42:
340:
205:
596:
464:
722:
Xu, Zhen-Hua; Otterness, Diane M.; Freimuth, Robert R.; Carlini, Edward J.; Wood, Thomas C.; Mitchell, Steve; Moon, Eunpyo; Kim, Ung-Jin; Xu, Jing-Ping; Siciliano, Michael J.; Weinshilboum, Richard M. (February 2000). "Human 3′-Phosphoadenosine 5′-Phosphosulfate
Synthetase 1 (PAPSS1) and PAPSS2: Gene
134:
563:
as an electron acceptor by aerobic organisms. Sulfate is not reduced directly but must be activated by the formation of APS or PAPS. These carriers of activated sulfate are produced by reaction with ATP. The first reaction is catalysed by
531:. It is endogenously synthesized by organisms via the phosphorylation of adenosine 5′-phosphosulfate (APS), an intermediary metabolite. In humans such reaction is performed by bifunctional 3′-phosphoadenosine 5′-phosphosulfate synthases (
363:
InChI=1S/C10H15N5O13P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(27-29(17,18)19)4(26-10)1-25-30(20,21)28-31(22,23)24/h2-4,6-7,10,16H,1H2,(H,20,21)(H2,11,12,13)(H2,17,18,19)(H,22,23,24)/t4-,6-,7-,10-/m1/s1
373:
InChI=1/C10H15N5O13P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(27-29(17,18)19)4(26-10)1-25-30(20,21)28-31(22,23)24/h2-4,6-7,10,16H,1H2,(H,20,21)(H2,11,12,13)(H2,17,18,19)(H,22,23,24)/t4-,6-,7-,10-/m1/s1
477:
389:
915:
911:
844:
907:
903:
813:
354:
971:
837:
241:
297:
893:
318:
760:"Human 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase: Biochemistry, molecular biology and genetic deficiency"
611:, leads also to hydrogen sulfide. But in this case, the product is used in biosynthesis, e.g. for the production of
607:, which is excreted. This process is called dissimilatory sulfate reduction. Reduction of PAPS, a more elaborated
1259:
484:
830:
556:
212:
41:
1124:
1254:
1137:
1029:
856:
584:
504:
200:
1037:
879:
615:. The latter process is called assimilatory sulfate reduction because the sulfate sulfur is assimilated.
540:
1047:
1042:
889:
528:
68:
54:
28:
1244:
955:
335:
1129:
935:
100:
1249:
1151:
789:
808:
M. T. Madigan, J. M. Martinko, J. Parker “Brock
Biology of Microorganisms” Prentice Hall, 1997.
17:
1222:
981:
963:
809:
781:
740:
704:
665:
771:
732:
696:
655:
645:
604:
524:
412:
306:
985:
565:
182:
110:
339:
204:
162:
1001:
660:
633:
508:
455:
1238:
1114:
1067:
1019:
608:
193:
794:
1119:
1109:
1077:
559:. In these organisms, sulfate serves as an electron acceptor, akin to the use of O
286:
1104:
1057:
776:
759:
1184:
1072:
1062:
925:
650:
443:
231:
173:
1179:
1174:
1009:
995:
516:
785:
744:
736:
708:
700:
669:
612:
520:
595:
1215:
1159:
871:
552:
512:
273:
213:
1189:
1164:
1098:
1094:
1090:
1086:
945:
853:
536:
532:
684:
454:
Except where otherwise noted, data are given for materials in their
822:
261:
594:
153:
133:
123:
1194:
1169:
1082:
252:
826:
603:
Reduction of APS leads to sulfite, which is further reduced to
397:
C1=NC2=C(C(=N1)N)N=CN23(((O3)COP(=O)(O)OS(=O)(=O)O)OP(=O)(O)O)O
551:
APS and PAPS are intermediates in the reduction of sulfate to
683:
Negishi M; Pedersen LG; Petrotchenko E; et al. (2001).
323:
723:
Cloning, Characterization and
Chromosomal Localization".
472:
1207:
1150:
1028:
870:
863:
725:
240:
632:Günal S; Hardman R; Kopriva S; Mueller JW (2019).
555:, an exothermic conversion that is carried out by
285:
599:Structure of adenosine 5′-phosphosulfate (APS).
109:
583:The conversion of APS to PAPS is catalysed by
838:
685:"Structure and function of sulfotransferases"
8:
27:"PAPS" redirects here. For other uses, see
867:
845:
831:
823:
338:
203:
181:
33:
793:
775:
659:
649:
305:
624:
394:
359:
334:
62:-Phosphono-5′-adenylyl hydrogen sulfate
634:"Sulfation pathways from red to green"
194:
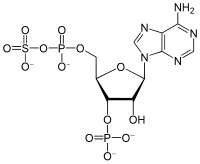
35:3′-Phosphoadenosine-5′-phosphosulfate
497:3′-Phosphoadenosine-5′-phosphosulfate
366:Key: GACDQMDRPRGCTN-KQYNXXCUSA-N
161:
73:methyl hydrogen (sulfooxy)phosphonate
7:
376:Key: GACDQMDRPRGCTN-KQYNXXCUBK
276:
260:
25:
462:
40:
458:(at 25 °C , 100 kPa).
86:Phosphoadenosine phosphosulfate
18:3'-phosphoadenylyl sulfate
88:3′-Phospho-5′-adenylyl sulfate
1:
758:Venkatachalam, K. V. (2003).
511:at the 3′ position and has a
527:reactions and hence part of
777:10.1080/1521654031000072148
591:APS + ATP ⇌ PAPS + ADP
1276:
84:3′-Phosphoadenylyl sulfate
26:
651:10.1074/jbc.REV119.007422
557:sulfate-reducing bacteria
519:. It is the most common
515:group attached to the 5′
452:
405:
385:
350:
93:
79:
67:
53:
48:
39:
543:as the phosphate donor.
547:Formation and reduction
505:adenosine monophosphate
737:10.1006/bbrc.2000.2123
701:10.1006/abbi.2001.2368
689:Arch. Biochem. Biophys
600:
576:+ ATP ⇌ APS + PP
598:
503:) is a derivative of
448:507.266
69:Systematic IUPAC name
29:Paps (disambiguation)
644:(33): 12293–12312.
36:
601:
529:sulfation pathways
485:Infobox references
34:
1260:Sulfur metabolism
1232:
1231:
1203:
1202:
493:Chemical compound
491:
490:
319:CompTox Dashboard
135:Interactive image
16:(Redirected from
1267:
868:
847:
840:
833:
824:
817:
806:
800:
799:
797:
779:
755:
749:
748:
719:
713:
712:
680:
674:
673:
663:
653:
629:
605:hydrogen sulfide
525:sulfotransferase
475:
469:
466:
465:
413:Chemical formula
343:
342:
327:
325:
309:
289:
278:
264:
244:
215:
207:
196:
185:
165:
137:
113:
44:
37:
21:
1275:
1274:
1270:
1269:
1268:
1266:
1265:
1264:
1235:
1234:
1233:
1228:
1199:
1146:
1141:
1133:
1024:
1015:
1007:
991:
977:
967:
959:
951:
941:
931:
921:
899:
885:
859:
851:
821:
820:
807:
803:
757:
756:
752:
721:
720:
716:
682:
681:
677:
631:
630:
626:
621:
579:
575:
566:ATP sulfurylase
562:
549:
494:
487:
482:
481:
480: ?)
471:
467:
463:
459:
437:
433:
429:
425:
421:
415:
401:
398:
393:
392:
381:
378:
377:
374:
368:
367:
364:
358:
357:
346:
328:
321:
312:
292:
279:
267:
247:
234:
225:
188:
168:
140:
127:
116:
103:
89:
87:
85:
83:
75:
74:
63:
32:
23:
22:
15:
12:
11:
5:
1273:
1271:
1263:
1262:
1257:
1255:Sulfate esters
1252:
1247:
1237:
1236:
1230:
1229:
1227:
1226:
1211:
1209:
1205:
1204:
1201:
1200:
1198:
1197:
1192:
1187:
1182:
1177:
1172:
1167:
1162:
1156:
1154:
1148:
1147:
1145:
1144:
1139:
1135:
1131:
1127:
1122:
1117:
1112:
1107:
1102:
1080:
1075:
1070:
1065:
1060:
1055:
1050:
1045:
1040:
1034:
1032:
1026:
1025:
1023:
1022:
1017:
1013:
1005:
999:
993:
989:
979:
975:
965:
957:
953:
949:
943:
939:
933:
929:
923:
919:
901:
897:
887:
883:
876:
874:
865:
861:
860:
852:
850:
849:
842:
835:
827:
819:
818:
801:
750:
731:(2): 437–444.
714:
675:
623:
622:
620:
617:
593:
592:
581:
580:
577:
573:
560:
548:
545:
509:phosphorylated
507:(AMP) that is
492:
489:
488:
483:
461:
460:
456:standard state
453:
450:
449:
446:
440:
439:
435:
431:
427:
423:
419:
416:
411:
408:
407:
403:
402:
400:
399:
396:
388:
387:
386:
383:
382:
380:
379:
375:
372:
371:
369:
365:
362:
361:
353:
352:
351:
348:
347:
345:
344:
336:DTXSID40894869
331:
329:
317:
314:
313:
311:
310:
302:
300:
294:
293:
291:
290:
282:
280:
272:
269:
268:
266:
265:
257:
255:
249:
248:
246:
245:
237:
235:
230:
227:
226:
224:
223:
219:
217:
209:
208:
198:
190:
189:
187:
186:
178:
176:
170:
169:
167:
166:
158:
156:
150:
149:
146:
145:Abbreviations
142:
141:
139:
138:
130:
128:
121:
118:
117:
115:
114:
106:
104:
99:
96:
95:
91:
90:
81:
77:
76:
72:
71:
65:
64:
57:
51:
50:
46:
45:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1272:
1261:
1258:
1256:
1253:
1251:
1248:
1246:
1243:
1242:
1240:
1225:
1224:
1218:
1217:
1213:
1212:
1210:
1206:
1196:
1193:
1191:
1188:
1186:
1183:
1181:
1178:
1176:
1173:
1171:
1168:
1166:
1163:
1161:
1158:
1157:
1155:
1153:
1149:
1143:
1136:
1134:
1128:
1126:
1123:
1121:
1118:
1116:
1115:Molybdopterin
1113:
1111:
1108:
1106:
1103:
1100:
1096:
1092:
1088:
1084:
1081:
1079:
1076:
1074:
1071:
1069:
1068:Cofactor F430
1066:
1064:
1061:
1059:
1056:
1054:
1051:
1049:
1046:
1044:
1041:
1039:
1036:
1035:
1033:
1031:
1027:
1021:
1020:Coenzyme F420
1018:
1011:
1003:
1002:Phylloquinone
1000:
997:
996:Ascorbic acid
994:
987:
983:
980:
973:
969:
961:
954:
947:
944:
937:
934:
927:
924:
917:
913:
909:
905:
902:
895:
891:
888:
881:
878:
877:
875:
873:
869:
866:
862:
858:
855:
848:
843:
841:
836:
834:
829:
828:
825:
815:
814:0-13-520875-0
811:
805:
802:
796:
791:
787:
783:
778:
773:
769:
765:
761:
754:
751:
746:
742:
738:
734:
730:
726:
718:
715:
710:
706:
702:
698:
695:(2): 149–57.
694:
690:
686:
679:
676:
671:
667:
662:
657:
652:
647:
643:
639:
638:J. Biol. Chem
635:
628:
625:
618:
616:
614:
610:
609:sulfate ester
606:
597:
590:
589:
588:
586:
571:
570:
569:
567:
558:
554:
546:
544:
542:
538:
534:
530:
526:
522:
518:
514:
510:
506:
502:
498:
486:
479:
474:
457:
451:
447:
445:
442:
441:
417:
414:
410:
409:
404:
395:
391:
384:
370:
360:
356:
349:
341:
337:
333:
332:
330:
320:
316:
315:
308:
304:
303:
301:
299:
296:
295:
288:
284:
283:
281:
275:
271:
270:
263:
259:
258:
256:
254:
251:
250:
243:
239:
238:
236:
233:
229:
228:
221:
220:
218:
216:
211:
210:
206:
202:
199:
197:
195:ECHA InfoCard
192:
191:
184:
180:
179:
177:
175:
172:
171:
164:
160:
159:
157:
155:
152:
151:
147:
144:
143:
136:
132:
131:
129:
125:
120:
119:
112:
108:
107:
105:
102:
98:
97:
92:
78:
70:
66:
61:
56:
52:
47:
43:
38:
30:
19:
1220:
1214:
1120:Mycofactocin
1110:Methanofuran
1052:
1030:non-vitamins
864:Active forms
804:
767:
763:
753:
728:
724:
717:
692:
688:
678:
641:
637:
627:
602:
582:
550:
500:
496:
495:
94:Identifiers
80:Other names
59:
1245:Nucleotides
1105:Lipoic Acid
1083:Heme / Haem
1010:Menaquinone
770:(1): 1–11.
406:Properties
201:100.222.927
163:CHEBI:17980
1239:Categories
1208:Base forms
1152:metal ions
1078:Coenzyme Q
1073:Coenzyme M
1063:Coenzyme B
926:Coenzyme A
880:TPP / ThDP
764:IUBMB Life
619:References
585:APS kinase
444:Molar mass
307:5TH3ERG159
232:IUPHAR/BPS
174:ChemSpider
122:3D model (
101:CAS Number
55:IUPAC name
1250:Coenzymes
1138:THMPT / H
936:PLP / P5P
857:cofactors
517:phosphate
222:694-699-5
214:EC Number
1223:vitamins
1216:vitamins
1130:THB / BH
964:DHFA / H
956:THFA / H
872:vitamins
795:37733913
786:12716056
745:10679223
709:11396917
670:31270211
613:cysteine
539:) using
521:coenzyme
438:S
111:482-67-7
661:6699852
553:sulfite
513:sulfate
478:what is
476: (
274:PubChem
1170:Fe, Fe
982:AdoCbl
946:Biotin
854:Enzyme
812:
792:
784:
743:
707:
668:
658:
537:PAPSS2
533:PAPSS1
473:verify
470:
390:SMILES
262:C00053
49:Names
986:MeCbl
916:NADPH
790:S2CID
355:InChI
287:10214
154:ChEBI
148:PAPS
124:JSmol
1221:see
1053:PAPS
1048:SAMe
972:MTHF
912:NADP
908:NADH
810:ISBN
782:PMID
741:PMID
705:PMID
666:PMID
535:and
501:PAPS
298:UNII
253:KEGG
242:1719
183:9799
82:PAPS
1142:MPT
1125:PQQ
1058:GSH
1043:CTP
1038:ATP
1008:),
998:(C)
904:NAD
894:FAD
890:FMN
772:doi
733:doi
729:268
697:doi
693:390
656:PMC
646:doi
642:294
541:ATP
523:in
324:EPA
277:CID
58:3′-
1241::
1219::
1195:Zn
1190:Ni
1185:Mo
1180:Mn
1175:Mg
1165:Cu
1160:Ca
1097:,
1093:,
1089:,
1012:(K
1004:(K
990:12
988:(B
984:,
974:(B
970:,
968:FA
962:,
960:FA
948:(B
938:(B
928:(B
918:(B
914:,
910:,
906:,
896:(B
892:,
882:(B
788:.
780:.
768:55
766:.
762:.
739:.
727:.
703:.
691:.
687:.
664:.
654:.
640:.
636:.
587::
572:SO
568::
432:13
424:15
420:10
1140:4
1132:4
1101:)
1099:O
1095:C
1091:B
1087:A
1085:(
1016:)
1014:2
1006:1
992:)
978:)
976:9
966:2
958:4
952:)
950:7
942:)
940:6
932:)
930:5
922:)
920:3
900:)
898:2
886:)
884:1
846:e
839:t
832:v
816:.
798:.
774::
747:.
735::
711:.
699::
672:.
648::
578:i
574:4
561:2
499:(
468:N
436:2
434:P
430:O
428:5
426:N
422:H
418:C
326:)
322:(
126:)
60:O
31:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.