337:
485:
167:
289:
500:
470:
318:
304:
394:
227:
showed that it was in the zwitterionic form in the solid state and confirmed the presence of hydrogen bonds. Theoretical calculations have been used to show that zwitterions may also be present in the gas phase for some cases different from the simple carboxylic acid-to-amine transfer.
411:
Because tautomers are different compounds, they sometimes have different enough structures that they can be detected independently in their mixture. This allows experimental analysis of the equilibrium.
451:
Other examples of permanent zwitterions include phosphatidylcholines, which also contain a quaternary nitrogen atom, but with a negatively-charged phosphate group in place of a carboxylate group;
444:
link. At the present time, all compounds whose structure includes this motif are known as betaines. Betaines do not isomerize because the chemical groups attached to the nitrogen atom are not
484:
852:
Cotrait, Par Michel (1972). "La structure cristalline de l'acide éthylènediamine tétraacétique, EDTA" [The crystalline structure of ethylenediamine tetraacetic acid, EDTA].
914:
Nagy, Peter I.; Takács-Novák, Krisztina (1997). "Theoretical and
Experimental Studies of the Zwitterion ⇌ Neutral Form Equilibrium of Ampholytes in Pure Solvents and Mixtures".
575:
879:
Kiruba, G. S. M.; Ming, Wah Wong (2003). "Tautomeric
Equilibria of Pyridoxal-5′-phosphate and 3-Hydroxypyridine Derivatives: A Theoretical Study of Solvation Effects".
408:, in aqueous solution is predicted to have an equilibrium favoring a tautomeric form in which a proton is transferred from the phenolic -OH group to the nitrogen atom.
685:"Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid α-glycine"
521:
character, activate strong bonds and small molecules, and serve as transient intermediates in catalysis. Donor-acceptor entities are of vast use in photochemistry (
336:
288:
662:
250:. This is also consistent with the zwitterion being the predominant isomer that is present in an aqueous solution. For comparison, the simple carboxylic acid
448:. These compounds may be classed as permanent zwitterions, as isomerisation to a molecule with no electrical charges does not occur, or is very slow.
401:
Insight to the equilibrium in solution may be gained from the results of theoretical calculations. For example, pyridoxal phosphate, a form of
623:
707:
950:
605:
85:
469:
567:
522:
460:
174:
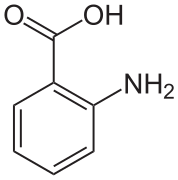
contains both acidic (carboxylic acid fragment) and basic (amine fragment) centres. The isomer on the right is a zwitterion.
215:
It has been suggested, on the basis of theoretical analysis, that the zwitterion is stabilized in aqueous solution by
499:
166:
642:
317:
232:
102:
374:
is a zwitterion with two protons having been transferred from carboxylic acid groups to the nitrogen atoms.
303:
1013:
Munz, Dominik; Karsten, Meyer (2021). "Charge frustration in ligand design and functional group transfer".
428:, was named as "betaine". Later, other compounds were discovered that contain the same structural motif, a
505:
429:
133:
1054:
456:
517:
Strongly polarized conjugated compounds (conjugated zwitterions) are typically very reactive, share
526:
491:
220:
147:
1030:
684:
530:
995:
987:
946:
896:
762:
619:
601:
1022:
979:
923:
888:
861:
834:
793:
752:
744:
699:
654:
547:
475:
452:
421:
356:
309:
216:
125:
41:
437:
94:
455:, which contain a quaternary nitrogen atom and a negatively charged sulfonate group; and
757:
732:
251:
968:"Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants"
967:
1048:
1034:
983:
349:
294:
143:
109:
393:
385:
and may jump to the phosphate group to form a compound which is not a zwitterion.
596:
Skoog, Douglas A.; West, Donald M.; Holler, F. James; Crouch, Stanley R. (2004).
208:
The ratio of the concentrations of the two species in solution is independent of
643:"On the Number of Water Molecules Necessary to Stabilize the Glycine Zwitterion"
542:
433:
155:
151:
1026:
865:
838:
798:
781:
703:
425:
402:
378:
342:
171:
129:
27:
Molecule containing an equal number of positive and negative functional groups
991:
518:
463:. Lauramidopropyl betaine is the major component of cocamidopropyl betaine.
360:
31:
900:
766:
17:
999:
382:
146:
to an all-neutral form, such as when the positive charge is located on a
121:
658:
782:"A neutron diffraction study on the crystal structure of sulfamic acid"
242:
for deprotonation of the common amino acids span the approximate range
224:
139:
927:
892:
748:
136:
will be established between the "parent" molecule and the zwitterion.
445:
731:
Price, William D.; Jockusch, Rebecca A.; Williams, Evan R. (1997).
124:
that contains an equal number of positively and negatively charged
600:(8th ed.). Thomson/Brooks/Cole. pp. 231, 385, 419, 460.
367:
324:
363:. One molecule is in the zwitterion form, the other is not.
209:
74:
71:
62:
56:
813:
Brown, C. J.; Ehrenberg, M. (1985). "Anthranilic acid, C
178:
Tautomerism of amino acids follows this stoichiometry:
86:
77:
65:
50:
68:
59:
53:
47:
44:
8:
966:Gonenne, Amnon; Ernst, Robert (1978-06-15).
945:(3rd ed.). New York: Worth Publishing.
733:"Is Arginine a Zwitterion in the Gas Phase?"
381:, the proton on the dimethyl amino group is
219:with solvent water molecules. Analysis of
150:group. Similarly, a molecule containing a
797:
756:
737:Journal of the American Chemical Society
647:Journal of the American Chemical Society
641:Jensen, Jan H.; Gordon, Mark S. (1995).
618:(9th ed.). 2013. pp. 415–416.
392:
265:
261:
257:
192:
188:
184:
165:
559:
465:
284:
943:Lehninger, Principles of Biochemistry
352:crystallizes in the zwitterion form.
101:
7:
616:Fundamentals of Analytical Chemistry
598:Fundamentals of Analytical Chemistry
297:isomers, with the zwitterion (right)
941:Nelson, D. L.; Cox, M. M. (2000).
683:Jönsson, P.-G.; Kvick, Å. (1972).
25:
692:Acta Crystallographica Section B
498:
483:
468:
335:
316:
302:
287:
40:
713:from the original on 2020-03-14
665:from the original on 2020-12-02
578:from the original on 2023-06-21
359:there are two molecules in the
523:photoinduced electron transfer
461:dipalmitoylphosphatidylcholine
416:Betaines and similar compounds
1:
132:, for example, in solution a
984:10.1016/0003-2697(78)90565-1
881:Journal of Organic Chemistry
142:are zwitterions that cannot
825:, by neutron diffraction".
436:group attached to it via a
1071:
1027:10.1038/s41570-021-00276-3
424:, which was isolated from
866:10.1107/S056774087200319X
839:10.1107/S0108270185004206
799:10.1107/S0365110X60000789
704:10.1107/S0567740872005096
854:Acta Crystallographica B
827:Acta Crystallographica C
158:group cannot isomerize.
972:Analytical Biochemistry
786:Acta Crystallographica
513:Conjugated zwitterions
506:cocamidopropyl betaine
478:(trivial name betaine)
398:
175:
457:pulmonary surfactants
432:nitrogen atom with a
396:
169:
780:Sass, R. L. (1960).
572:Chemistry LibreTexts
366:In the solid state,
134:chemical equilibrium
743:(49): 11988–11989.
659:10.1021/ja00136a013
527:organic electronics
492:phosphatidylcholine
397:pyridoxal phosphate
389:Theoretical studies
221:neutron diffraction
148:quaternary ammonium
112:'), also called an
399:
176:
928:10.1021/ja963512f
922:(21): 4999–5006.
893:10.1021/jo0266792
749:10.1021/ja9711627
653:(31): 8159–8170.
625:978-1-285-60719-1
126:functional groups
103:[ˈtsvɪtɐ]
16:(Redirected from
1062:
1039:
1038:
1010:
1004:
1003:
963:
957:
956:
938:
932:
931:
916:J. Am. Chem. Soc
911:
905:
904:
887:(7): 2874–2881.
876:
870:
869:
849:
843:
842:
810:
804:
803:
801:
777:
771:
770:
760:
728:
722:
721:
719:
718:
712:
698:(6): 1827–1833.
689:
680:
674:
673:
671:
670:
638:
632:
629:
611:
593:
587:
586:
584:
583:
564:
548:Azomethine ylide
529:, switching and
502:
487:
476:Trimethylglycine
472:
422:trimethylglycine
357:anthranilic acid
339:
320:
310:Anthranilic acid
306:
291:
269:
249:
247:
217:hydrogen bonding
204:
203:
202:
199:
105:
89:
84:
83:
80:
79:
76:
73:
70:
67:
64:
61:
58:
55:
52:
49:
46:
21:
1070:
1069:
1065:
1064:
1063:
1061:
1060:
1059:
1045:
1044:
1043:
1042:
1012:
1011:
1007:
965:
964:
960:
953:
940:
939:
935:
913:
912:
908:
878:
877:
873:
851:
850:
846:
824:
820:
816:
812:
811:
807:
779:
778:
774:
730:
729:
725:
716:
714:
710:
687:
682:
681:
677:
668:
666:
640:
639:
635:
626:
614:
608:
595:
594:
590:
581:
579:
566:
565:
561:
556:
539:
515:
508:
503:
494:
488:
479:
473:
441:
418:
406:
391:
371:
355:In crystals of
345:
340:
331:
328:
321:
312:
307:
298:
292:
283:
281:Other compounds
277:value of 4.88.
276:
267:
263:
259:
255:
245:
243:
239:
200:
197:
196:
194:
190:
186:
182:
164:
87:
43:
39:
28:
23:
22:
15:
12:
11:
5:
1068:
1066:
1058:
1057:
1047:
1046:
1041:
1040:
1021:(6): 422–439.
1015:Nat. Rev. Chem
1005:
958:
951:
933:
906:
871:
860:(3): 781–785.
844:
833:(3): 441–443.
822:
818:
814:
805:
792:(4): 320–324.
772:
723:
675:
633:
631:
630:
624:
606:
588:
574:. 2015-11-03.
558:
557:
555:
552:
551:
550:
545:
538:
535:
514:
511:
510:
509:
504:
497:
495:
489:
482:
480:
474:
467:
439:
417:
414:
404:
390:
387:
369:
347:
346:
341:
334:
332:
326:
322:
315:
313:
308:
301:
299:
293:
286:
282:
279:
274:
252:propionic acid
237:
206:
205:
163:
160:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1067:
1056:
1053:
1052:
1050:
1036:
1032:
1028:
1024:
1020:
1016:
1009:
1006:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
962:
959:
954:
952:1-57259-153-6
948:
944:
937:
934:
929:
925:
921:
917:
910:
907:
902:
898:
894:
890:
886:
882:
875:
872:
867:
863:
859:
855:
848:
845:
840:
836:
832:
828:
809:
806:
800:
795:
791:
787:
783:
776:
773:
768:
764:
759:
754:
750:
746:
742:
738:
734:
727:
724:
709:
705:
701:
697:
693:
686:
679:
676:
664:
660:
656:
652:
648:
644:
637:
634:
627:
621:
617:
613:
612:
609:
607:0-03-035523-0
603:
599:
592:
589:
577:
573:
569:
563:
560:
553:
549:
546:
544:
541:
540:
536:
534:
532:
528:
524:
520:
512:
507:
501:
496:
493:
490:Example of a
486:
481:
477:
471:
466:
464:
462:
458:
454:
453:sulfobetaines
449:
447:
443:
435:
431:
427:
423:
420:The compound
415:
413:
409:
407:
395:
388:
386:
384:
380:
375:
373:
364:
362:
358:
353:
351:
350:Sulfamic acid
344:
338:
333:
330:
323:Structure of
319:
314:
311:
305:
300:
296:
295:Sulfamic acid
290:
285:
280:
278:
273:
253:
241:
236:
229:
226:
222:
218:
213:
211:
181:
180:
179:
173:
168:
161:
159:
157:
153:
149:
145:
141:
137:
135:
131:
127:
123:
119:
115:
111:
110:hermaphrodite
107:
104:
100:
96:
92:
91:
82:
37:
33:
19:
1018:
1014:
1008:
978:(1): 28–38.
975:
971:
961:
942:
936:
919:
915:
909:
884:
880:
874:
857:
853:
847:
830:
826:
808:
789:
785:
775:
740:
736:
726:
715:. Retrieved
695:
691:
678:
667:. Retrieved
650:
646:
636:
615:
597:
591:
580:. Retrieved
571:
568:"Zwitterion"
562:
516:
450:
419:
410:
400:
376:
365:
354:
348:
271:
234:
230:
214:
207:
191:H ⇌ RCH(NH
177:
154:group and a
138:
117:
113:
106:
98:
35:
29:
1055:Zwitterions
543:Amphoterism
434:carboxylate
162:Amino acids
156:carboxylate
152:phosphonium
130:amino acids
118:dipolar ion
93:; from
18:Zwitterions
717:2019-09-03
669:2020-08-28
582:2022-02-11
554:References
430:quaternary
426:sugar beet
379:psilocybin
343:Psilocybin
172:amino acid
114:inner salt
36:zwitterion
1035:235220781
992:0003-2697
519:diradical
403:vitamin B
361:unit cell
270:) has a p
223:data for
144:isomerize
90:-ə-rye-ən
32:chemistry
1049:Category
901:12662064
767:16479267
708:Archived
663:Archived
576:Archived
537:See also
459:such as
140:Betaines
122:molecule
99:Zwitter
758:1364450
531:sensing
225:glycine
128:. With
120:, is a
108: '
1033:
1000:677454
998:
990:
949:
899:
765:
755:
622:
604:
446:labile
383:labile
240:values
183:RCH(NH
95:German
1031:S2CID
711:(PDF)
688:(PDF)
97:
88:TSVIT
996:PMID
988:ISSN
947:ISBN
897:PMID
763:PMID
620:ISBN
602:ISBN
372:EDTA
329:EDTA
244:2.15
231:The
34:, a
1023:doi
980:doi
924:doi
920:119
889:doi
862:doi
835:doi
794:doi
753:PMC
745:doi
741:119
700:doi
655:doi
651:117
525:),
438:–CH
377:In
248:0.2
195:)CO
187:)CO
170:An
116:or
30:In
1051::
1029:.
1017:.
994:.
986:.
976:87
974:.
970:.
918:.
895:.
885:68
883:.
858:28
856:.
831:41
829:.
821:NO
790:13
788:.
784:.
761:.
751:.
739:.
735:.
706:.
696:28
694:.
690:.
661:.
649:.
645:.
570:.
533:.
264:CO
260:CH
256:CH
212:.
210:pH
72:aɪ
1037:.
1025::
1019:5
1002:.
982::
955:.
930:.
926::
903:.
891::
868:.
864::
841:.
837::
823:2
819:7
817:H
815:7
802:.
796::
769:.
747::
720:.
702::
672:.
657::
628:.
610:.
585:.
442:–
440:2
405:6
370:4
368:H
327:4
325:H
275:a
272:K
268:H
266:2
262:2
258:3
254:(
246:±
238:a
235:K
233:p
201:2
198:−
193:3
189:2
185:2
81:/
78:n
75:ə
69:r
66:ˌ
63:ə
60:t
57:ɪ
54:v
51:s
48:t
45:ˈ
42:/
38:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.