:
784:
779:
778:
767:
764:
759:
758:
747:
722:
709:
703:
694:
688:
649:
625:
617:
589:
569:
549:
529:
509:
489:
469:
449:
432:
424:
404:
401:
388:
387:
365:
362:
359:
358:
356:
349:
346:
343:
342:
340:
324:
296:Bioavailability
288:Pharmacokinetic
282:
266:
203:
171:
164:
145:
138:
68:Xarelto, others
28:
23:
22:
15:
12:
11:
5:
4065:
4063:
4055:
4054:
4049:
4044:
4039:
4034:
4029:
4024:
4019:
4014:
4004:
4003:
3999:
3998:
3975:
3974:
3971:
3970:
3969:
3968:
3965:
3954:
3948:
3942:
3941:
3938:
3937:
3935:
3934:
3929:
3924:
3918:
3916:
3910:
3909:
3907:
3906:
3901:
3896:
3886:
3881:
3876:
3871:
3870:
3869:
3864:
3856:
3855:
3854:
3849:
3844:
3839:
3824:
3822:
3811:
3810:
3807:
3806:
3804:
3803:
3798:
3793:
3792:
3791:
3781:
3776:
3771:
3766:
3760:
3758:
3754:
3753:
3751:
3750:
3745:
3740:
3735:
3730:
3725:
3716:
3715:
3714:
3709:
3704:
3689:
3687:
3681:
3680:
3677:
3676:
3674:
3673:
3668:
3663:
3658:
3653:
3648:
3642:
3640:
3633:
3632:
3630:
3629:
3628:
3627:
3622:
3617:
3607:
3606:
3605:
3600:
3590:
3589:
3588:
3583:
3578:
3573:
3568:
3563:
3558:
3553:
3542:
3540:
3519:
3510:
3509:
3507:
3506:
3500:
3495:
3490:
3481:
3476:
3471:
3466:
3461:
3456:
3446:
3444:
3417:
3415:Anticoagulants
3411:
3410:
3407:
3406:
3404:
3403:
3398:
3393:
3387:
3385:
3381:
3380:
3378:
3377:
3372:
3367:
3361:
3359:
3353:
3352:
3350:
3349:
3348:
3347:
3342:
3332:
3331:
3330:
3325:
3320:
3304:
3302:
3296:
3295:
3293:
3292:
3287:
3282:
3277:
3272:
3266:
3264:
3262:COX inhibitors
3258:
3257:
3255:
3254:
3249:
3244:
3239:
3233:
3231:
3220:
3219:
3217:
3216:
3215:
3214:
3209:
3204:
3189:
3188:
3187:
3182:
3177:
3166:
3164:
3157:
3152:
3151:
3149:
3148:
3143:
3138:
3133:
3128:
3123:
3117:
3115:
3106:
3100:
3099:
3087:anticoagulants
3077:
3075:
3074:
3067:
3060:
3052:
3044:
3043:
2992:
2962:
2910:
2888:
2859:
2832:
2806:
2776:
2746:
2720:
2688:
2645:
2628:
2607:(2): 264–269.
2587:
2559:
2540:(7): 549–561.
2521:
2480:
2439:
2421:
2406:
2380:
2361:(19): 5900–8.
2344:
2322:
2294:
2267:(2): 198–207.
2251:
2225:
2199:
2180:(2): 293–300.
2157:
2108:
2086:
2064:
2039:
2020:(4): 636–643.
1999:
1970:(3): 455–466.
1950:
1898:
1846:
1817:
1766:
1717:
1692:
1662:
1634:
1612:
1587:
1562:
1555:
1535:
1498:
1457:
1437:"Xarelto EPAR"
1425:
1395:
1356:
1345:
1316:
1287:
1286:
1284:
1281:
1252:
1249:
1243:
1240:
1186:
1183:
1174:
1171:
1161:
1158:
1137:
1134:
1093:
1090:
1002:
999:
987:andexanet alfa
978:
977:Reversal agent
975:
954:
951:
946:P-glycoprotein
933:
930:
909:in preventing
898:
895:
812:
811:
794:
793:
791:
790:
787:
785:
782:
774:
773:
772:
769:
768:
766:
765:
762:
754:
753:
752:
749:
748:
746:
745:
737:
735:
727:
726:
720:
714:
713:
707:
701:
692:
686:
681:
675:
674:
670:
669:
659:
651:
650:
648:
647:
634:
632:
619:
618:
616:
615:
599:
597:
591:
590:
588:
587:
579:
577:
571:
570:
568:
567:
559:
557:
551:
550:
548:
547:
539:
537:
531:
530:
528:
527:
519:
517:
511:
510:
508:
507:
499:
497:
491:
490:
488:
487:
479:
477:
471:
470:
468:
467:
459:
457:
451:
450:
448:
447:
439:
437:
426:
425:
423:
422:
414:
412:
406:
405:
403:
402:
391:
383:
382:
381:
378:
377:
373:
372:
338:
332:
331:
328:
319:
318:
308:
302:
301:
298:
292:
291:
284:
283:
281:
280:
271:
256:
243:
232:
218:
216:
210:
209:
205:
204:
202:
201:
188:
186:
180:
179:
174:
172:administration
166:
165:
163:
162:
160:
150:
148:
140:
139:
137:
136:
118:
116:
110:
109:
102:
96:
95:
88:
78:
77:
74:
70:
69:
66:
60:
59:
55:
54:
46:
45:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
4064:
4053:
4050:
4048:
4045:
4043:
4040:
4038:
4035:
4033:
4030:
4028:
4025:
4023:
4020:
4018:
4017:Morpholinones
4015:
4013:
4010:
4009:
4007:
3997:
3987:
3983:
3966:
3964:
3961:
3960:
3958:
3955:
3952:
3949:
3947:
3944:
3943:
3939:
3933:
3930:
3928:
3925:
3923:
3920:
3919:
3917:
3915:
3914:Non-medicinal
3911:
3905:
3902:
3900:
3897:
3895:
3891:
3887:
3885:
3884:Streptokinase
3882:
3880:
3877:
3875:
3872:
3868:
3865:
3863:
3860:
3859:
3857:
3853:
3850:
3848:
3845:
3843:
3840:
3838:
3835:
3834:
3833:
3829:
3826:
3825:
3823:
3821:
3820:fibrinolytics
3816:
3812:
3802:
3799:
3797:
3794:
3790:
3787:
3786:
3785:
3782:
3780:
3777:
3775:
3772:
3770:
3767:
3765:
3762:
3761:
3759:
3755:
3749:
3746:
3744:
3741:
3739:
3736:
3734:
3731:
3729:
3726:
3724:
3720:
3717:
3713:
3710:
3708:
3705:
3703:
3700:
3699:
3698:
3694:
3691:
3690:
3688:
3686:
3682:
3672:
3669:
3667:
3664:
3662:
3659:
3657:
3654:
3652:
3649:
3647:
3644:
3643:
3641:
3638:
3634:
3626:
3623:
3621:
3618:
3616:
3613:
3612:
3611:
3608:
3604:
3601:
3599:
3596:
3595:
3594:
3591:
3587:
3584:
3582:
3579:
3577:
3574:
3572:
3569:
3567:
3564:
3562:
3559:
3557:
3554:
3552:
3549:
3548:
3547:
3544:
3543:
3541:
3538:
3532:
3527:
3523:
3520:
3515:
3511:
3505:
3501:
3499:
3496:
3494:
3491:
3489:
3485:
3482:
3480:
3477:
3475:
3474:Phenprocoumon
3472:
3470:
3467:
3465:
3462:
3460:
3459:Coumatetralyl
3457:
3455:
3454:Acenocoumarol
3451:
3448:
3447:
3445:
3442:
3438:
3434:
3430:
3425:
3421:
3418:
3416:
3412:
3402:
3399:
3397:
3394:
3392:
3389:
3388:
3386:
3382:
3376:
3373:
3371:
3368:
3366:
3363:
3362:
3360:
3358:
3354:
3346:
3343:
3341:
3338:
3337:
3336:
3333:
3329:
3326:
3324:
3321:
3318:
3314:
3311:
3310:
3309:
3306:
3305:
3303:
3301:
3297:
3291:
3288:
3286:
3283:
3281:
3278:
3276:
3273:
3271:
3268:
3267:
3265:
3263:
3259:
3253:
3250:
3248:
3245:
3243:
3240:
3238:
3235:
3234:
3232:
3229:
3225:
3221:
3213:
3210:
3208:
3205:
3203:
3200:
3199:
3197:
3193:
3190:
3186:
3183:
3181:
3178:
3176:
3173:
3172:
3171:
3168:
3167:
3165:
3163:
3160:
3153:
3147:
3144:
3142:
3139:
3137:
3134:
3132:
3129:
3127:
3124:
3122:
3119:
3118:
3116:
3114:
3110:
3107:
3105:
3101:
3096:
3092:
3088:
3084:
3083:thrombolytics
3080:
3073:
3068:
3066:
3061:
3059:
3054:
3053:
3050:
3039:
3035:
3030:
3025:
3020:
3015:
3011:
3007:
3003:
2996:
2993:
2980:
2976:
2972:
2966:
2963:
2958:
2954:
2949:
2944:
2940:
2936:
2932:
2928:
2924:
2917:
2915:
2911:
2906:
2905:The Chronicle
2902:
2895:
2893:
2889:
2877:
2873:
2866:
2864:
2860:
2848:
2847:
2842:
2836:
2833:
2820:
2816:
2810:
2807:
2794:
2790:
2786:
2780:
2777:
2764:
2760:
2756:
2750:
2747:
2734:
2730:
2724:
2721:
2705:
2698:
2692:
2689:
2684:
2680:
2676:
2672:
2668:
2664:
2660:
2656:
2649:
2646:
2641:
2635:
2633:
2629:
2624:
2620:
2615:
2610:
2606:
2602:
2598:
2591:
2588:
2573:
2566:
2564:
2560:
2555:
2551:
2547:
2543:
2539:
2535:
2528:
2526:
2522:
2517:
2513:
2508:
2503:
2499:
2495:
2491:
2484:
2481:
2476:
2472:
2467:
2462:
2459:(2): 155–65.
2458:
2454:
2450:
2443:
2440:
2435:
2428:
2426:
2422:
2417:
2413:
2409:
2403:
2399:
2395:
2391:
2384:
2381:
2376:
2372:
2368:
2364:
2360:
2356:
2348:
2345:
2332:
2326:
2323:
2311:
2304:
2298:
2295:
2290:
2286:
2282:
2278:
2274:
2270:
2266:
2262:
2255:
2252:
2240:
2236:
2233:Schroeder C.
2229:
2226:
2214:
2210:
2207:Schroeder C.
2203:
2200:
2195:
2191:
2187:
2183:
2179:
2175:
2168:
2161:
2158:
2153:
2149:
2144:
2139:
2135:
2131:
2128:(2): 285–93.
2127:
2123:
2119:
2112:
2109:
2096:
2090:
2087:
2074:
2068:
2065:
2049:
2043:
2040:
2035:
2031:
2027:
2023:
2019:
2015:
2008:
2006:
2004:
2000:
1995:
1991:
1986:
1981:
1977:
1973:
1969:
1965:
1961:
1954:
1951:
1946:
1942:
1937:
1932:
1928:
1924:
1920:
1916:
1912:
1905:
1903:
1899:
1894:
1890:
1885:
1880:
1876:
1872:
1868:
1864:
1860:
1853:
1851:
1847:
1834:
1832:
1827:
1821:
1818:
1813:
1809:
1804:
1799:
1794:
1789:
1785:
1781:
1777:
1770:
1767:
1762:
1758:
1753:
1748:
1744:
1740:
1736:
1732:
1728:
1721:
1718:
1706:
1702:
1696:
1693:
1680:
1676:
1672:
1666:
1663:
1657:
1652:
1648:
1644:
1638:
1635:
1622:
1616:
1613:
1601:
1597:
1591:
1588:
1576:
1572:
1566:
1563:
1558:
1556:9783319546438
1552:
1548:
1547:
1539:
1536:
1523:
1519:
1513:
1511:
1509:
1507:
1505:
1503:
1499:
1494:
1490:
1485:
1480:
1477:(5): 238–44.
1476:
1472:
1468:
1461:
1458:
1445:
1443:
1438:
1432:
1430:
1426:
1414:
1410:
1404:
1402:
1400:
1396:
1383:
1379:
1373:
1371:
1369:
1367:
1365:
1363:
1361:
1357:
1354:
1349:
1346:
1334:
1330:
1326:
1320:
1317:
1305:
1301:
1295:
1293:
1289:
1282:
1280:
1277:
1275:
1271:
1267:
1263:
1262:Robert Califf
1258:
1250:
1248:
1241:
1239:
1237:
1233:
1228:
1226:
1222:
1218:
1213:
1211:
1206:
1204:
1200:
1196:
1192:
1191:Health Canada
1184:
1182:
1180:
1172:
1166:
1159:
1157:
1155:
1151:
1147:
1143:
1135:
1133:
1131:
1127:
1123:
1119:
1118:oxazolidinone
1115:
1111:
1103:
1098:
1091:
1089:
1086:
1082:
1081:dose response
1078:
1073:
1071:
1067:
1063:
1062:acenocoumarol
1059:
1058:phenprocoumon
1055:
1051:
1047:
1043:
1039:
1034:
1032:
1028:
1024:
1020:
1016:
1012:
1008:
1000:
998:
996:
992:
988:
984:
976:
974:
971:
966:
964:
960:
952:
950:
947:
943:
938:
931:
929:
927:
923:
918:
916:
912:
908:
904:
896:
894:
888:
884:
879:
877:
873:
870:it has fewer
869:
865:
864:breastfeeding
861:
857:
853:
848:
846:
842:
838:
834:
830:
826:
822:
818:
808:
801:
795:
786:
781:
777:
770:
761:
757:
750:
743:
739:
738:
736:
733:
728:
721:
719:
715:
682:
680:
676:
671:
667:
663:
660:
658:
656:ECHA InfoCard
652:
644:
640:
639:DTXSID3057723
636:
635:
633:
624:
620:
612:
611:RCSB PDB
606:
601:
600:
598:
596:
592:
585:
581:
580:
578:
576:
572:
565:
561:
560:
558:
556:
552:
545:
541:
540:
538:
536:
532:
525:
521:
520:
518:
516:
512:
505:
501:
500:
498:
496:
492:
485:
481:
480:
478:
476:
472:
465:
461:
460:
458:
456:
452:
445:
441:
440:
438:
431:
427:
420:
416:
415:
413:
411:
407:
399:
395:
390:
386:
379:
374:
339:
337:
333:
329:
327:
320:
316:
312:
309:
307:
303:
299:
297:
293:
289:
285:
279: Rx-only
272:
269:
257:
254:
244:
242:
233:
230:
220:
219:
217:
215:
211:
206:
198:
193:
190:
189:
187:
185:
181:
178:
175:
173:
167:
161:
152:
151:
149:
147:
141:
134:
129:
120:
119:
117:
115:
111:
107:
103:
101:
97:
93:
89:
87:
83:
79:
75:
71:
67:
65:
61:
58:Clinical data
56:
52:
47:
43:
38:
30:
19:
4027:Chloroarenes
3904:Fibrinolysin
3874:Anistreplase
3852:Desmoteplase
3847:Tenecteplase
3748:Ximelagatran
3670:
3598:Fondaparinux
3537:antithrombin
3493:Diphenadione
3370:Dipyridamole
3313:Dipyridamole
3252:Treprostinil
3247:Prostacyclin
3126:Eptifibatide
3012:(8): 785–8.
3009:
3005:
2995:
2983:. Retrieved
2979:the original
2974:
2965:
2930:
2926:
2904:
2879:. Retrieved
2875:
2850:. Retrieved
2844:
2835:
2823:. Retrieved
2818:
2809:
2797:. Retrieved
2793:the original
2779:
2767:. Retrieved
2763:the original
2749:
2737:. Retrieved
2723:
2711:. Retrieved
2704:the original
2691:
2658:
2654:
2648:
2604:
2600:
2590:
2578:. Retrieved
2537:
2533:
2497:
2493:
2483:
2456:
2452:
2442:
2389:
2383:
2358:
2354:
2347:
2335:. Retrieved
2325:
2313:. Retrieved
2309:
2297:
2264:
2260:
2254:
2242:. Retrieved
2238:
2228:
2216:. Retrieved
2212:
2202:
2177:
2173:
2160:
2125:
2121:
2111:
2101:September 1,
2099:. Retrieved
2089:
2079:September 1,
2077:. Retrieved
2067:
2057:September 1,
2055:. Retrieved
2042:
2017:
2013:
1967:
1963:
1953:
1918:
1914:
1866:
1862:
1837:. Retrieved
1829:
1820:
1783:
1779:
1769:
1734:
1730:
1720:
1708:. Retrieved
1704:
1695:
1683:. Retrieved
1674:
1665:
1656:10665/345533
1646:
1637:
1625:. Retrieved
1615:
1603:. Retrieved
1599:
1590:
1578:. Retrieved
1574:
1565:
1545:
1538:
1526:. Retrieved
1521:
1474:
1470:
1460:
1450:November 13,
1448:. Retrieved
1440:
1418:November 13,
1416:. Retrieved
1412:
1386:. Retrieved
1381:
1348:
1336:. Retrieved
1328:
1319:
1307:. Retrieved
1303:
1278:
1254:
1245:
1242:Legal action
1229:
1214:
1207:
1188:
1185:Legal status
1176:
1139:
1129:
1107:
1074:
1050:antithrombin
1046:fondaparinux
1044:(LMWH), and
1035:
1004:
994:
980:
968:As of 2015,
967:
956:
939:
935:
919:
900:
897:Medical uses
880:
849:
820:
816:
815:
804:
798:
584:ChEMBL198362
397:
393:
323:Elimination
214:Legal status
208:Legal status
114:License data
29:
3953:from market
3774:Defibrotide
3702:Bivalirudin
3671:Rivaroxaban
3610:Heparinoids
3603:Idraparinux
3504:Tioclomarol
3498:Phenindione
3488:Clorindione
3391:Cloricromen
3185:Ticlopidine
3175:Clopidogrel
2769:January 31,
2739:January 31,
2713:January 19,
2494:Circulation
1710:January 14,
1685:January 14,
1388:November 9,
1338:October 22,
1148:(a part of
856:anaphylaxis
829:blood clots
817:Rivaroxaban
725: g·mol
662:100.210.589
564:CHEBI:68579
419:366789-02-8
396:)-5-chloro-
376:Identifiers
133:Rivaroxaban
100:MedlinePlus
76:BAY 59-7939
73:Other names
64:Trade names
34:Rivaroxaban
4022:Thiophenes
4006:Categories
3879:Monteplase
3796:Ramatroban
3779:Nafamostat
3764:Abelacimab
3743:Melagatran
3728:Dabigatran
3723:Argatroban
3666:Otamixaban
3651:Betrixaban
3639:("xabans")
3625:Sulodexide
3615:Danaparoid
3586:Tinzaparin
3576:Parnaparin
3571:Nadroparin
3566:Enoxaparin
3561:Dalteparin
3556:Certoparin
3516:inhibitors
3464:Dicoumarol
3365:Cilostazol
3345:Terutroban
3323:Picotamide
3212:Ticagrelor
3196:nucleoside
3192:Nucleotide
3162:inhibitors
3141:Sibrafiban
2436:. Reuters.
2244:August 20,
2218:August 10,
1839:January 1,
1786:: 640723.
1780:Thrombosis
1283:References
1110:structural
730:3D model (
718:Molar mass
595:PDB ligand
524:9NDF7JZ4M3
495:ChemSpider
455:IUPHAR/BPS
410:CAS Number
385:IUPAC name
306:Metabolism
3963:Phase III
3951:Withdrawn
3867:Urokinase
3862:Saruplase
3842:Reteplase
3837:Alteplase
3784:Protein C
3738:Inogatran
3733:Efegatran
3719:Univalent
3712:Lepirudin
3707:Desirudin
3656:Darexaban
3581:Reviparin
3551:Bemiparin
3514:Factor Xa
3450:Coumarins
3427:(inhibit
3401:Vorapaxar
3375:Triflusal
3340:Terbogrel
3328:Terbogrel
3317:+ aspirin
3290:Triflusal
3285:Indobufen
3275:Aloxiprin
3237:Beraprost
3207:Elinogrel
3202:Cangrelor
3180:Prasugrel
3146:Tirofiban
3136:Roxifiban
3131:Orbofiban
3121:Abciximab
2899:Patel V.
2825:April 27,
2623:220129323
2337:August 1,
2315:August 1,
1869:: 27–32.
1627:April 24,
1605:April 24,
1575:Drugs.com
1522:Drugs.com
1471:P & T
1304:Drugs.com
1205:surgery.
1201:or total
1173:Economics
1114:linezolid
1102:linezolid
1092:Chemistry
1027:platelets
1007:Factor Xa
876:factor Xa
860:pregnancy
336:Excretion
325:half-life
170:Routes of
144:Pregnancy
92:Monograph
86:Drugs.com
3996:Medicine
3693:Bivalent
3661:Edoxaban
3646:Apixaban
3479:Warfarin
3396:Ditazole
3242:Iloprost
3198:analogs
3038:26839968
2957:21830957
2852:April 7,
2683:23498967
2675:19925048
2580:June 11,
2554:23458226
2516:17116766
2475:18096568
2416:80951298
2375:16161994
2289:22593448
2281:25644580
2239:DrugNews
2213:DrugNews
2194:24681117
2152:25689417
2097:. RxList
2034:31925665
1994:23305158
1945:31380891
1893:33906063
1812:24455237
1761:30383133
1705:ClinCalc
1679:Archived
1675:ClinCalc
1645:(2021).
1528:March 3,
1493:19561868
1413:DailyMed
1309:March 3,
1251:Research
1130:in vitro
1070:factor X
1054:warfarin
1023:thrombin
959:bleeding
907:warfarin
868:warfarin
845:by mouth
807:(verify)
475:DrugBank
184:ATC code
177:By mouth
146:category
128:DailyMed
3932:Oxalate
3922:Citrate
3899:Brinase
3697:Hirudin
3526:Heparin
3502:Other:
2948:3860773
2799:July 1,
2143:4541976
2075:. WebMD
1985:3769672
1936:6686768
1884:8225570
1803:3885278
1752:6825839
1484:2697099
1136:History
1040:(UFH),
1038:heparin
1009:in the
995:AndexXa
911:strokes
821:Xarelto
679:Formula
504:8051086
484:DB06228
444:6433119
430:PubChem
369:
357:
353:
341:
270:Rx-only
267:WARNING
239::
200:)
194: (
192:B01AF01
159: C
130::
106:a611049
18:Xarelto
3982:Portal
3946:WHO-EM
3894:Ancrod
3888:Other
3535:(bind
3528:group/
3036:
2985:May 3,
2955:
2945:
2881:May 3,
2681:
2673:
2621:
2552:
2514:
2473:
2414:
2404:
2373:
2287:
2279:
2192:
2150:
2140:
2032:
1992:
1982:
1943:
1933:
1891:
1881:
1833:(NICE)
1810:
1800:
1759:
1749:
1580:May 9,
1553:
1491:
1481:
1060:, and
942:CYP3A4
891:
756:SMILES
723:435.88
575:ChEMBL
544:D07086
315:CYP2J2
311:CYP3A4
264:
251:
241:℞-only
227:
126:
3832:r-tPA
3757:Other
3384:Other
2759:Bayer
2733:Bayer
2707:(PDF)
2700:(PDF)
2679:S2CID
2619:S2CID
2575:(PDF)
2412:S2CID
2306:(PDF)
2285:S2CID
2170:(PDF)
2051:(PDF)
1444:(EMA)
1382:(emc)
1266:Bayer
1142:Bayer
776:InChI
732:JSmol
602:RIV (
555:ChEBI
3927:EDTA
3858:UPA
3801:REG1
3228:PGI2
3089:and
3034:PMID
2987:2016
2953:PMID
2883:2016
2854:2019
2827:2016
2801:2011
2771:2010
2741:2010
2715:2017
2671:PMID
2582:2009
2550:PMID
2512:PMID
2471:PMID
2402:ISBN
2371:PMID
2339:2018
2317:2018
2277:PMID
2246:2015
2220:2016
2190:PMID
2148:PMID
2103:2014
2081:2014
2059:2014
2030:PMID
1990:PMID
1941:PMID
1889:PMID
1841:2020
1808:PMID
1784:2013
1757:PMID
1712:2024
1687:2024
1629:2019
1607:2019
1582:2017
1551:ISBN
1530:2019
1489:PMID
1452:2020
1420:2020
1390:2022
1340:2023
1311:2019
1268:and
1064:are
862:and
854:and
835:and
605:PDBe
535:KEGG
515:UNII
464:6388
290:data
82:AHFS
3433:VII
3095:B01
3093:) (
3024:hdl
3014:doi
3010:374
2943:PMC
2935:doi
2931:365
2663:doi
2609:doi
2542:doi
2502:doi
2498:114
2461:doi
2394:doi
2363:doi
2310:FDA
2269:doi
2182:doi
2138:PMC
2130:doi
2022:doi
1980:PMC
1972:doi
1931:PMC
1923:doi
1919:179
1879:PMC
1871:doi
1867:203
1798:PMC
1788:doi
1747:PMC
1739:doi
1735:169
1651:hdl
1479:PMC
1333:FDA
1232:FDA
917:.
628:EPA
434:CID
253:POM
197:WHO
4008::
3959::
3892::
3830::
3721::
3695::
3486::
3452::
3439:,
3437:IX
3435:,
3431:,
3429:II
3158:12
3085:,
3032:.
3022:.
3008:.
3004:.
2973:.
2951:.
2941:.
2929:.
2925:.
2913:^
2903:.
2891:^
2874:.
2862:^
2843:.
2817:.
2677:.
2669:.
2659:10
2657:.
2631:^
2617:.
2603:.
2599:.
2562:^
2548:.
2538:51
2536:.
2524:^
2510:.
2496:.
2492:.
2469:.
2457:29
2455:.
2451:.
2424:^
2410:.
2400:.
2369:.
2359:48
2357:.
2308:.
2283:.
2275:.
2265:35
2263:.
2237:.
2211:.
2188:.
2178:61
2176:.
2172:.
2146:.
2136:.
2126:80
2124:.
2120:.
2028:.
2018:49
2016:.
2002:^
1988:.
1978:.
1968:76
1966:.
1962:.
1939:.
1929:.
1917:.
1913:.
1901:^
1887:.
1877:.
1865:.
1861:.
1849:^
1828:.
1806:.
1796:.
1782:.
1778:.
1755:.
1745:.
1733:.
1729:.
1703:.
1677:.
1673:.
1598:.
1573:.
1520:.
1501:^
1487:.
1475:34
1473:.
1469:.
1439:.
1428:^
1411:.
1398:^
1380:.
1359:^
1331:.
1327:.
1302:.
1291:^
1238:.
1072:.
1056:,
997:.
965:.
928:.
878:.
847:.
696:Cl
693:18
687:19
608:,
313:,
275:EU
260:US
247:UK
236:CA
229:S4
223:AU
155:AU
123:US
3984::
3817:/
3539:)
3533:/
3443:)
3441:X
3319:)
3315:(
3230:)
3226:(
3194:/
3097:)
3081:(
3071:e
3064:t
3057:v
3040:.
3026::
3016::
2989:.
2959:.
2937::
2885:.
2856:.
2829:.
2803:.
2773:.
2743:.
2717:.
2685:.
2665::
2625:.
2611::
2605:3
2584:.
2556:.
2544::
2518:.
2504::
2477:.
2463::
2418:.
2396::
2377:.
2365::
2319:.
2291:.
2271::
2248:.
2222:.
2196:.
2184::
2154:.
2132::
2105:.
2083:.
2061:.
2036:.
2024::
1996:.
1974::
1947:.
1925::
1895:.
1873::
1843:.
1814:.
1790::
1763:.
1741::
1714:.
1689:.
1653::
1631:.
1609:.
1584:.
1559:.
1532:.
1495:.
1454:.
1422:.
1392:.
1342:.
1313:.
944:/
734:)
711:S
708:5
705:O
702:3
699:N
690:H
684:C
630:)
626:(
614:)
398:N
394:S
392:(
366:3
363:/
360:1
350:3
347:/
344:2
277::
262::
249::
225::
157::
84:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.