686:
663:
42:
3469:
1137:
and decreases the amount of cholesterol normally available to liver cells. The lower levels of cholesterol in the liver cells leads them to absorb more cholesterol from circulation and thus lowering the levels of circulating cholesterol. It blocks the critical mediator of cholesterol absorption, the
2240:
Basha SJ, Naveed SA, Tiwari NK, Shashikumar D, Muzeeb S, Kumar TR, et al. (June 2007). "Concurrent determination of ezetimibe and its phase-I and II metabolites by HPLC with UV detection: quantitative application to various in vitro metabolic stability studies and for qualitative estimation in
1195:
Ezetimibe is primarily metabolized in the liver and the small intestine via glucuronide conjugation with subsequent renal and biliary excretion. Both the parent compound and its active metabolite are eliminated from plasma with a half-life around 22 hours, allowing for once-daily dosing. Ezetimibe
1811:
Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. (July 2011). "ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the
European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS)".
953:
Initiation of ezetimibe along with high-intensity statin therapy at the time of an acute coronary syndrome (ACS) event was associated with significantly better cholesterol reduction at day-7, 1-month, 3-months and 1-year post ACS event; which translated into significantly lower recurrent
2016:
Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M (March 2018). "Effect of
Ezetimibe Monotherapy on Plasma Lipoprotein(a) Concentrations in Patients with Primary Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials".
1184:(45–71 ng/ml) of ezetimibe-glucuronide is attained within 1–2 hours. The concomitant administration of food (high-fat vs. nonfat meals) has no effect on the extent of absorption of ezetimibe. However, coadministration with a high-fat meal increases its C
1208:
5–6). Due to insufficient data, the manufacturer does not recommend ezetimibe for patients with moderate to severe hepatic impairment (Child-Pugh score 7–15). In patients with mild, moderate, or severe hepatic impairment, the mean
1107:
The incidence of overdose with ezetimibe is rare; subsequently, few data exist on the effects of overdose. However, an acute overdose of ezetimibe is expected to produce an exaggeration of its usual effects, leading to
1917:"Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease | Guidance and guidelines | NICE"
758:
310:
2153:
Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, et al. (February 2015). "NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy".
1192:
cannot be determined, since ezetimibe is insoluble in aqueous media suitable for injection. Ezetimibe and its active metabolites are highly bound to human plasma proteins (90%).
235:
1863:"Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version"
1475:
1415:
1311:
2308:
950:. Several treatment guidelines recommend adding ezetimibe in select high risk persons in whom LDL goals cannot be achieved by maximally tolerated statin alone.
810:
InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2/t21-,22+,23-/m1/s1
121:
1934:
Grundy SM, Arai H, Barter P, Bersot TP, Betteridge DJ, Carmena R, et al. (Expert
Dyslipidemia Panel of the International Atherosclerosis Society) (2014).
1180:) was 3.4–5.5 ng/ml. Following oral administration, ezetimibe is absorbed and extensively conjugated to a phenolic glucuronide (active metabolite). Mean C
954:
cardiovascular events (death from any cause, major ACS, non-fatal stroke, non-fatal myocardial infarction, and ischemic stroke) post the index event of ACS.
1445:
782:
3514:
3499:
2218:
2975:
2560:
1210:
946:. Combining ezetimibe with simvastatin had no effect on overall mortality but did lower the risk of heart attack or stroke in people with prior
2276:
2196:
2109:
1902:
1847:
1748:
3442:
3428:
3408:
3085:
3029:
2131:
2301:
1173:
Within 4–12 hours of the oral administration of a 10-mg dose to fasting adults, the attained mean ezetimibe peak plasma concentration (
3108:
888:
3504:
1391:
1002:
802:
2130:. U.S. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services. 27 October 2014.
1213:
values for total ezetimibe are increased about 1.7-fold, 3-to-4-fold, and 5-to-6-fold, respectively, compared to healthy subjects.
2339:
1936:"An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia--full report"
908:
228:
1497:
1467:
1407:
1303:
2294:
432:
295:
153:
1608:
Savarese G, De
Ferrari GM, Rosano GM, Perrone-Filardi P (December 2015). "Safety and efficacy of ezetimibe: A meta-analysis".
1561:
1531:
1354:
3489:
981:
1977:"Impact of early initiation of ezetimibe in patients with acute coronary syndrome: A systematic review and meta-analysis"
2564:
2330:
562:
3391:
3046:
642:
172:
3459:
1916:
1065:
1437:
1200:
isoenzymes, which explains its limited number of drug interactions. No dose adjustment is needed in patients with
1013:
The two contraindications to taking ezetimibe are a previous allergic reaction to it, including symptoms of rash,
3078:
2990:
2889:
2855:
2770:
2512:
1061:
881:
938:
has no effect on overall mortality or cardiovascular mortality, although it significantly reduces the risk of
511:
2210:
3435:
3118:
3034:
2914:
2909:
2884:
2851:
2813:
2597:
2516:
2452:
2379:
947:
209:
681:
3174:
2899:
2894:
1201:
1154:
1143:
935:
877:
873:
849:
378:
2681:
1260:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
957:
Ezetimibe is indicated in the United States as an add-on to dietary measures to reduce levels of certain
3494:
3331:
3225:
3179:
2904:
2371:
2270:
2190:
2103:
1896:
1841:
1742:
1045:
939:
869:
502:
1005:
activity score but the available evidence indicates it does not improve outcomes of hepatic steatosis.
631:
3071:
2945:
2671:
2530:
1259:
922:. In 2022, it was the 79th most commonly prescribed medication in the United States, with more than 8
401:
3153:
2317:
1174:
1057:
861:
658:
457:
216:
3138:
3356:
3280:
2929:
2375:
2178:
2123:
2042:
919:
183:
2062:"Ezetimibe decreased nonalcoholic fatty liver disease activity score but not hepatic steatosis"
3415:
3123:
2924:
2593:
2550:
2448:
2258:
2170:
2091:
2034:
1998:
1957:
1884:
1829:
1793:
1730:
1679:
1625:
1387:
1117:
611:
334:
322:
111:
1697:
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. (June 2015).
3509:
3169:
3128:
2250:
2162:
2081:
2073:
2026:
1988:
1947:
1874:
1821:
1783:
1775:
1720:
1710:
1669:
1659:
1617:
1205:
1069:
994:
A 2018 review found that ezetimibe used as sole treatment slightly lowered plasma levels of
698:
551:
466:
360:
59:
1233:
571:
3473:
3316:
3205:
3184:
2809:
2625:
1825:
1197:
1189:
1134:
918:
Ezetimibe was approved for medical use in the United States in 2002. It is available as a
388:
368:
2243:
Journal of
Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences
1438:"Liptruzet (ezetimibe and atorvastatin) tablets for oral useInitial U.S. Approval: 2013"
685:
662:
3371:
3113:
3094:
3040:
2766:
2606:
2086:
2061:
1788:
1763:
1674:
1647:
1468:"Roszet- rosuvastatin and ezetimibe tablet Roszet (- rosuvastatin and ezetimibe tablet"
1113:
1081:
995:
988:
966:
896:
1048:(≥1% of patients) associated with ezetimibe therapy include headache and/or diarrhea (
3483:
3321:
3235:
3148:
2413:
2403:
2046:
904:
769:
674:
347:
145:
3361:
3260:
3143:
3133:
3015:
2691:
2686:
2653:
2644:
2616:
2545:
2496:
2486:
2466:
2461:
2321:
2182:
853:
491:
278:
273:
268:
263:
258:
253:
248:
243:
2254:
1553:
2166:
1861:
Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. (2013).
1621:
1583:
1523:
1346:
131:
3421:
3336:
3290:
3285:
3270:
3265:
3255:
3230:
2995:
2970:
2950:
2919:
2827:
2799:
2794:
2789:
2784:
2779:
2731:
2726:
2716:
2711:
2676:
2583:
2573:
2501:
2491:
2471:
2421:
2388:
1049:
1033:
1025:
1018:
973:
892:
139:
1993:
1976:
1952:
1935:
3351:
3346:
3341:
3300:
3295:
3250:
3210:
3189:
3010:
2985:
2965:
2960:
2955:
2864:
2837:
2832:
2822:
2736:
2706:
2701:
2696:
2630:
2578:
2540:
2481:
2476:
2398:
2393:
2356:
2286:
2030:
1162:
1158:
1150:
1146:
1084:) have been reported and are included as warnings on the label for ezetimibe.
1073:
1014:
978:
865:
845:
734:
542:
223:
3275:
3240:
3215:
3063:
2721:
2408:
2361:
1920:
1092:
912:
900:
414:
125:
2262:
2174:
2095:
2038:
2002:
1961:
1888:
1833:
1797:
1779:
1734:
1683:
1629:
2077:
1715:
1698:
33:
3366:
3245:
3005:
3000:
2980:
2752:
2525:
2417:
2060:
Lee HY, Jun DW, Kim HJ, Oh H, Saeed WK, Ahn H, et al. (March 2019).
1725:
1109:
1096:
1077:
522:
167:
1664:
891:, joint pain, diarrhea, and tiredness. Serious side effects may include
531:
2847:
2663:
1053:
1029:
477:
1879:
1862:
41:
2535:
2444:
2343:
1139:
1088:
958:
943:
857:
622:
17:
591:
2435:
1699:"Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes"
757:
748:
602:
394:
1021:, and severe liver disease, especially when taken with a statin.
582:
328:
3067:
2290:
341:
162:
1267:
1584:"Ezetimibe Drug Usage Statistics, United States, 2013 - 2022"
1648:"Ezetimibe therapy: mechanism of action and clinical update"
1498:"Nexlizet- bempedoic acid and ezetimibe tablet, film coated"
1024:
Ezetimibe may have significant medication interactions with
647:
1052:). Infrequent adverse effects (0.1–1% of patients) include
856:. Generally it is used together with dietary changes and a
304:
194:
95:
71:
62:
1133:
Ezetimibe inhibits the absorption of cholesterol from the
1975:
Mahajan K, Nagendra L, Dhall A, Dutta D (February 2024).
1095:
uptake, the use of ezetimibe can lead to side effects in
860:. Alone, it is less preferred than a statin. It is taken
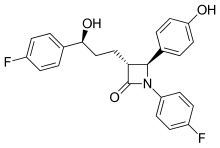
448:)-1-(4-fluorophenyl)-3--4-(4-hydroxyphenyl)azetidin-2-one
83:
77:
2211:"Ezetimibe - National Library of Medicine HSDB Database"
1386:(76 ed.). Pharmaceutical Press. 2018. p. 196.
317:
998:, but the effect was not large enough to be important.
827:
3457:
68:
98:
86:
74:
3400:
3384:
3309:
3198:
3162:
3101:
2938:
2877:
2846:
2808:
2765:
2745:
2662:
2652:
2643:
2615:
2592:
2559:
2510:
2443:
2434:
2370:
2338:
2329:
768:
746:
733:
697:
692:
673:
641:
621:
601:
581:
561:
541:
521:
510:
501:
476:
456:
423:
413:
400:
387:
377:
367:
359:
294:
289:
234:
222:
208:
182:
152:
138:
120:
110:
92:
80:
65:
53:
48:
1228:
1226:
1196:lacks significant inhibitor or inducer effects on
887:The most commonly reported adverse events include
1353:. American Society of Health-System Pharmacists.
1298:
1296:
1294:
1292:
1290:
1288:
1286:
1284:
1080:may occur. Cases of muscle problems (myalgia and
790:Fc1ccc(cc1)(O)CC4C(=O)N(c2ccc(F)cc2)4c3ccc(O)cc3
490:
465:
3079:
2302:
1165:complex involved in trafficking cholesterol.
8:
171:
32:
1408:"Vytorin- ezetimibe and simvastatin tablet"
899:, depression, and muscle breakdown. Use in
3306:
3086:
3072:
3064:
2659:
2649:
2440:
2335:
2309:
2295:
2287:
1341:
1339:
1337:
1335:
1333:
1331:
1329:
1068:) results. Rarely (<0.1% of patients),
972:Mixed hyperlipidemia, in combination with
684:
661:
550:
40:
2976:Magnesium pyridoxal 5-phosphate glutamate
2085:
1992:
1951:
1878:
1867:Journal of Atherosclerosis and Thrombosis
1787:
1724:
1714:
1673:
1663:
907:is of unclear safety. Ezetimibe works by
570:
27:Medication used to treat high cholesterol
1524:"Ezetimibe (Zetia) Use During Pregnancy"
1384:British national formulary : BNF 76
934:Adding ezetimibe to statin treatment of
3464:
2066:The Korean Journal of Internal Medicine
1762:Alenghat FJ, Davis AM (February 2019).
1646:Phan BA, Dayspring TD, Toth PP (2012).
1641:
1639:
1378:
1376:
1374:
1372:
1347:"Ezetimibe Monograph for Professionals"
1222:
807:
787:
774:164 to 166 °C (327 to 331 °F)
657:
530:
437:
144:
2268:
2188:
2101:
1894:
1839:
1740:
1238:Therapeutic Goods Administration (TGA)
984:, in combination with specific statins
675:
31:
3409:Merck Manual of Diagnosis and Therapy
1826:10.1016/j.atherosclerosis.2011.06.011
630:
610:
130:
7:
277:
267:
257:
247:
1703:The New England Journal of Medicine
1652:Vascular Health and Risk Management
1610:International Journal of Cardiology
1564:from the original on 30 August 2024
1478:from the original on 14 August 2022
1448:from the original on 14 August 2022
1418:from the original on 14 August 2022
590:
481:
3515:Drugs developed by Schering-Plough
3500:Drugs developed by Merck & Co.
2275:: CS1 maint: overridden setting (
2195:: CS1 maint: overridden setting (
2108:: CS1 maint: overridden setting (
1901:: CS1 maint: overridden setting (
1846:: CS1 maint: overridden setting (
1747:: CS1 maint: overridden setting (
1534:from the original on 13 April 2019
889:upper respiratory tract infections
25:
2340:Cholesterol absorption inhibitors
2221:from the original on 12 June 2018
1764:"Management of Blood Cholesterol"
1357:from the original on 17 June 2019
1003:non-alcoholic fatty liver disease
909:decreasing cholesterol absorption
3467:
2217:. National Library of Medicine.
2134:from the original on 5 July 2016
1314:from the original on 10 May 2021
721:
715:
709:
229:Cholesterol absorption inhibitor
58:
815:Key:OLNTVTPDXPETLC-XPWALMASSA-N
2155:Science Translational Medicine
1940:Journal of Clinical Lipidology
864:. It is also available in the
724:
703:
1:
2255:10.1016/j.jchromb.2007.02.053
1204:or mild hepatic dysfunction (
982:familial hypercholesterolemia
2167:10.1126/scitranslmed.3010329
1622:10.1016/j.ijcard.2015.08.103
840:, sold under the brand name
3392:Merck Headquarters Building
3531:
1994:10.1016/j.ejim.2024.02.004
1953:10.1016/j.jacl.2013.12.005
693:Chemical and physical data
3024:
2991:Omega−3-acid ethyl esters
2031:10.1007/s40265-018-0870-1
1987:: S0953-6205(24)00049-9.
1304:"Zetia- ezetimibe tablet"
823:
798:
778:
428:
39:
3505:4-Fluorophenyl compounds
2890:Bempedoic acid/ezetimibe
2511:Niacin and derivatives (
1142:(NPC1L1) protein on the
969:, alone or with a statin
882:ezetimibe/bempedoic acid
2915:Fenofibrate/simvastatin
2910:Fenofibrate/pravastatin
2885:Amlodipine/atorvastatin
1001:Ezetimibe improves the
926:million prescriptions.
3175:Cubist Pharmaceuticals
2900:Ezetimibe/rosuvastatin
2895:Ezetimibe/atorvastatin
2372:Bile acid sequestrants
1780:10.1001/jama.2019.0015
1202:chronic kidney disease
1144:gastrointestinal tract
1140:Niemann-Pick C1-like 1
1046:adverse drug reactions
936:high blood cholesterol
878:ezetimibe/rosuvastatin
874:ezetimibe/atorvastatin
850:high blood cholesterol
3332:Ezetimibe/simvastatin
3226:Ezetimibe/simvastatin
3180:H. K. Mulford Company
3095:Merck & Co., Inc.
2905:Ezetimibe/simvastatin
2318:Lipid-lowering agents
2078:10.3904/kjim.2017.194
1716:10.1056/NEJMoa1410489
1554:"The Top 300 of 2022"
1474:. 15 September 2021.
1444:. 30 September 2016.
1188:by 38%. The absolute
1149:cells, as well as in
940:myocardial infarction
870:ezetimibe/simvastatin
419:Kidney 11%, fecal 78%
3490:Hypolipidemic agents
2946:Alipogene tiparvovec
2672:Aluminium clofibrate
2531:Aluminium nicotinate
1923:on 19 November 2014.
3102:Corporate directors
2682:Choline fenofibrate
1665:10.2147/VHRM.S33664
1504:. 24 September 2021
1310:. 26 January 2011.
1234:"AusPAR: Ezetimibe"
1129:Mechanism of action
1058:liver function test
854:lipid abnormalities
844:among others, is a
337:(Prescription only)
313:(Prescription only)
36:
3357:Rocuronium bromide
3281:rVSV-ZEBOV vaccine
3051:Never to phase III
2930:Niacin/simvastatin
2920:Niacin/laropiprant
2215:toxnet.nlm.nih.gov
920:generic medication
852:and certain other
3455:
3454:
3416:The Merck Manuals
3380:
3379:
3061:
3060:
2925:Niacin/lovastatin
2873:
2872:
2761:
2760:
2639:
2638:
2594:ATP citrate lyase
2551:Nicotinyl alcohol
2449:HMG-CoA reductase
2430:
2429:
1880:10.5551/jat.15792
1709:(25): 2387–2397.
1157:and interrupts a
1072:reactions (rash,
1009:Contraindications
835:
834:
759:Interactive image
643:CompTox Dashboard
393:Intestinal wall,
345:
332:
320:
308:
198:
165:
16:(Redirected from
3522:
3472:
3471:
3470:
3463:
3307:
3170:Acceleron Pharma
3129:Rochelle Lazarus
3119:William Harrison
3088:
3081:
3074:
3065:
2810:PCSK9 inhibitors
2660:
2650:
2441:
2336:
2311:
2304:
2297:
2288:
2281:
2280:
2274:
2266:
2237:
2231:
2230:
2228:
2226:
2207:
2201:
2200:
2194:
2186:
2161:(275): 275ra23.
2150:
2144:
2143:
2141:
2139:
2120:
2114:
2113:
2107:
2099:
2089:
2057:
2051:
2050:
2013:
2007:
2006:
1996:
1981:Eur J Intern Med
1972:
1966:
1965:
1955:
1931:
1925:
1924:
1919:. Archived from
1913:
1907:
1906:
1900:
1892:
1882:
1858:
1852:
1851:
1845:
1837:
1808:
1802:
1801:
1791:
1759:
1753:
1752:
1746:
1738:
1728:
1718:
1694:
1688:
1687:
1677:
1667:
1643:
1634:
1633:
1605:
1599:
1598:
1596:
1594:
1580:
1574:
1573:
1571:
1569:
1550:
1544:
1543:
1541:
1539:
1520:
1514:
1513:
1511:
1509:
1494:
1488:
1487:
1485:
1483:
1464:
1458:
1457:
1455:
1453:
1434:
1428:
1427:
1425:
1423:
1404:
1398:
1397:
1380:
1367:
1366:
1364:
1362:
1343:
1324:
1323:
1321:
1319:
1300:
1279:
1278:
1276:
1274:
1264:nctr-crs.fda.gov
1256:
1250:
1249:
1247:
1245:
1230:
1206:Child-Pugh score
1169:Pharmacokinetics
1155:aminopeptidase N
1070:hypersensitivity
961:in people with:
925:
831:
830:
761:
741:
726:
723:
717:
711:
705:
688:
677:
666:
665:
651:
649:
634:
614:
594:
574:
554:
534:
514:
494:
484:
483:
469:
405:
350:
343:
340:
330:
327:
319:
316:
306:
303:
281:
271:
261:
251:
196:
193:
175:
164:
161:
148:
134:
105:
104:
101:
100:
97:
94:
89:
88:
85:
82:
79:
76:
73:
70:
67:
64:
44:
37:
35:
21:
3530:
3529:
3525:
3524:
3523:
3521:
3520:
3519:
3480:
3479:
3478:
3468:
3466:
3458:
3456:
3451:
3396:
3376:
3317:Coppertone sign
3310:Schering-Plough
3305:
3194:
3185:Schering-Plough
3158:
3097:
3092:
3062:
3057:
3056:
3041:Clinical trials
3020:
2934:
2869:
2842:
2804:
2767:CETP inhibitors
2757:
2741:
2635:
2626:Dextrothyroxine
2611:
2588:
2555:
2506:
2426:
2366:
2325:
2315:
2285:
2284:
2267:
2239:
2238:
2234:
2224:
2222:
2209:
2208:
2204:
2187:
2152:
2151:
2147:
2137:
2135:
2122:
2121:
2117:
2100:
2059:
2058:
2054:
2015:
2014:
2010:
1974:
1973:
1969:
1933:
1932:
1928:
1915:
1914:
1910:
1893:
1860:
1859:
1855:
1838:
1814:Atherosclerosis
1810:
1809:
1805:
1761:
1760:
1756:
1739:
1696:
1695:
1691:
1645:
1644:
1637:
1607:
1606:
1602:
1592:
1590:
1582:
1581:
1577:
1567:
1565:
1552:
1551:
1547:
1537:
1535:
1522:
1521:
1517:
1507:
1505:
1496:
1495:
1491:
1481:
1479:
1466:
1465:
1461:
1451:
1449:
1436:
1435:
1431:
1421:
1419:
1414:. 1 June 2022.
1406:
1405:
1401:
1394:
1382:
1381:
1370:
1360:
1358:
1345:
1344:
1327:
1317:
1315:
1302:
1301:
1282:
1272:
1270:
1258:
1257:
1253:
1243:
1241:
1232:
1231:
1224:
1219:
1198:cytochrome P450
1190:bioavailability
1187:
1183:
1178:
1171:
1135:small intestine
1131:
1126:
1105:
1091:also regulates
1042:
1040:Adverse effects
1011:
932:
923:
826:
824:
819:
816:
811:
806:
805:
794:
791:
786:
785:
764:
739:
729:
720:
714:
708:
669:
645:
637:
617:
597:
577:
557:
537:
517:
497:
480:
472:
452:
449:
436:
435:
403:
379:Protein binding
369:Bioavailability
361:Pharmacokinetic
355:
348:
285:
211:
204:
185:
178:
91:
61:
57:
28:
23:
22:
15:
12:
11:
5:
3528:
3526:
3518:
3517:
3512:
3507:
3502:
3497:
3492:
3482:
3481:
3477:
3476:
3453:
3452:
3450:
3449:
3448:
3447:
3440:
3433:
3426:
3412:
3404:
3402:
3398:
3397:
3395:
3394:
3388:
3386:
3382:
3381:
3378:
3377:
3375:
3374:
3372:Urofollitropin
3369:
3364:
3359:
3354:
3349:
3344:
3339:
3334:
3329:
3324:
3319:
3313:
3311:
3304:
3303:
3298:
3293:
3288:
3283:
3278:
3273:
3268:
3263:
3258:
3253:
3248:
3243:
3238:
3233:
3228:
3223:
3218:
3213:
3208:
3202:
3200:
3196:
3195:
3193:
3192:
3187:
3182:
3177:
3172:
3166:
3164:
3160:
3159:
3157:
3156:
3151:
3146:
3141:
3136:
3131:
3126:
3124:William Kelley
3121:
3116:
3114:Johnnetta Cole
3111:
3105:
3103:
3099:
3098:
3093:
3091:
3090:
3083:
3076:
3068:
3059:
3058:
3055:
3054:
3053:
3052:
3049:
3038:
3032:
3026:
3025:
3022:
3021:
3019:
3018:
3013:
3008:
3003:
2998:
2993:
2988:
2983:
2978:
2973:
2968:
2963:
2958:
2953:
2948:
2942:
2940:
2936:
2935:
2933:
2932:
2927:
2922:
2917:
2912:
2907:
2902:
2897:
2892:
2887:
2881:
2879:
2875:
2874:
2871:
2870:
2868:
2867:
2861:
2859:
2844:
2843:
2841:
2840:
2835:
2830:
2825:
2819:
2817:
2806:
2805:
2803:
2802:
2797:
2792:
2787:
2782:
2776:
2774:
2763:
2762:
2759:
2758:
2756:
2755:
2749:
2747:
2743:
2742:
2740:
2739:
2734:
2729:
2724:
2719:
2714:
2709:
2704:
2699:
2694:
2689:
2684:
2679:
2674:
2668:
2666:
2657:
2647:
2641:
2640:
2637:
2636:
2634:
2633:
2628:
2622:
2620:
2613:
2612:
2610:
2609:
2607:Bempedoic acid
2603:
2601:
2590:
2589:
2587:
2586:
2581:
2576:
2570:
2568:
2557:
2556:
2554:
2553:
2548:
2543:
2538:
2533:
2528:
2522:
2520:
2508:
2507:
2505:
2504:
2499:
2494:
2489:
2484:
2479:
2474:
2469:
2464:
2458:
2456:
2438:
2432:
2431:
2428:
2427:
2425:
2424:
2411:
2406:
2401:
2396:
2391:
2385:
2383:
2368:
2367:
2365:
2364:
2359:
2354:
2348:
2346:
2333:
2327:
2326:
2316:
2314:
2313:
2306:
2299:
2291:
2283:
2282:
2249:(1–2): 88–96.
2232:
2202:
2145:
2115:
2072:(2): 296–304.
2052:
2025:(4): 453–462.
2008:
1967:
1926:
1908:
1873:(6): 517–523.
1853:
1803:
1774:(8): 800–801.
1754:
1689:
1635:
1600:
1575:
1545:
1515:
1489:
1459:
1429:
1399:
1392:
1368:
1325:
1280:
1251:
1240:. 21 June 2022
1221:
1220:
1218:
1215:
1185:
1181:
1176:
1170:
1167:
1130:
1127:
1125:
1122:
1114:abdominal pain
1104:
1101:
1082:rhabdomyolysis
1056:and/or raised
1041:
1038:
1010:
1007:
996:lipoprotein(a)
992:
991:
989:sitosterolemia
985:
976:
970:
967:hyperlipidemia
931:
928:
897:liver problems
848:used to treat
833:
832:
821:
820:
818:
817:
814:
812:
809:
801:
800:
799:
796:
795:
793:
792:
789:
781:
780:
779:
776:
775:
772:
766:
765:
763:
762:
754:
752:
744:
743:
737:
731:
730:
727:
718:
712:
706:
701:
695:
694:
690:
689:
679:
671:
670:
668:
667:
654:
652:
639:
638:
636:
635:
627:
625:
619:
618:
616:
615:
607:
605:
599:
598:
596:
595:
587:
585:
579:
578:
576:
575:
567:
565:
559:
558:
556:
555:
547:
545:
539:
538:
536:
535:
527:
525:
519:
518:
516:
515:
507:
505:
499:
498:
496:
495:
487:
485:
474:
473:
471:
470:
462:
460:
454:
453:
451:
450:
439:
431:
430:
429:
426:
425:
421:
420:
417:
411:
410:
407:
398:
397:
391:
385:
384:
381:
375:
374:
371:
365:
364:
357:
356:
354:
353:
338:
325:
314:
300:
298:
292:
291:
287:
286:
284:
283:
240:
238:
232:
231:
226:
220:
219:
214:
212:administration
206:
205:
203:
202:
200:
190:
188:
180:
179:
177:
176:
158:
156:
150:
149:
142:
136:
135:
128:
118:
117:
114:
108:
107:
55:
51:
50:
46:
45:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3527:
3516:
3513:
3511:
3508:
3506:
3503:
3501:
3498:
3496:
3493:
3491:
3488:
3487:
3485:
3475:
3465:
3461:
3446:
3445:
3441:
3439:
3438:
3434:
3432:
3431:
3427:
3425:
3424:
3420:
3419:
3418:
3417:
3413:
3411:
3410:
3406:
3405:
3403:
3399:
3393:
3390:
3389:
3387:
3383:
3373:
3370:
3368:
3365:
3363:
3360:
3358:
3355:
3353:
3350:
3348:
3345:
3343:
3340:
3338:
3335:
3333:
3330:
3328:
3325:
3323:
3322:Desloratadine
3320:
3318:
3315:
3314:
3312:
3308:
3302:
3299:
3297:
3294:
3292:
3289:
3287:
3284:
3282:
3279:
3277:
3274:
3272:
3269:
3267:
3264:
3262:
3259:
3257:
3254:
3252:
3249:
3247:
3244:
3242:
3239:
3237:
3236:Fosaprepitant
3234:
3232:
3229:
3227:
3224:
3222:
3219:
3217:
3214:
3212:
3209:
3207:
3204:
3203:
3201:
3197:
3191:
3188:
3186:
3183:
3181:
3178:
3176:
3173:
3171:
3168:
3167:
3165:
3161:
3155:
3154:Peter Wendell
3152:
3150:
3149:Wendell Weeks
3147:
3145:
3142:
3140:
3137:
3135:
3132:
3130:
3127:
3125:
3122:
3120:
3117:
3115:
3112:
3110:
3109:Richard Clark
3107:
3106:
3104:
3100:
3096:
3089:
3084:
3082:
3077:
3075:
3070:
3069:
3066:
3050:
3048:
3045:
3044:
3042:
3039:
3036:
3033:
3031:
3028:
3027:
3023:
3017:
3014:
3012:
3009:
3007:
3004:
3002:
2999:
2997:
2994:
2992:
2989:
2987:
2984:
2982:
2979:
2977:
2974:
2972:
2969:
2967:
2964:
2962:
2959:
2957:
2954:
2952:
2949:
2947:
2944:
2943:
2941:
2937:
2931:
2928:
2926:
2923:
2921:
2918:
2916:
2913:
2911:
2908:
2906:
2903:
2901:
2898:
2896:
2893:
2891:
2888:
2886:
2883:
2882:
2880:
2876:
2866:
2863:
2862:
2860:
2857:
2853:
2849:
2845:
2839:
2836:
2834:
2831:
2829:
2826:
2824:
2821:
2820:
2818:
2815:
2811:
2807:
2801:
2798:
2796:
2793:
2791:
2788:
2786:
2783:
2781:
2778:
2777:
2775:
2772:
2768:
2764:
2754:
2751:
2750:
2748:
2744:
2738:
2735:
2733:
2730:
2728:
2725:
2723:
2720:
2718:
2715:
2713:
2710:
2708:
2705:
2703:
2700:
2698:
2695:
2693:
2690:
2688:
2685:
2683:
2680:
2678:
2675:
2673:
2670:
2669:
2667:
2665:
2661:
2658:
2655:
2654:PPAR agonists
2651:
2648:
2646:
2645:Blood vessels
2642:
2632:
2629:
2627:
2624:
2623:
2621:
2618:
2617:Thyromimetics
2614:
2608:
2605:
2604:
2602:
2599:
2595:
2591:
2585:
2582:
2580:
2577:
2575:
2572:
2571:
2569:
2566:
2562:
2558:
2552:
2549:
2547:
2544:
2542:
2539:
2537:
2534:
2532:
2529:
2527:
2524:
2523:
2521:
2518:
2514:
2509:
2503:
2500:
2498:
2495:
2493:
2490:
2488:
2485:
2483:
2480:
2478:
2475:
2473:
2470:
2468:
2465:
2463:
2460:
2459:
2457:
2454:
2450:
2446:
2442:
2439:
2437:
2433:
2423:
2419:
2415:
2414:Soluble fiber
2412:
2410:
2407:
2405:
2404:Colestyramine
2402:
2400:
2397:
2395:
2392:
2390:
2387:
2386:
2384:
2381:
2377:
2373:
2369:
2363:
2360:
2358:
2355:
2353:
2350:
2349:
2347:
2345:
2341:
2337:
2334:
2332:
2328:
2323:
2319:
2312:
2307:
2305:
2300:
2298:
2293:
2292:
2289:
2278:
2272:
2264:
2260:
2256:
2252:
2248:
2244:
2236:
2233:
2220:
2216:
2212:
2206:
2203:
2198:
2192:
2184:
2180:
2176:
2172:
2168:
2164:
2160:
2156:
2149:
2146:
2133:
2129:
2125:
2119:
2116:
2111:
2105:
2097:
2093:
2088:
2083:
2079:
2075:
2071:
2067:
2063:
2056:
2053:
2048:
2044:
2040:
2036:
2032:
2028:
2024:
2020:
2012:
2009:
2004:
2000:
1995:
1990:
1986:
1982:
1978:
1971:
1968:
1963:
1959:
1954:
1949:
1945:
1941:
1937:
1930:
1927:
1922:
1918:
1912:
1909:
1904:
1898:
1890:
1886:
1881:
1876:
1872:
1868:
1864:
1857:
1854:
1849:
1843:
1835:
1831:
1827:
1823:
1819:
1815:
1807:
1804:
1799:
1795:
1790:
1785:
1781:
1777:
1773:
1769:
1765:
1758:
1755:
1750:
1744:
1736:
1732:
1727:
1726:11573/1150638
1722:
1717:
1712:
1708:
1704:
1700:
1693:
1690:
1685:
1681:
1676:
1671:
1666:
1661:
1657:
1653:
1649:
1642:
1640:
1636:
1631:
1627:
1623:
1619:
1615:
1611:
1604:
1601:
1589:
1585:
1579:
1576:
1563:
1559:
1555:
1549:
1546:
1533:
1529:
1525:
1519:
1516:
1503:
1499:
1493:
1490:
1477:
1473:
1469:
1463:
1460:
1447:
1443:
1439:
1433:
1430:
1417:
1413:
1409:
1403:
1400:
1395:
1393:9780857113382
1389:
1385:
1379:
1377:
1375:
1373:
1369:
1356:
1352:
1348:
1342:
1340:
1338:
1336:
1334:
1332:
1330:
1326:
1313:
1309:
1305:
1299:
1297:
1295:
1293:
1291:
1289:
1287:
1285:
1281:
1269:
1265:
1261:
1255:
1252:
1239:
1235:
1229:
1227:
1223:
1216:
1214:
1212:
1207:
1203:
1199:
1193:
1191:
1179:
1168:
1166:
1164:
1160:
1156:
1152:
1148:
1145:
1141:
1136:
1128:
1123:
1121:
1119:
1115:
1111:
1102:
1100:
1098:
1094:
1090:
1085:
1083:
1079:
1075:
1071:
1067:
1063:
1059:
1055:
1051:
1047:
1039:
1037:
1035:
1031:
1027:
1022:
1020:
1016:
1008:
1006:
1004:
999:
997:
990:
986:
983:
980:
977:
975:
971:
968:
964:
963:
962:
960:
955:
951:
949:
945:
941:
937:
929:
927:
921:
916:
914:
910:
906:
905:breastfeeding
902:
898:
894:
890:
885:
883:
879:
875:
871:
868:combinations
867:
863:
859:
855:
851:
847:
843:
839:
829:
822:
813:
808:
804:
797:
788:
784:
777:
773:
771:
770:Melting point
767:
760:
756:
755:
753:
750:
745:
738:
736:
732:
702:
700:
696:
691:
687:
683:
680:
678:
676:ECHA InfoCard
672:
664:
660:
659:DTXSID1044223
656:
655:
653:
644:
640:
633:
629:
628:
626:
624:
620:
613:
609:
608:
606:
604:
600:
593:
589:
588:
586:
584:
580:
573:
569:
568:
566:
564:
560:
553:
549:
548:
546:
544:
540:
533:
529:
528:
526:
524:
520:
513:
509:
508:
506:
504:
500:
493:
489:
488:
486:
479:
475:
468:
464:
463:
461:
459:
455:
447:
443:
438:
434:
427:
422:
418:
416:
412:
408:
406:
399:
396:
392:
390:
386:
382:
380:
376:
372:
370:
366:
362:
358:
351:
339:
336:
326:
324:
315:
312:
302:
301:
299:
297:
293:
288:
280:
275:
270:
265:
260:
255:
250:
245:
242:
241:
239:
237:
233:
230:
227:
225:
221:
218:
215:
213:
207:
201:
192:
191:
189:
187:
181:
174:
169:
160:
159:
157:
155:
151:
147:
143:
141:
137:
133:
129:
127:
123:
119:
116:Zetia, others
115:
113:
109:
103:
56:
54:Pronunciation
52:
49:Clinical data
47:
43:
38:
30:
19:
3495:Beta-lactams
3443:
3436:
3429:
3422:
3414:
3407:
3401:Publications
3362:Temozolomide
3326:
3261:Omarigliptin
3220:
3163:Subsidiaries
3144:Samuel Thier
3139:Anne Tatlock
3134:Thomas Shenk
3016:Volanesorsen
2878:Combinations
2850:inhibitors (
2692:Clinofibrate
2687:Ciprofibrate
2596:inhibitors (
2563:inhibitors (
2546:Nicofuranose
2497:Rosuvastatin
2487:Pitavastatin
2467:Cerivastatin
2462:Atorvastatin
2351:
2271:cite journal
2246:
2242:
2235:
2223:. Retrieved
2214:
2205:
2191:cite journal
2158:
2154:
2148:
2136:. Retrieved
2128:Medline Plus
2127:
2118:
2104:cite journal
2069:
2065:
2055:
2022:
2018:
2011:
1984:
1980:
1970:
1946:(1): 29–60.
1943:
1939:
1929:
1921:the original
1911:
1897:cite journal
1870:
1866:
1856:
1842:cite journal
1817:
1813:
1806:
1771:
1767:
1757:
1743:cite journal
1706:
1702:
1692:
1655:
1651:
1613:
1609:
1603:
1591:. Retrieved
1587:
1578:
1566:. Retrieved
1557:
1548:
1536:. Retrieved
1527:
1518:
1506:. Retrieved
1501:
1492:
1480:. Retrieved
1471:
1462:
1450:. Retrieved
1441:
1432:
1420:. Retrieved
1411:
1402:
1383:
1359:. Retrieved
1350:
1316:. Retrieved
1307:
1271:. Retrieved
1263:
1254:
1242:. Retrieved
1237:
1194:
1172:
1153:; it blocks
1132:
1124:Pharmacology
1110:loose stools
1106:
1086:
1043:
1023:
1012:
1000:
993:
956:
952:
948:heart attack
933:
930:Medical uses
917:
886:
841:
837:
836:
825:
445:
441:
409:19 h to 30 h
402:Elimination
296:Legal status
290:Legal status
154:License data
29:
3337:Famciclovir
3291:Sitagliptin
3286:Simvastatin
3271:Rizatriptan
3266:Raltegravir
3256:Montelukast
3231:Finasteride
3206:Alendronate
3037:from market
2996:Policosanol
2971:Lapaquistat
2951:Azacosterol
2828:Bococizumab
2800:Torcetrapib
2795:Obicetrapib
2790:Evacetrapib
2785:Dalcetrapib
2780:Anacetrapib
2732:Ronifibrate
2727:Pemafibrate
2717:Gemfibrozil
2712:Fenofibrate
2677:Bezafibrate
2584:Mitratapide
2574:Dirlotapide
2502:Simvastatin
2492:Pravastatin
2472:Fluvastatin
2422:glucomannan
2389:Colesevelam
2124:"Ezetimibe"
1820:(1): 3–46.
1658:: 415–427.
1616:: 247–252.
1151:hepatocytes
1050:steatorrhea
1034:fenofibrate
1032:other than
1026:ciclosporin
1019:anaphylaxis
987:Homozygous
974:fenofibrate
893:anaphylaxis
742: g·mol
682:100.207.996
612:CHEBI:49040
467:163222-33-1
424:Identifiers
140:MedlinePlus
112:Trade names
3484:Categories
3444:Geriatrics
3437:Veterinary
3385:Facilities
3352:Mometasone
3347:Loratadine
3342:Infliximab
3301:Vorinostat
3296:Vericiguat
3251:Lovastatin
3211:Aprepitant
3190:Viralytics
3011:Triparanol
2986:Mipomersen
2966:Darapladib
2961:Benfluorex
2956:Azalanstat
2865:Evinacumab
2838:Inclisiran
2833:Evolocumab
2823:Alirocumab
2737:Simfibrate
2707:Etofibrate
2702:Clofibride
2697:Clofibrate
2631:Resmetirom
2579:Lomitapide
2541:Niceritrol
2482:Mevastatin
2477:Lovastatin
2399:Colestipol
2394:Colestilan
2357:Hyzetimibe
1273:22 October
1217:References
1163:annexin A2
1159:caveolin 1
1147:epithelial
1074:angioedema
1015:angioedema
979:Homozygous
913:intestines
866:fixed-dose
846:medication
747:3D model (
735:Molar mass
632:ChEMBL1138
572:EOR26LQQ24
543:ChemSpider
503:IUPHAR/BPS
458:CAS Number
433:IUPAC name
389:Metabolism
373:35% to 65%
224:Drug class
3327:Ezetimibe
3276:Rofecoxib
3241:Indinavir
3221:Ezetimibe
3216:Ertapenem
3047:Phase III
3035:Withdrawn
2722:Nafenopin
2409:Colextran
2362:SCH-48461
2352:Ezetimibe
2047:207489460
1593:30 August
1568:30 August
1528:Drugs.com
1508:13 August
1482:13 August
1452:13 August
1422:13 August
1351:Drugs.com
1318:13 August
1099:therapy.
1093:vitamin K
1028:and with
901:pregnancy
838:Ezetimibe
415:Excretion
404:half-life
210:Routes of
184:Pregnancy
173:Ezetimibe
132:Monograph
126:Drugs.com
34:Ezetimibe
3474:Medicine
3367:Tibolone
3246:Losartan
3199:Products
3006:Tiadenol
3001:Probucol
2981:Meglutol
2753:GW501516
2664:Fibrates
2526:Acipimox
2418:psyllium
2416:such as
2331:GI tract
2263:17442643
2219:Archived
2175:25696002
2138:21 March
2132:Archived
2096:29551054
2039:29396832
2003:38336550
1962:24528685
1889:23665881
1834:21882396
1798:30715135
1735:26039521
1684:22910633
1630:26301648
1588:ClinCalc
1562:Archived
1558:ClinCalc
1538:13 April
1532:Archived
1502:DailyMed
1476:Archived
1472:DailyMed
1446:Archived
1442:DailyMed
1416:Archived
1412:DailyMed
1361:13 April
1355:Archived
1312:Archived
1308:DailyMed
1244:20 April
1103:Overdose
1097:warfarin
1078:myopathy
1030:fibrates
965:Primary
862:by mouth
828:(verify)
523:DrugBank
236:ATC code
217:By mouth
199: B3
186:category
168:DailyMed
3510:Phenols
2848:ANGPTL3
2445:Statins
2241:bile".
2183:5951911
2087:6406097
1789:6679800
1675:3402055
1118:fatigue
1054:myalgia
1044:Common
911:in the
740:409.433
699:Formula
532:DB00973
478:PubChem
383:>90%
352:Rx-only
349:WARNING
321::
276: (
274:C10BA02
266: (
264:C10BA06
256: (
254:C10BA05
252:)
246: (
244:C10AX09
170::
146:a603015
3460:Portal
3430:Manual
3030:WHO-EM
2746:Others
2619:(VLDL)
2536:Niacin
2376:resins
2344:NPC1L1
2261:
2225:29 May
2181:
2173:
2094:
2084:
2045:
2037:
2001:
1960:
1887:
1832:
1796:
1786:
1733:
1682:
1672:
1628:
1390:
1116:, and
1089:NPC1L1
1087:Since
1017:, and
959:lipids
944:stroke
924:
880:, and
858:statin
783:SMILES
623:ChEMBL
592:D01966
552:132493
492:150311
346:
333:
323:℞-only
309:
166:
106:
3423:Index
2939:Other
2656:(LDL)
2436:Liver
2179:S2CID
2043:S2CID
2019:Drugs
1076:) or
842:Zetia
803:InChI
749:JSmol
603:ChEBI
395:liver
18:Zetia
2565:VLDL
2561:MTTP
2515:and
2420:and
2277:link
2259:PMID
2227:2018
2197:link
2171:PMID
2140:2018
2110:link
2092:PMID
2035:PMID
1999:PMID
1958:PMID
1903:link
1885:PMID
1848:link
1830:PMID
1794:PMID
1768:JAMA
1749:link
1731:PMID
1680:PMID
1626:PMID
1595:2024
1570:2024
1540:2019
1510:2022
1484:2022
1454:2022
1424:2022
1388:ISBN
1363:2019
1320:2022
1275:2023
1246:2024
942:and
903:and
583:KEGG
563:UNII
512:6816
363:data
122:AHFS
2856:HDL
2852:LDL
2814:LDL
2771:HDL
2598:LDL
2517:LDL
2513:HDL
2453:LDL
2380:LDL
2322:C10
2251:doi
2247:853
2163:doi
2082:PMC
2074:doi
2027:doi
1989:doi
1985:124
1948:doi
1875:doi
1822:doi
1818:217
1784:PMC
1776:doi
1772:321
1721:hdl
1711:doi
1707:372
1670:PMC
1660:doi
1618:doi
1614:201
1268:FDA
1211:AUC
1186:max
1182:max
1177:max
1066:AST
1062:ALT
648:EPA
482:CID
335:POM
279:WHO
269:WHO
259:WHO
249:WHO
3486::
3043::
2451:,
2342:,
2273:}}
2269:{{
2257:.
2245:.
2213:.
2193:}}
2189:{{
2177:.
2169:.
2157:.
2126:.
2106:}}
2102:{{
2090:.
2080:.
2070:34
2068:.
2064:.
2041:.
2033:.
2023:78
2021:.
1997:.
1983:.
1979:.
1956:.
1942:.
1938:.
1899:}}
1895:{{
1883:.
1871:20
1869:.
1865:.
1844:}}
1840:{{
1828:.
1816:.
1792:.
1782:.
1770:.
1766:.
1745:}}
1741:{{
1729:.
1719:.
1705:.
1701:.
1678:.
1668:.
1654:.
1650:.
1638:^
1624:.
1612:.
1586:.
1560:.
1556:.
1530:.
1526:.
1500:.
1470:.
1440:.
1410:.
1371:^
1349:.
1328:^
1306:.
1283:^
1266:.
1262:.
1236:.
1225:^
1120:.
1112:,
1036:.
915:.
895:,
884:.
876:,
872:,
713:21
707:24
444:,4
440:(3
342:US
329:UK
318:CA
311:S4
305:AU
272:)
262:)
195:AU
163:US
96:aɪ
90:,-
3462::
3087:e
3080:t
3073:v
2858:)
2854:/
2816:)
2812:(
2773:)
2769:(
2600:)
2567:)
2519:)
2455:)
2447:(
2382:)
2378:(
2374:/
2324:)
2320:(
2310:e
2303:t
2296:v
2279:)
2265:.
2253::
2229:.
2199:)
2185:.
2165::
2159:7
2142:.
2112:)
2098:.
2076::
2049:.
2029::
2005:.
1991::
1964:.
1950::
1944:8
1905:)
1891:.
1877::
1850:)
1836:.
1824::
1800:.
1778::
1751:)
1737:.
1723::
1713::
1686:.
1662::
1656:8
1632:.
1620::
1597:.
1572:.
1542:.
1512:.
1486:.
1456:.
1426:.
1396:.
1365:.
1322:.
1277:.
1248:.
1175:C
1161:–
1064:/
1060:(
751:)
728:3
725:O
722:N
719:2
716:F
710:H
704:C
650:)
646:(
446:S
442:R
344::
331::
307::
282:)
197::
124:/
102:/
99:b
93:m
87:b
84:ɪ
81:m
78:ɪ
75:t
72:ɛ
69:z
66:ˈ
63:ɛ
60:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.