558:
535:
982:
42:
3073:
729:. It can also be used as a preventative medication in patients over 55 years old to reduce the risk of having a heart attack, stroke or cardiovascular death in patients shown to be at high risk, such as some diabetics and patients with vascular disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth.
898:
above baseline is expected after initiation of therapy with
Ramipril, however, monitoring serum biochemistry and renal function after initiation is crucial. Treatment with Ramipril in some patients with significant narrowing in both kidneys can increase serum creatinine concentration (measured in the
1106:
in the United States, Novapril by
Pharmanova in Ghana, Ramitens by PharmaSwiss, Ampril by Krka in Slovenia, Corpril by Cemelog-BRS in Hungary, Piramil and Prilinda by Hemofarm in Serbia, by Lek in Poland and by Novartis and Opsonin Pharma Limited as Ramace in Bangladesh, and in Canada as Altace
1118:
The 2001 Heart
Outcomes and Prevention Evaluation trial seemed to show ramipril possessed cardioprotective qualities which extended beyond its qualities as an antihypertensive. However, the trial and the interpretation of its results have been criticised.
850:
Prevent the onset and/or delay the progression of diabetic kidney disease, with or without proteinuria. Randomized trial evidence suggests that a maximum tolerable dose prevents cardiovascular events and death in patients with diabetic kidney
2003:
627:
1996:
1897:"Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators".
1989:
2633:
1579:
Ahmed A (July 2002). "Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: how concerned should we be by the rise in serum creatinine?".
679:
InChI=1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17-,18-,19-,20-/m0/s1
795:. This carboxylate then interacts with the positive Zn+2 to inhibit the ACE enzyme. The COOH helps orient it with the enzyme. Ramipril is similar in structure to another ACE Inhibitor,
140:
1940:
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. (April 2008). "Telmisartan, ramipril, or both in patients at high risk for vascular events".
963:
to this drug are unlikely, but immediate medical attention must be sought if they occur. Symptoms of a serious allergic reaction include, but are not limited to a
2626:
64:
1110:
Ramipril is marketed in India under the brand names
Cardace, Zigpril, Ramistar, Odipril and Zorem . Ramipril is marketed in Myanmar under brand name Endpril .
1082:
reversed a district court trial verdict and found that
Aventis's patent on ramipril was invalid for "obviousness", opening this drug to generic manufacturers.
1122:
The Acute
Infarction Ramipril Efficacy (AIRE) trial showed a 27% reduction in mortality for patients receiving ramipril for chronic heart failure following a
1727:
Thomsen R, Rasmussen HB, Linnet K (January 2014). "In vitro drug metabolism by human carboxylesterase 1: focus on angiotensin-converting enzyme inhibitors".
651:
1772:"24-hour blood pressure profiles in hypertensive patients administered ramipril or placebo once daily: magnitude and duration of antihypertensive effects"
3040:
2372:
1236:"Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy"
3050:
2619:
2475:
2423:
1079:
1824:
1275:"A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure"
3133:
2577:
1684:
Frampton JE, Peters DH (March 1995). "Ramipril. An updated review of its therapeutic use in essential hypertension and heart failure".
3128:
2281:
1134:
1074:) on 29 October 1991. The patent was scheduled to expire on 29 October 2008. On 11 September 2007, in an appeal by the Indian company
1374:
1350:
1014:, reducing blood pressure as the blood is pumped through widened vessels. Its effect on bradykinin is responsible for the dry cough
671:
2177:
2398:
2307:
1447:"Effect of different angiotensin-converting-enzyme inhibitors on mortality among elderly patients with congestive heart failure"
2402:
2334:
296:
170:
96:
2376:
2338:
2262:
2207:
2139:
1398:
967:
or swelling of the face, mouth, tongue, or throat. In extreme cases, ramipril may lead to potentially fatal liver problems.
3045:
2492:
2471:
2427:
2190:
995:
2016:
976:
757:
2355:
1053:. Peak effect occurs between 3 and 6 hours after dosing, with approximately 50% of this effect retained after 24 hours.
434:
2173:
2169:
732:
Common side effects include headaches, dizziness, fatigue, and cough. Serious side effects may include liver problems,
2594:
1981:
1011:
514:
887:. It is also recommended to avoid using salt-substitutes as this can further increase potassium levels in the blood.
115:
3108:
3063:
3103:
1195:"Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients"
1828:
383:
2431:
2078:
3093:
2582:
2303:
2161:
2082:
726:
126:
2330:
2099:
1091:
553:
2394:
2238:
2165:
2157:
2062:
237:
2415:
2351:
2215:
2211:
2203:
2117:
3113:
3098:
3035:
2368:
2135:
2095:
2012:
1123:
891:
830:
374:
503:
1663:
1095:
767:. In 2021, it was the 201st most commonly prescribed medication in the United States, with more than 2
3030:
2653:
2642:
2546:
2467:
2439:
2419:
1103:
947:
741:
262:
2435:
530:
329:
1273:
Savarese G, Costanzo P, Cleland JG, Vassallo E, Ruggiero D, Rosano G, et al. (January 2013).
2789:
2779:
2764:
2754:
2645:
2520:
1922:
1752:
1709:
1624:"Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease"
1604:
1540:"Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease"
1255:
1235:
1030:
960:
764:
2992:
2977:
2962:
2814:
2784:
2774:
2719:
981:
2972:
2967:
2714:
2510:
1833:
Ramipril significantly reduced the high cardiovascular risk associated with renal insufficiency
1066:
The compound was protected by a patent which was assigned to the German pharmaceutical company
3118:
2997:
2769:
2729:
1967:
1914:
1793:
1744:
1701:
1645:
1596:
1561:
1517:
1476:
1370:
1346:
1340:
1296:
1216:
844:
209:
196:
184:
54:
483:
423:
1957:
1949:
1906:
1858:
1783:
1736:
1693:
1635:
1588:
1551:
1507:
1466:
1458:
1286:
1247:
1206:
895:
861:
570:
219:
1496:"Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study"
443:
338:
3123:
3077:
3007:
2674:
2450:
737:
249:
227:
1880:"Debate: Do ACE Inhibitors Have Unique Properties, Beyond Their Antihypertensive Effect?"
763:
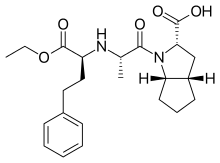
Ramipril was patented in 1981 and approved for medical use in 1989. It is available as a
557:
534:
2679:
2669:
2588:
1471:
1446:
1099:
1050:
999:
1251:
3087:
3012:
2245:
2069:
2040:
2029:
1910:
1756:
1713:
1697:
1592:
1046:
991:
869:
834:
753:
749:
722:
714:
638:
546:
88:
1608:
363:
2947:
2937:
2917:
2867:
2505:
2231:
2020:
1926:
1259:
953:
884:
820:
800:
796:
718:
153:
148:
2611:
1445:
Pilote L, Abrahamowicz M, Eisenberg M, Humphries K, Behlouli H, Tu JV (May 2008).
1390:
41:
2927:
2902:
2892:
2887:
2857:
2804:
2709:
2699:
2515:
2387:
2296:
2150:
1622:
Chrysochou C, Foley RN, Young JF, Khavandi K, Cheung CM, Kalra PA (April 2012).
1538:
Chrysochou C, Foley RN, Young JF, Khavandi K, Cheung CM, Kalra PA (April 2012).
1420:
1314:
1211:
1194:
1167:
1130:
1015:
941:
890:
Ramipril can be considered in patients with bilateral or unilateral significant
877:
804:
792:
82:
74:
1291:
1274:
2942:
2932:
2912:
2877:
2862:
2842:
2832:
2799:
2794:
2759:
2744:
2739:
2734:
2724:
2704:
2563:
2539:
2530:
2496:
2382:
2361:
2323:
2318:
2313:
2291:
2255:
2250:
2226:
2128:
2105:
2088:
2055:
2050:
1811:
1075:
1067:
1026:
1003:
865:
784:
733:
603:
414:
1879:
1847:"Ramipril reduced mortality and cardiovascular morbidity in high risk adults"
1770:
McCarron D, et al. (Ramipril
Multicenter Study Group) (September 1991).
2987:
2982:
2957:
2922:
2897:
2882:
2872:
2852:
2837:
2827:
2809:
2689:
2684:
2481:
2460:
2408:
2271:
2221:
2183:
2145:
2123:
2074:
2045:
1042:
1034:
1007:
911:
873:
745:
275:
68:
1971:
1788:
1771:
1748:
1740:
1649:
1600:
1565:
1521:
1512:
1495:
1480:
1300:
1220:
1193:
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (January 2000).
1953:
1918:
1797:
1705:
2847:
2749:
2344:
2110:
1640:
1623:
1556:
1539:
930:
788:
780:
394:
133:
110:
33:
1462:
403:
1863:
1846:
1071:
1022:
349:
17:
1962:
1038:
925:
883:
People should not take ramipril (or any ACE inhibitors) if they have
840:
494:
280:
463:
980:
626:
617:
474:
254:
964:
935:
Signs of infection (e.g., fever, chills, persistent sore throat)
454:
190:
2615:
1985:
899:
blood test), which returns to baseline upon therapy cessation.
839:
People over 55 years at high risk: prevention of heart attack,
203:
105:
1369:(76 ed.). Pharmaceutical Press. 2018. pp. 172–173.
519:
1006:. The decrease in angiotensin II results in relaxation of
1045:
is variable (3–16 hours), and is prolonged by heart and
179:
916:
Dizziness and lightheadedness due to low blood pressure
696:
3061:
2822:
1827:. Hypertension Online. 13 August 2001. Archived from
3021:
2652:
2556:
2529:
2490:
2449:
2280:
2028:
637:
615:
602:
569:
564:
545:
513:
493:
473:
453:
433:
413:
393:
382:
373:
348:
328:
287:
274:
261:
248:
236:
226:
218:
169:
164:
139:
125:
95:
81:
63:
53:
48:
1825:"HOPE Trial: Main Outcomes and Serum Creatinine"
362:
1494:Remuzzi G, Macia M, Ruggenenti P (April 2006).
1315:"Ramipril Pregnancy and Breastfeeding Warnings"
1174:. American Society of Health-System Pharmacists
1107:(Sanofi-Aventis) and Ramipril (Pharmascience).
337:
1129:Ramipril was found to have similar results as
1025:or precursor drug, is converted to the active
659:O=C(OCC)(N(C(=O)N1(C(=O)O)C2CCC12)C)CCc3ccccc3
316:)-1-amino]propanoyl]-3,3a,4,5,6,6a-hexahydro-2
2627:
1997:
1500:Journal of the American Society of Nephrology
1279:Journal of the American College of Cardiology
8:
2654:
114:
32:
1679:
1677:
2634:
2620:
2612:
2004:
1990:
1982:
1668:eMedTV: Health Information Brought To Life
1581:Journal of the American Geriatrics Society
1162:
1160:
1158:
1156:
1154:
1152:
1150:
998:(ACE), thereby lowering the production of
556:
533:
422:
40:
3041:Olmesartan/amlodipine/hydrochlorothiazide
1961:
1862:
1787:
1639:
1555:
1511:
1470:
1290:
1234:HOPE study investigators (January 2000).
1210:
1102:in Italy and United States and Altace by
442:
3051:Valsartan/hydrochlorothiazide/amlodipine
1367:British national formulary : BNF 76
1080:Court of Appeals for the Federal Circuit
3068:
2178:+bisoprolol, amlodipine, and indapamide
1146:
1010:smooth muscle leading to a decrease in
919:Fatigue, especially in the early stages
676:
656:
529:
402:
301:
87:
1345:. John Wiley & Sons. p. 469.
1168:"Ramipril Monograph for Professionals"
843:, cardiovascular death, or in need of
547:
31:
1628:Nephrology, Dialysis, Transplantation
1544:Nephrology, Dialysis, Transplantation
1533:
1531:
860:Contraindications to its use include
502:
482:
73:
7:
1878:Jafary F, Yusuf S, Nissen S (2002).
1401:from the original on 15 January 2024
758:renin-angiotensin-aldosterone system
320:-cyclopentapyrrole-2-carboxylic acid
152:
1942:The New England Journal of Medicine
1857:(2). BMJ Publishing Group Ltd: 47.
1199:The New England Journal of Medicine
1090:Ramipril is marketed as Prilace by
462:
353:
1845:Malmberg K, Rydén L (March 2000).
1421:"Ramipril - Drug Usage Statistics"
25:
1886:. Controversies in Cardiology II.
987:letter codes and icons may differ
922:Mouth dryness in the early stages
3071:
1698:10.2165/00003495-199549030-00008
1593:10.1046/j.1532-5415.2002.50321.x
1002:and decreasing the breakdown of
587:
581:
1729:Drug Metabolism and Disposition
1339:Fischer J, Ganellin CR (2006).
1135:angiotensin II receptor blocker
1098:in the Philippines, Triatec by
985:Ramipril 1.25-mg oral capsule,
684:Key:HDACQVRGBOVJII-JBDAPHQKSA-N
3134:Drugs developed by AstraZeneca
717:type medication used to treat
593:
575:
1:
3046:Valsartan/hydrochlorothiazide
1905:(8875): 821–8. October 1993.
1342:Analogue-based Drug Discovery
1252:10.1016/S0140-6736(99)12323-7
996:angiotensin converting enzyme
752:is not recommended. It is an
2659:Tooltip Angiotensin receptor
1911:10.1016/0140-6736(93)92693-N
709:, sold under the brand name
1851:BMJ Evidence-Based Medicine
1212:10.1056/NEJM200001203420301
1012:total peripheral resistance
833:in people with evidence of
3150:
2174:+bisoprolol and amlodipine
2170:+amlodipine and indapamide
1292:10.1016/j.jacc.2012.10.011
974:
791:at the OCH2CH3 and form a
565:Chemical and physical data
3129:Drugs developed by Pfizer
2572:
1094:in Australia, Ramipro by
692:
667:
647:
643:109 °C (228 °F)
292:
39:
2070:Dicarboxylate-containing
2017:renin–angiotensin system
977:Renin–angiotensin system
894:(RAS). An early rise in
826:Congestive heart failure
756:and works by decreasing
27:ACE inhibitor medication
1664:"Ramipril Side Effects"
1033:. Ramiprilat is mostly
994:inhibit the actions of
944:(low white blood cells)
864:patients, a history of
783:. The molecule must be
771:million prescriptions.
727:diabetic kidney disease
2246:Phosphonate-containing
2013:Antihypertensive drugs
1789:10.1002/clc.4960140908
1741:10.1124/dmd.113.053512
1513:10.1681/ASN.2005121324
988:
950:(erectile dysfunction)
815:Medical uses include:
799:, but it has a second
775:Activation and binding
3036:Olmesartan/amlodipine
2041:Sulfhydryl-containing
1954:10.1056/NEJMoa0801317
1812:U.S. patent 5,061,722
1782:(9). Wiley: 737–742.
1391:"The Top 300 of 2021"
1124:myocardial infarction
1092:Arrow Pharmaceuticals
984:
892:renal artery stenosis
283:(60%) and fecal (40%)
3031:Amlodipine/valsartan
2643:Angiotensin receptor
1506:(4 Suppl 2): S90-7.
1104:King Pharmaceuticals
1078:, the United States
819:High blood pressure
742:high blood potassium
713:among others, is an
2476:+amlodipine and HCT
2424:+amlodipine and HCT
2399:+amlodipine and HCT
2373:+amlodipine and HCT
2335:+amlodipine and HCT
2308:+amlodipine and HCT
2208:+amlodipine and HCT
1776:Clinical Cardiology
1463:10.1503/cmaj.060068
1070:(since merged into
1057:Society and culture
971:Mechanism of action
719:high blood pressure
199:(Prescription only)
36:
2599:Never to phase III
1864:10.1136/ebm.5.2.47
1831:on 2 August 2012.
1641:10.1093/ndt/gfr496
1557:10.1093/ndt/gfr496
1031:carboxylesterase 1
989:
961:allergic reactions
803:ring instead of a
765:generic medication
3109:Enantiopure drugs
3059:
3058:
2955:Renin inhibitors:
2609:
2608:
1246:(9200): 253–259.
856:Contraindications
845:revascularization
704:
703:
628:Interactive image
515:CompTox Dashboard
207:
194:
182:
108:
16:(Redirected from
3141:
3104:Carboxylic acids
3076:
3075:
3074:
3067:
2824:
2660:
2656:
2636:
2629:
2622:
2613:
2544:
2465:
2451:Renin inhibitors
2413:
2392:
2366:
2349:
2328:
2301:
2269:Other/ungrouped:
2260:
2236:
2201:
2188:
2155:
2133:
2115:
2093:
2060:
2006:
1999:
1992:
1983:
1976:
1975:
1965:
1937:
1931:
1930:
1894:
1888:
1887:
1875:
1869:
1868:
1866:
1842:
1836:
1835:
1821:
1815:
1814:
1808:
1802:
1801:
1791:
1767:
1761:
1760:
1724:
1718:
1717:
1681:
1672:
1671:
1660:
1654:
1653:
1643:
1634:(4): 1403–1409.
1619:
1613:
1612:
1587:(7): 1297–1300.
1576:
1570:
1569:
1559:
1550:(4): 1403–1409.
1535:
1526:
1525:
1515:
1491:
1485:
1484:
1474:
1442:
1436:
1435:
1433:
1431:
1417:
1411:
1410:
1408:
1406:
1387:
1381:
1380:
1363:
1357:
1356:
1336:
1330:
1329:
1327:
1325:
1311:
1305:
1304:
1294:
1270:
1264:
1263:
1231:
1225:
1224:
1214:
1190:
1184:
1183:
1181:
1179:
1164:
1096:Westfield Pharma
896:serum creatinine
770:
700:
699:
630:
610:
595:
589:
583:
577:
560:
549:
538:
537:
523:
521:
506:
486:
466:
446:
426:
406:
386:
366:
356:
355:
341:
266:
244:56% (ramiprilat)
205:
202:
192:
189:
181:
178:
156:
118:
107:
104:
91:
77:
44:
37:
35:
21:
3149:
3148:
3144:
3143:
3142:
3140:
3139:
3138:
3084:
3083:
3082:
3072:
3070:
3062:
3060:
3055:
3017:
3008:Angiotensinogen
2675:Angiotensin III
2658:
2648:
2640:
2610:
2605:
2604:
2589:Clinical trials
2568:
2552:
2542:
2525:
2486:
2463:
2453:
2445:
2411:
2390:
2364:
2347:
2326:
2299:
2284:
2276:
2258:
2234:
2199:
2186:
2153:
2131:
2113:
2091:
2058:
2032:
2024:
2010:
1980:
1979:
1948:(15): 1547–59.
1939:
1938:
1934:
1896:
1895:
1891:
1877:
1876:
1872:
1844:
1843:
1839:
1823:
1822:
1818:
1810:
1809:
1805:
1769:
1768:
1764:
1726:
1725:
1721:
1683:
1682:
1675:
1662:
1661:
1657:
1621:
1620:
1616:
1578:
1577:
1573:
1537:
1536:
1529:
1493:
1492:
1488:
1457:(10): 1303–11.
1444:
1443:
1439:
1429:
1427:
1419:
1418:
1414:
1404:
1402:
1389:
1388:
1384:
1377:
1365:
1364:
1360:
1353:
1338:
1337:
1333:
1323:
1321:
1313:
1312:
1308:
1272:
1271:
1267:
1233:
1232:
1228:
1192:
1191:
1187:
1177:
1175:
1166:
1165:
1148:
1143:
1116:
1088:
1064:
1059:
986:
979:
973:
905:
903:Adverse effects
862:volume-depleted
858:
813:
777:
768:
738:kidney problems
695:
693:
688:
685:
680:
675:
674:
663:
660:
655:
654:
633:
608:
598:
592:
586:
580:
541:
517:
509:
489:
469:
449:
429:
409:
389:
369:
352:
344:
324:
321:
300:
299:
264:
257:, to ramiprilat
243:
238:Protein binding
228:Bioavailability
220:Pharmacokinetic
214:
160:
128:
121:
28:
23:
22:
15:
12:
11:
5:
3147:
3145:
3137:
3136:
3131:
3126:
3121:
3116:
3111:
3106:
3101:
3096:
3094:ACE inhibitors
3086:
3085:
3081:
3080:
3057:
3056:
3054:
3053:
3048:
3043:
3038:
3033:
3027:
3025:
3019:
3018:
3016:
3015:
3010:
3001:
3000:
2995:
2990:
2985:
2980:
2975:
2970:
2965:
2960:
2951:
2950:
2945:
2940:
2935:
2930:
2925:
2920:
2915:
2910:
2905:
2900:
2895:
2890:
2885:
2880:
2875:
2870:
2865:
2860:
2855:
2850:
2845:
2840:
2835:
2830:
2818:
2817:
2812:
2807:
2802:
2797:
2792:
2787:
2782:
2777:
2772:
2767:
2762:
2757:
2752:
2747:
2742:
2737:
2732:
2727:
2722:
2717:
2712:
2707:
2702:
2693:
2692:
2687:
2682:
2680:Angiotensin IV
2677:
2672:
2670:Angiotensin II
2663:
2661:
2650:
2649:
2641:
2639:
2638:
2631:
2624:
2616:
2607:
2606:
2603:
2602:
2601:
2600:
2597:
2586:
2580:
2574:
2573:
2570:
2569:
2567:
2566:
2560:
2558:
2554:
2553:
2551:
2550:
2536:
2534:
2527:
2526:
2524:
2523:
2518:
2513:
2508:
2502:
2500:
2488:
2487:
2485:
2484:
2479:
2457:
2455:
2447:
2446:
2444:
2443:
2432:+lercanidipine
2406:
2385:
2380:
2359:
2342:
2321:
2316:
2311:
2294:
2288:
2286:
2278:
2277:
2275:
2274:
2266:
2253:
2242:
2229:
2224:
2219:
2194:
2181:
2148:
2143:
2126:
2121:
2108:
2103:
2086:
2079:+lercanidipine
2066:
2053:
2048:
2036:
2034:
2030:ACE inhibitors
2026:
2025:
2015:acting on the
2011:
2009:
2008:
2001:
1994:
1986:
1978:
1977:
1932:
1889:
1870:
1837:
1816:
1803:
1762:
1735:(1): 126–133.
1719:
1692:(3): 440–466.
1673:
1655:
1614:
1571:
1527:
1486:
1437:
1412:
1382:
1375:
1358:
1351:
1331:
1306:
1265:
1226:
1185:
1145:
1144:
1142:
1139:
1115:
1112:
1100:Sanofi-Aventis
1087:
1084:
1063:
1060:
1058:
1055:
1051:kidney failure
1029:ramiprilat by
1000:angiotensin II
992:ACE inhibitors
972:
969:
957:
956:
951:
945:
939:
936:
933:
928:
923:
920:
917:
914:
909:
904:
901:
857:
854:
853:
852:
848:
837:
827:
824:
812:
809:
779:Ramipril is a
776:
773:
702:
701:
690:
689:
687:
686:
683:
681:
678:
670:
669:
668:
665:
664:
662:
661:
658:
650:
649:
648:
645:
644:
641:
635:
634:
632:
631:
623:
621:
613:
612:
606:
600:
599:
596:
590:
584:
578:
573:
567:
566:
562:
561:
551:
543:
542:
540:
539:
526:
524:
511:
510:
508:
507:
499:
497:
491:
490:
488:
487:
479:
477:
471:
470:
468:
467:
459:
457:
451:
450:
448:
447:
439:
437:
431:
430:
428:
427:
419:
417:
411:
410:
408:
407:
399:
397:
391:
390:
388:
387:
379:
377:
371:
370:
368:
367:
359:
357:
346:
345:
343:
342:
334:
332:
326:
325:
323:
322:
303:
295:
294:
293:
290:
289:
285:
284:
278:
272:
271:
270:13 to 17 hours
268:
259:
258:
252:
246:
245:
242:73% (ramipril)
240:
234:
233:
230:
224:
223:
216:
215:
213:
212:
200:
187:
175:
173:
167:
166:
162:
161:
159:
158:
145:
143:
137:
136:
131:
129:administration
123:
122:
120:
119:
101:
99:
93:
92:
85:
79:
78:
71:
61:
60:
59:Altace, others
57:
51:
50:
46:
45:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3146:
3135:
3132:
3130:
3127:
3125:
3122:
3120:
3117:
3115:
3112:
3110:
3107:
3105:
3102:
3100:
3097:
3095:
3092:
3091:
3089:
3079:
3069:
3065:
3052:
3049:
3047:
3044:
3042:
3039:
3037:
3034:
3032:
3029:
3028:
3026:
3024:
3023:Combinations:
3020:
3014:
3013:Angiotensin I
3011:
3009:
3006:
3003:
3002:
2999:
2996:
2994:
2991:
2989:
2986:
2984:
2981:
2979:
2976:
2974:
2971:
2969:
2966:
2964:
2961:
2959:
2956:
2953:
2952:
2949:
2946:
2944:
2941:
2939:
2936:
2934:
2931:
2929:
2926:
2924:
2921:
2919:
2916:
2914:
2911:
2909:
2906:
2904:
2901:
2899:
2896:
2894:
2891:
2889:
2886:
2884:
2881:
2879:
2876:
2874:
2871:
2869:
2866:
2864:
2861:
2859:
2856:
2854:
2851:
2849:
2846:
2844:
2841:
2839:
2836:
2834:
2831:
2829:
2826:
2820:
2819:
2816:
2813:
2811:
2808:
2806:
2803:
2801:
2798:
2796:
2793:
2791:
2788:
2786:
2783:
2781:
2778:
2776:
2773:
2771:
2768:
2766:
2763:
2761:
2758:
2756:
2753:
2751:
2748:
2746:
2743:
2741:
2738:
2736:
2733:
2731:
2728:
2726:
2723:
2721:
2718:
2716:
2713:
2711:
2708:
2706:
2703:
2701:
2698:
2695:
2694:
2691:
2688:
2686:
2683:
2681:
2678:
2676:
2673:
2671:
2668:
2665:
2664:
2662:
2657:
2651:
2647:
2644:
2637:
2632:
2630:
2625:
2623:
2618:
2617:
2614:
2598:
2596:
2593:
2592:
2590:
2587:
2584:
2581:
2579:
2576:
2575:
2571:
2565:
2562:
2561:
2559:
2555:
2548:
2541:
2538:
2537:
2535:
2532:
2528:
2522:
2519:
2517:
2514:
2512:
2509:
2507:
2504:
2503:
2501:
2498:
2494:
2489:
2483:
2480:
2477:
2473:
2469:
2462:
2459:
2458:
2456:
2452:
2448:
2441:
2437:
2433:
2429:
2425:
2421:
2417:
2410:
2407:
2404:
2400:
2396:
2389:
2386:
2384:
2381:
2378:
2374:
2370:
2363:
2360:
2357:
2353:
2346:
2343:
2340:
2336:
2332:
2325:
2322:
2320:
2317:
2315:
2312:
2309:
2305:
2298:
2295:
2293:
2290:
2289:
2287:
2283:
2279:
2273:
2270:
2267:
2264:
2257:
2254:
2252:
2248:
2247:
2243:
2240:
2233:
2230:
2228:
2225:
2223:
2220:
2217:
2213:
2209:
2205:
2198:
2195:
2192:
2185:
2182:
2179:
2175:
2171:
2167:
2163:
2159:
2152:
2149:
2147:
2144:
2141:
2137:
2130:
2127:
2125:
2122:
2119:
2112:
2109:
2107:
2104:
2101:
2097:
2090:
2087:
2084:
2083:+nitrendipine
2080:
2076:
2073:
2071:
2067:
2064:
2057:
2054:
2052:
2049:
2047:
2044:
2042:
2038:
2037:
2035:
2031:
2027:
2022:
2018:
2014:
2007:
2002:
2000:
1995:
1993:
1988:
1987:
1984:
1973:
1969:
1964:
1959:
1955:
1951:
1947:
1943:
1936:
1933:
1928:
1924:
1920:
1916:
1912:
1908:
1904:
1900:
1893:
1890:
1885:
1881:
1874:
1871:
1865:
1860:
1856:
1852:
1848:
1841:
1838:
1834:
1830:
1826:
1820:
1817:
1813:
1807:
1804:
1799:
1795:
1790:
1785:
1781:
1777:
1773:
1766:
1763:
1758:
1754:
1750:
1746:
1742:
1738:
1734:
1730:
1723:
1720:
1715:
1711:
1707:
1703:
1699:
1695:
1691:
1687:
1680:
1678:
1674:
1669:
1665:
1659:
1656:
1651:
1647:
1642:
1637:
1633:
1629:
1625:
1618:
1615:
1610:
1606:
1602:
1598:
1594:
1590:
1586:
1582:
1575:
1572:
1567:
1563:
1558:
1553:
1549:
1545:
1541:
1534:
1532:
1528:
1523:
1519:
1514:
1509:
1505:
1501:
1497:
1490:
1487:
1482:
1478:
1473:
1468:
1464:
1460:
1456:
1452:
1448:
1441:
1438:
1426:
1422:
1416:
1413:
1400:
1396:
1392:
1386:
1383:
1378:
1376:9780857113382
1372:
1368:
1362:
1359:
1354:
1352:9783527607495
1348:
1344:
1343:
1335:
1332:
1320:
1316:
1310:
1307:
1302:
1298:
1293:
1288:
1285:(2): 131–42.
1284:
1280:
1276:
1269:
1266:
1261:
1257:
1253:
1249:
1245:
1241:
1237:
1230:
1227:
1222:
1218:
1213:
1208:
1205:(3): 145–53.
1204:
1200:
1196:
1189:
1186:
1173:
1169:
1163:
1161:
1159:
1157:
1155:
1153:
1151:
1147:
1140:
1138:
1136:
1132:
1127:
1125:
1120:
1113:
1111:
1108:
1105:
1101:
1097:
1093:
1085:
1083:
1081:
1077:
1073:
1069:
1061:
1056:
1054:
1052:
1049:, as well as
1048:
1047:liver failure
1044:
1040:
1036:
1032:
1028:
1024:
1019:
1017:
1013:
1009:
1005:
1001:
997:
993:
983:
978:
970:
968:
966:
962:
955:
952:
949:
946:
943:
940:
937:
934:
932:
929:
927:
924:
921:
918:
915:
913:
910:
907:
906:
902:
900:
897:
893:
888:
886:
881:
879:
875:
871:
870:ACE inhibitor
867:
863:
855:
849:
846:
842:
838:
836:
835:heart failure
832:
828:
825:
822:
821:(Hypertension
818:
817:
816:
810:
808:
806:
802:
798:
794:
790:
786:
782:
774:
772:
766:
761:
759:
755:
754:ACE inhibitor
751:
750:breastfeeding
747:
743:
739:
735:
730:
728:
724:
723:heart failure
720:
716:
715:ACE inhibitor
712:
708:
698:
691:
682:
677:
673:
666:
657:
653:
646:
642:
640:
639:Melting point
636:
629:
625:
624:
622:
619:
614:
607:
605:
601:
574:
572:
568:
563:
559:
555:
552:
550:
548:ECHA InfoCard
544:
536:
532:
531:DTXSID8023551
528:
527:
525:
516:
512:
505:
501:
500:
498:
496:
492:
485:
481:
480:
478:
476:
472:
465:
461:
460:
458:
456:
452:
445:
441:
440:
438:
436:
432:
425:
421:
420:
418:
416:
412:
405:
401:
400:
398:
396:
392:
385:
381:
380:
378:
376:
372:
365:
361:
360:
358:
351:
347:
340:
336:
335:
333:
331:
327:
319:
315:
311:
307:
302:
298:
291:
286:
282:
279:
277:
273:
269:
267:
260:
256:
253:
251:
247:
241:
239:
235:
231:
229:
225:
221:
217:
211:
201:
198:
188:
186:
177:
176:
174:
172:
168:
163:
155:
150:
147:
146:
144:
142:
138:
135:
132:
130:
124:
117:
112:
103:
102:
100:
98:
94:
90:
86:
84:
80:
76:
72:
70:
66:
62:
58:
56:
52:
49:Clinical data
47:
43:
38:
30:
19:
3114:Ethyl esters
3099:Carboxamides
3022:
3005:Propeptides:
3004:
2954:
2948:Zofenoprilat
2938:Trandolapril
2918:Rescinnamine
2907:
2868:Gemopatrilat
2821:
2697:Antagonists:
2696:
2666:
2506:Gemopatrilat
2268:
2244:
2232:Trandolapril
2196:
2068:
2039:
1945:
1941:
1935:
1902:
1898:
1892:
1883:
1873:
1854:
1850:
1840:
1832:
1829:the original
1819:
1806:
1779:
1775:
1765:
1732:
1728:
1722:
1689:
1685:
1667:
1658:
1631:
1627:
1617:
1584:
1580:
1574:
1547:
1543:
1503:
1499:
1489:
1454:
1450:
1440:
1428:. Retrieved
1424:
1415:
1403:. Retrieved
1394:
1385:
1366:
1361:
1341:
1334:
1322:. Retrieved
1318:
1309:
1282:
1278:
1268:
1243:
1239:
1229:
1202:
1198:
1188:
1176:. Retrieved
1171:
1128:
1121:
1117:
1109:
1089:
1065:
1021:Ramipril, a
1020:
990:
958:
954:Hyperkalemia
889:
885:hyperkalemia
882:
868:while on an
859:
831:heart attack
814:
811:Medical uses
801:cyclopentane
797:trandolapril
778:
762:
731:
710:
706:
705:
694:
317:
313:
309:
305:
263:Elimination
171:Legal status
165:Legal status
97:License data
29:
2928:Spiraprilat
2903:Quinaprilat
2893:Perindopril
2888:Omapatrilat
2858:Enalaprilat
2825:inhibitors:
2805:Telmisartan
2790:Saprisartan
2780:Pratosartan
2765:Olodanrigan
2755:Milfasartan
2710:Candesartan
2700:Abitesartan
2585:from market
2521:Sampatrilat
2516:Omapatrilat
2468:+amlodipine
2440:+sacubitril
2420:+amlodipine
2395:+amlodipine
2388:Telmisartan
2369:+amlodipine
2352:+amlodipine
2331:+amlodipine
2304:+amlodipine
2297:Candesartan
2285:("-sartan")
2216:+felodipine
2212:+bisoprolol
2204:+amlodipine
2166:+indapamide
2162:+bisoprolol
2158:+amlodipine
2151:Perindopril
2136:+amlodipine
2118:+manidipine
2100:+pimobendan
2096:+amlodipine
1131:telmisartan
1086:Brand names
1016:side effect
942:Neutropenia
878:hypotension
805:cyclohexane
793:carboxylate
611: g·mol
554:100.170.726
288:Identifiers
83:MedlinePlus
55:Trade names
3088:Categories
2993:Terlakiren
2978:Imarikiren
2963:Ciprokiren
2943:Zofenopril
2933:Temocapril
2913:Rentiapril
2878:Lisinopril
2863:Fosinopril
2843:Cilazapril
2833:Benazepril
2815:Zolasartan
2800:Tasosartan
2795:Sparsentan
2785:Ripisartan
2775:Pomisartan
2760:Olmesartan
2745:Irbesartan
2740:Forasartan
2735:Fimasartan
2725:Eprosartan
2720:Embusartan
2705:Azilsartan
2646:modulators
2564:Sparsentan
2547:+valsartan
2540:Sacubitril
2533:inhibitors
2531:Neprilysin
2499:inhibitors
2454:("-kiren")
2436:+nebivolol
2416:+aliskiren
2383:Tasosartan
2362:Olmesartan
2324:Irbesartan
2319:Fimasartan
2314:Eprosartan
2292:Azilsartan
2256:Fosinopril
2251:Ceronapril
2239:+verapamil
2227:Temocapril
2129:Lisinopril
2106:Cilazapril
2089:Benazepril
2063:+nebivolol
2056:Zofenopril
2051:Rentiapril
1963:2437/81925
1430:14 January
1405:14 January
1240:The Lancet
1141:References
1076:Lupin Ltd.
1068:Hoechst AG
1027:metabolite
1004:bradykinin
975:See also:
938:Chest pain
866:angioedema
847:procedures
829:Following
785:hydrolyzed
760:activity.
734:angioedema
616:3D model (
604:Molar mass
504:ChEMBL1168
484:CHEBI:8774
444:L35JN3I7SJ
415:ChemSpider
375:IUPHAR/BPS
339:87333-19-5
330:CAS Number
297:IUPAC name
250:Metabolism
2988:Remikiren
2983:Pepstatin
2973:Enalkiren
2968:Ditekiren
2958:Aliskiren
2923:Spirapril
2898:Quinapril
2883:Moexipril
2873:Imidapril
2853:Enalapril
2838:Captopril
2828:Alacepril
2810:Valsartan
2715:Elisartan
2690:Saralasin
2685:L-163,491
2667:Agonists:
2595:Phase III
2583:Withdrawn
2511:Ilepatril
2482:Remikiren
2461:Aliskiren
2409:Valsartan
2272:Alacepril
2222:Spirapril
2184:Quinapril
2146:Moexipril
2124:Imidapril
2075:Enalapril
2046:Captopril
2033:("-pril")
1757:206496779
1714:195691742
1319:Drugs.com
1172:Drugs.com
1062:US patent
1043:half-life
1008:arteriole
948:Impotence
912:Dry cough
908:Shakiness
874:pregnancy
746:pregnancy
744:. Use in
276:Excretion
265:half-life
127:Routes of
75:Monograph
69:Drugs.com
3119:Prodrugs
3078:Medicine
2998:Zankiren
2908:Ramipril
2848:Delapril
2770:PD123319
2750:Losartan
2730:EXP-3174
2345:Losartan
2197:Ramipril
2111:Delapril
1972:18378520
1884:MedScape
1749:24141856
1650:21993376
1609:31459410
1601:12133029
1566:21993376
1522:16565256
1481:18458262
1425:ClinCalc
1399:Archived
1395:ClinCalc
1301:23219304
1221:10639539
1114:Research
1035:excreted
959:Serious
931:Fainting
851:disease.
789:esterase
781:pro-drug
707:Ramipril
697:(verify)
395:DrugBank
141:ATC code
134:By mouth
116:Ramipril
111:DailyMed
34:Ramipril
1927:5770772
1919:8104270
1798:1835914
1706:7774515
1472:2335176
1324:3 March
1260:1863533
1178:3 March
1072:Aventis
1039:kidneys
1037:by the
1023:prodrug
787:by the
609:416.518
571:Formula
424:4514937
404:DB00178
364:5362129
350:PubChem
183::
157:)
151: (
149:C09AA05
113::
89:a692027
18:Cardace
3124:Sanofi
3064:Portal
2578:WHO-EM
2543:
2464:
2412:
2391:
2365:
2348:
2327:
2300:
2282:AIIRAs
2259:
2235:
2200:
2187:
2154:
2132:
2114:
2092:
2059:
1970:
1925:
1917:
1899:Lancet
1796:
1755:
1747:
1712:
1704:
1648:
1607:
1599:
1564:
1520:
1479:
1469:
1373:
1349:
1299:
1258:
1219:
1041:. Its
926:Nausea
841:stroke
807:ring.
769:
740:, and
725:, and
711:Altace
652:SMILES
495:ChEMBL
464:D00421
281:Kidney
210:℞-only
208:
195:
185:℞-only
109:
2557:Other
2491:Dual
1923:S2CID
1753:S2CID
1710:S2CID
1686:Drugs
1605:S2CID
1256:S2CID
1133:, an
672:InChI
618:JSmol
475:ChEBI
255:Liver
2472:+HCT
2428:+HCT
2403:+HCT
2377:+HCT
2356:+HCT
2339:+HCT
2263:+HCT
2191:+HCT
2140:+HCT
1968:PMID
1915:PMID
1794:PMID
1745:PMID
1702:PMID
1646:PMID
1597:PMID
1562:PMID
1518:PMID
1477:PMID
1451:CMAJ
1432:2024
1407:2024
1371:ISBN
1347:ISBN
1326:2019
1297:PMID
1217:PMID
1180:2019
965:rash
876:and
748:and
455:KEGG
435:UNII
384:6339
222:data
65:AHFS
2823:ACE
2655:ATR
2497:NEP
2493:ACE
2021:C09
1958:hdl
1950:doi
1946:358
1907:doi
1903:342
1859:doi
1784:doi
1737:doi
1694:doi
1636:doi
1589:doi
1552:doi
1508:doi
1467:PMC
1459:doi
1455:178
1287:doi
1248:doi
1244:355
1207:doi
1203:342
520:EPA
354:CID
312:,6a
308:,3a
232:28%
197:POM
154:WHO
3090::
2591::
2474:,
2470:,
2438:,
2434:,
2430:,
2426:,
2422:,
2418:,
2401:,
2397:,
2375:,
2371:,
2354:,
2337:,
2333:,
2306:,
2249::
2214:,
2210:,
2206:,
2176:,
2172:,
2168:,
2164:,
2160:,
2138:,
2098:,
2081:,
2072::
1966:.
1956:.
1944:.
1921:.
1913:.
1901:.
1882:.
1853:.
1849:.
1792:.
1780:14
1778:.
1774:.
1751:.
1743:.
1733:42
1731:.
1708:.
1700:.
1690:49
1688:.
1676:^
1666:.
1644:.
1632:27
1630:.
1626:.
1603:.
1595:.
1585:50
1583:.
1560:.
1548:27
1546:.
1542:.
1530:^
1516:.
1504:17
1502:.
1498:.
1475:.
1465:.
1453:.
1449:.
1423:.
1397:.
1393:.
1317:.
1295:.
1283:61
1281:.
1277:.
1254:.
1242:.
1238:.
1215:.
1201:.
1197:.
1170:.
1149:^
1137:.
1126:.
1018:.
880:.
872:,
736:,
721:,
585:32
579:23
304:(2
204:US
191:UK
180:CA
106:US
3066::
2635:e
2628:t
2621:v
2549:)
2545:(
2495:/
2478:)
2466:(
2442:)
2414:(
2405:)
2393:(
2379:)
2367:(
2358:)
2350:(
2341:)
2329:(
2310:)
2302:(
2265:)
2261:(
2241:)
2237:(
2218:)
2202:(
2193:)
2189:(
2180:)
2156:(
2142:)
2134:(
2120:)
2116:(
2102:)
2094:(
2085:)
2077:(
2065:)
2061:(
2043::
2023:)
2019:(
2005:e
1998:t
1991:v
1974:.
1960::
1952::
1929:.
1909::
1867:.
1861::
1855:5
1800:.
1786::
1759:.
1739::
1716:.
1696::
1670:.
1652:.
1638::
1611:.
1591::
1568:.
1554::
1524:.
1510::
1483:.
1461::
1434:.
1409:.
1379:.
1355:.
1328:.
1303:.
1289::
1262:.
1250::
1223:.
1209::
1182:.
823:)
620:)
597:5
594:O
591:2
588:N
582:H
576:C
522:)
518:(
318:H
314:S
310:S
306:S
206::
193::
67:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.