229:
154:
509:
35:
405:
447:
with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition acetanisole can sometimes smell like butter or caramel. Its chemical names are based on considering the structure as either an
418:
265:
618:
602:
650:
243:
425:
186:
111:
207:
65:-Acetanisole; 4-Methoxyacetophenone; Linarodin; Novatone; Vananote; Castoreum anisole; 4-Methoxyphenyl methyl ketone
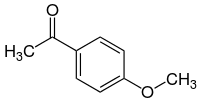
496:
640:
149:
645:
47:
224:
77:
599:
521:
492:
444:
584:
368:
288:
195:
606:
551:
500:
131:
87:
621:
228:
153:
396:
634:
588:
525:
357:
347:
142:
17:
508:
469:
465:
453:
566:
175:
384:
316:
122:
34:
476:
441:
570:
461:
337:
162:
480:
457:
449:
395:
Except where otherwise noted, data are given for materials in their
110:
100:
212:
413:
252:
InChI=1S/C9H10O2/c1-7(10)8-3-5-9(11-2)6-4-8/h3-6H,1-2H3
600:
Tobacco
Documents | Profiles | Additives | Acetanisole
174:
86:
8:
547:
545:
543:
541:
352:38.2 °C (100.8 °F; 311.3 K)
227:
152:
130:
26:
580:
578:
562:
560:
194:
464:. Other names It can also be seen as a
537:
270:
248:
223:
362:254 °C (489 °F; 527 K)
143:
7:
165:
524:, a fragrance, and a flavoring in
475:Acetanisole is found naturally in
25:
479:, the glandular secretion of the
507:
403:
300:
33:
399:(at 25 °C , 100 kPa).
332:White to pale yellow crystals
306:
294:
52:1-(4-Methoxyphenyl)ethan-1-one
1:
491:Acetanisole can be prepared
667:
389:138 °C (280 °F)
554:, The Good Scents Company
393:
378:
281:
261:
239:
70:
58:
46:
41:
32:
651:Sweet-smelling chemicals
497:Friedel-Crafts acylation
605:April 11, 2008, at the
567:4'-Methoxyacetophenone
273:CC(=O)C1=CC=C(C=C1)OC
18:4-methoxyacetophenone
48:Preferred IUPAC name
369:Solubility in water
324: g·mol
29:
522:cigarette additive
426:Infobox references
27:
445:chemical compound
434:Chemical compound
432:
431:
208:CompTox Dashboard
112:Interactive image
61:4-Acetylanisole;
16:(Redirected from
658:
625:
624:
615:
609:
597:
591:
582:
573:
564:
555:
552:Para-Acetanisole
549:
520:It is used as a
511:
499:of anisole with
416:
410:
407:
406:
323:
308:
302:
296:
289:Chemical formula
232:
231:
216:
214:
198:
178:
167:
156:
145:
134:
114:
90:
37:
30:
21:
666:
665:
661:
660:
659:
657:
656:
655:
631:
630:
629:
628:
617:
616:
612:
607:Wayback Machine
598:
594:
583:
576:
565:
558:
550:
539:
534:
518:
501:acetyl chloride
489:
435:
428:
423:
422:
421: ?)
412:
408:
404:
400:
371:
321:
311:
305:
299:
291:
277:
274:
269:
268:
257:
254:
253:
247:
246:
235:
217:
210:
201:
181:
168:
137:
117:
104:
93:
80:
66:
54:
53:
23:
22:
15:
12:
11:
5:
664:
662:
654:
653:
648:
643:
641:Food additives
633:
632:
627:
626:
610:
592:
574:
556:
536:
535:
533:
530:
517:
514:
513:
512:
488:
485:
433:
430:
429:
424:
402:
401:
397:standard state
394:
391:
390:
387:
381:
380:
376:
375:
372:
367:
364:
363:
360:
354:
353:
350:
344:
343:
340:
334:
333:
330:
326:
325:
319:
313:
312:
309:
303:
297:
292:
287:
284:
283:
279:
278:
276:
275:
272:
264:
263:
262:
259:
258:
256:
255:
251:
250:
242:
241:
240:
237:
236:
234:
233:
220:
218:
206:
203:
202:
200:
199:
191:
189:
183:
182:
180:
179:
171:
169:
161:
158:
157:
147:
139:
138:
136:
135:
127:
125:
119:
118:
116:
115:
107:
105:
98:
95:
94:
92:
91:
83:
81:
76:
73:
72:
68:
67:
60:
56:
55:
51:
50:
44:
43:
39:
38:
24:
14:
13:
10:
9:
6:
4:
3:
2:
663:
652:
649:
647:
646:Piceol ethers
644:
642:
639:
638:
636:
623:
620:
614:
611:
608:
604:
601:
596:
593:
590:
589:Sigma-Aldrich
586:
581:
579:
575:
572:
568:
563:
561:
557:
553:
548:
546:
544:
542:
538:
531:
529:
527:
523:
515:
510:
506:
505:
504:
502:
498:
494:
493:synthetically
486:
484:
482:
478:
473:
471:
467:
463:
459:
455:
451:
446:
443:
439:
427:
420:
415:
398:
392:
388:
386:
383:
382:
377:
373:
370:
366:
365:
361:
359:
358:Boiling point
356:
355:
351:
349:
348:Melting point
346:
345:
341:
339:
336:
335:
331:
328:
327:
320:
318:
315:
314:
293:
290:
286:
285:
280:
271:
267:
260:
249:
245:
238:
230:
226:
225:DTXSID2044347
222:
221:
219:
209:
205:
204:
197:
193:
192:
190:
188:
185:
184:
177:
173:
172:
170:
164:
160:
159:
155:
151:
148:
146:
144:ECHA InfoCard
141:
140:
133:
129:
128:
126:
124:
121:
120:
113:
109:
108:
106:
102:
97:
96:
89:
85:
84:
82:
79:
75:
74:
69:
64:
57:
49:
45:
40:
36:
31:
19:
613:
595:
519:
490:
474:
470:acetophenone
466:methyl ether
460:) analog of
437:
436:
71:Identifiers
62:
59:Other names
28:Acetanisole
619:21 CFR
585:Acetanisole
516:Application
487:Preparation
438:Acetanisole
385:Flash point
342:1.094 g/cm
329:Appearance
282:Properties
150:100.002.560
635:Categories
532:References
468:analog of
374:2470 mg/L
317:Molar mass
196:0IRH2BR587
123:ChemSpider
99:3D model (
78:CAS Number
477:castoreum
603:Archived
442:aromatic
379:Hazards
88:100-06-1
622:172.515
571:PubChem
462:anisole
419:what is
417: (
338:Density
322:150.177
163:PubChem
481:beaver
458:ketone
454:methyl
450:acetyl
440:is an
414:verify
411:
266:SMILES
42:Names
569:from
244:InChI
101:JSmol
526:food
187:UNII
176:7476
132:7196
63:para
587:at
495:by
213:EPA
166:CID
637::
577:^
559:^
540:^
528:.
503::
483:.
472:.
304:10
456:-
452:(
409:Y
310:2
307:O
301:H
298:9
295:C
215:)
211:(
103:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.