42:
247:
146:
51:
367:
370:
372:
467:
371:
839:
There are several different grades of PVA depending on the degrees of polymerization and hydrolysis, which will affect their physical and chemical properties as well as their biodegradability. Aqueous solutions of PVA degrade faster, which is why PVA grades that are highly water-soluble tend to have
1479:
Byrne, Dominic, Boeije, Geert, Croft, Ian, Hüttmann, Gerd, Luijkx, Gerard, Meier, Frank, Parulekar, Yash and
Stijntjes, Gerard. "Biodegradability of Polyvinyl Alcohol Based Film Used for Liquid Detergent Capsules: Biologische Abbaubarkeit der für Flüssigwaschmittelkapseln verwendeten Folie auf
616:
PVA-based polymers are used widely in additive manufacturing. For example, 3D printed oral dosage forms demonstrate great potential in the pharmaceutical industry. It is possible to create drug-loaded tablets with modified drug-release characteristics where PVA is used as a binder substance.
547:. It is colourless (white) and odorless. It is commonly supplied as beads or as solutions in water. Without an externally added crosslinking agent, PVA solution can be gelled through repeated freezing-thawing, yielding highly strong, ultrapure,
840:
a faster biodegradation. Not all PVA grades are readily biodegradable, but studies show that high water-soluble PVA grades such as the ones used in detergents can be readily biodegradable according to OECD screening test conditions.
835:
Polyvinyl alcohol is widely used, thus its toxicity and biodegradation are of interest. Tests showed that fish (guppies) are not harmed, even at a poly(vinyl alcohol) concentration of 500 mg/L of water.
635:
particles to be used for peripheral hypervascular tumors. It may also used as the embolic agent in a
Uterine Fibroid Embolectomy (UFE). In biomedical engineering research, PVA has also been studied for
804:. In terms of microstructure, it is composed mainly of 1,3-diol linkages , but a few percent of 1,2-diols occur, depending on the conditions for the polymerization of the vinyl ester precursor.
65:
PVOH; Poly(Ethenol), Ethenol, homopolymer; PVA; Polyviol; Vinol; Alvyl; Alcotex; Covol; Gelvatol; Lemol; Mowiol; Mowiflex, Alcotex, Elvanol, Gelvatol, Lemol, Nelfilcon A, Polyviol und
Rhodoviol
585:
PVA is used in a variety of medical applications because of its biocompatibility, low tendency for protein adhesion, and low toxicity. Specific uses include cartilage replacements,
480:
998:
1263:
373:
1349:
Chaouat, Marc; Le Visage, Catherine; Baille, Wilms E.; Escoubet, Brigitte; Chaubet, Frédéric; Mateescu, Mircea
Alexandru; Letourneur, Didier (2008-10-09).
381:
788:, while mainland China has installed a number of very large production facilities in the past decade and currently accounts for 45% of world capacity.
395:
819:. It has high tensile strength and flexibility, as well as high oxygen and aroma barrier properties. However, these properties are dependent on
1190:
1123:
Baker MI, Walsh SP, Schwartz Z, Boyan BD (July 2012). "A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications".
1288:
1031:
Tang X, Alavi S (2011). "Recent
Advances in Starch, Polyvinyl Alcohol Based Polymer Blends, Nanocomposites and Their Biodegradability".
1390:"Comparison of Polyvinyl Alcohol Fixative with Three Less Hazardous Fixatives for Detection and Identification of Intestinal Parasites"
1015:
388:
1388:
Jensen, B.; Kepley, W.; Guarner, J.; Anderson, K.; Anderson, D.; Clairmont, J.; De l'aune, W.; Austin, E.H.; Austin, G.E. (2000).
1583:
1558:
698:(PVF), respectively. Preparation of polyvinyl butyral is the largest use for polyvinyl alcohol in the US and Western Europe.
487:
625:
204:
1173:
Lampman, Steve, ed. (2003). "Effects of
Composition, Processing, and Structure on Properties of Engineering Plastics".
1593:
225:
730:. Instead, PVA is prepared by hydrolysis of polyvinyl acetate, or sometimes other vinyl ester-derived polymers with
594:
41:
780:
Worldwide consumption of polyvinyl alcohol was over one million metric tons in 2006. Large producers include
1578:
141:
862:
738:
groups instead of acetate. The conversion of the polyvinyl esters is usually conducted by base-catalysed
315:
107:
1059:
1219:
1071:
855:
843:
739:
242:
77:
1462:
1370:
1105:
641:
598:
827:, which reduces the polymer's tensile strength, but increases its elongation and tear strength.
449:
1588:
1525:
1517:
1454:
1419:
1331:
1245:
1186:
1140:
1097:
1011:
969:
902:
897:
691:
574:
540:
522:
443:
50:
28:
1350:
1509:
1446:
1409:
1401:
1362:
1323:
1296:
1235:
1227:
1178:
1160:
1132:
1087:
1079:
1040:
1003:
959:
949:
785:
695:
329:
264:
1058:
Adelnia, Hossein; Ensandoost, Reza; Shebbrin
Moonshi, Shehzahdi; et al. (2022-02-05).
213:
1311:
87:
1480:
Polyvinylalkoholbasis." Tenside
Surfactants Detergents, vol. 58, no. 2, 2021, pp. 88-96.
1553:
1437:
Kawai F, Hu X (August 2009). "Biochemistry of microbial polyvinyl alcohol degradation".
1223:
1075:
246:
145:
1240:
1207:
922:
711:
645:
458:
1513:
1572:
1414:
1389:
1312:"A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications"
1109:
964:
892:
735:
707:
683:
621:
548:
305:
134:
1466:
1405:
1374:
1083:
1310:
Baker, Maribel I.; Walsh, Steven P.; Schwartz, Zvi; Boyan, Barbara D. (July 2012).
1182:
727:
687:
652:
632:
586:
563:
1044:
777:
The properties of the polymer are affected by the degree of transesterification.
880:
824:
606:
544:
532:
431:
407:
1231:
1450:
869:
659:
118:
1521:
1101:
1007:
1497:
876:
663:
637:
610:
559:
1529:
1458:
1423:
1366:
1335:
1249:
1144:
973:
858:
of polyvinyl alcohol is very low, with LD(50)s in the range of 15-20 g/kg;
820:
812:
808:
723:
679:
590:
551:
394:
387:
380:
353:
277:
161:
153:
1481:
1327:
1136:
1092:
1563:
954:
937:
816:
801:
797:
781:
731:
719:
715:
675:
602:
570:
519:
295:
17:
651:
PVA is commonly used in household sponges that absorb more water than
601:
to make PVAc dispersions. In Japan its major use is the production of
1316:
Journal of
Biomedical Materials Research Part B: Applied Biomaterials
1208:"3D Printed Polyvinyl Alcohol Tablets with Multiple Release Profiles"
1125:
Journal of
Biomedical Materials Research Part B: Applied Biomaterials
1060:"Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future"
850:
of polyvinyl alcohol is based on some of the following observations:
847:
628:
613:
is required to produce it. Another application is photographic film.
536:
283:
271:
98:
1351:"A Novel Cross-linked Poly(vinyl alcohol) (PVA) for Vascular Grafts"
457:
Except where otherwise noted, data are given for materials in their
177:
555:
515:
188:
168:
543:(PVAc) adhesive formulations, in a variety of coatings, and
230:
554:
which have been used for a variety of applications such as
1163:, published March 2007, abstract retrieved July 30, 2008.
936:
Schnepf MJ, Mayer M, Kuttner C, et al. (July 2017).
861:
Orally administered PVA is very poorly absorbed from the
365:
1498:"Review of the oral toxicity of polyvinyl alcohol (PVA)"
577:, which is commonly used as a wood adhesive and sealer.
996:
Hallensleben ML (2000). "Polyvinyl Compounds, Others".
569:
Although polyvinyl alcohol is often referred to by the
475:
938:"Nanorattles with tailored electric field enhancement"
823:: water absorbed at higher humidity levels acts as a
658:
PVA may be used as an adhesive during preparation of
154:
815:
properties. It is also resistant to oil, grease and
722:, is thermodynamically unstable with respect to its
597:. Its largest application in China is its use as a
1206:Xu X, Zhao J, Wang M, et al. (August 2019).
1175:Characterization and Failure Analysis of Plastics
1496:DeMerlis, C. C.; Schoneker, D. R. (March 2003).
369:
86:
999:Ullmann's Encyclopedia of Industrial Chemistry
807:Polyvinyl alcohol has excellent film-forming,
412:79.44 °C (174.99 °F; 352.59 K)
8:
539:, as a thickener and emulsion stabilizer in
1161:SRI Consulting CEH Report Polyvinyl Alcohol
1289:"Uterine Fibroid Embolization and Imaging"
644:applications, and potential materials for
605:fiber. This fiber is also manufactured in
245:
144:
33:
1491:
1489:
1413:
1239:
1091:
991:
989:
987:
985:
983:
963:
953:
609:for self-sufficiency reasons, because no
593:. Polyvinyl alcohol is used as an aid in
212:
914:
831:Safety and environmental considerations
241:
1439:Applied Microbiology and Biotechnology
310:200 °C (392 °F; 473 K)
135:
1482:https://doi.org/10.1515/tsd-2020-2326
1156:
1154:
106:
7:
176:
678:are prepared by treating PVA with
573:PVA, more generally PVA refers to
25:
1564:Forming PVA layers in PET bottles
1177:. ASM International. p. 29.
1394:Journal of Clinical Microbiology
846:PVA is relatively harmless. The
465:
49:
40:
1406:10.1128/jcm.38.4.1592-1598.2000
1084:10.1016/j.eurpolymj.2021.110974
868:PVA does not accumulate in the
662:for microscopic examination in
525:. It has the idealized formula
461:(at 25 °C , 100 kPa).
1183:10.31399/asm.tb.cfap.t69780028
1:
1514:10.1016/s0278-6915(02)00258-2
1355:Advanced Functional Materials
1045:10.1016/j.carbpol.2011.01.030
1502:Food and Chemical Toxicology
1295:. WebMD LLC. Archived from
786:Sekisui Specialty Chemicals
420:or concentration (LD, LC):
1610:
1264:"Contour™ - Brief Summary"
1232:10.1038/s41598-019-48921-8
595:suspension polymerizations
26:
1451:10.1007/s00253-009-2113-6
1287:Siskin GP. Cho KJ (ed.).
965:10067/1447970151162165141
875:Polyvinyl alcohol is not
872:when administered orally;
710:, PVA is not prepared by
455:
416:
347:
257:
70:
62:
57:
48:
39:
1268:www.bostonscientific.com
1064:European Polymer Journal
1008:10.1002/14356007.a21_743
792:Structure and properties
27:Not to be confused with
1002:. Weinheim: Wiley-VCH.
800:material that exhibits
631:approval to be used as
1584:Biodegradable plastics
1367:10.1002/adfm.200701261
863:gastrointestinal tract
376:
162:(additional chemicals)
1033:Carbohydrate Polymers
923:"Poly(vinyl alcohol)"
718:, since the monomer,
714:of the corresponding
620:Medically, PVA-based
438:14,700 mg/kg (mouse)
375:
358:(fire diamond)
1328:10.1002/jbm.b.32694
1224:2019NatSR...912487X
1137:10.1002/jbm.b.32694
1076:2022EurPJ.16410974A
856:acute oral toxicity
844:Orally administered
740:transesterification
537:textile warp sizing
36:
1594:E-number additives
1212:Scientific Reports
955:10.1039/C7NR02952G
599:protective colloid
488:Infobox references
377:
35:Polyvinyl alcohol
34:
1361:(19): 2855–2861.
1192:978-0-87170-789-5
948:(27): 9376–9385.
903:Polyvinyl acetate
898:Polyvinyl nitrate
692:polyvinyl butyral
670:Polyvinyl acetals
575:polyvinyl acetate
541:polyvinyl acetate
523:synthetic polymer
500:Polyvinyl alcohol
496:Chemical compound
494:
493:
444:Safety data sheet
226:CompTox Dashboard
29:polyvinyl acetate
16:(Redirected from
1601:
1541:
1540:
1538:
1536:
1493:
1484:
1477:
1471:
1470:
1434:
1428:
1427:
1417:
1400:(4): 1592–1598.
1385:
1379:
1378:
1346:
1340:
1339:
1322:(5): 1451–1457.
1307:
1301:
1300:
1284:
1278:
1277:
1275:
1274:
1260:
1254:
1253:
1243:
1203:
1197:
1196:
1170:
1164:
1158:
1149:
1148:
1120:
1114:
1113:
1095:
1055:
1049:
1048:
1028:
1022:
1021:
993:
978:
977:
967:
957:
933:
927:
926:
919:
696:polyvinyl formal
531:. It is used in
478:
472:
469:
468:
397:
390:
383:
368:
330:Refractive index
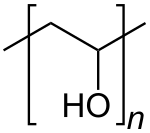
265:Chemical formula
250:
249:
234:
232:
216:
180:
156:
148:
137:
110:
90:
53:
44:
37:
21:
1609:
1608:
1604:
1603:
1602:
1600:
1599:
1598:
1569:
1568:
1550:
1545:
1544:
1534:
1532:
1495:
1494:
1487:
1478:
1474:
1436:
1435:
1431:
1387:
1386:
1382:
1348:
1347:
1343:
1309:
1308:
1304:
1286:
1285:
1281:
1272:
1270:
1262:
1261:
1257:
1205:
1204:
1200:
1193:
1172:
1171:
1167:
1159:
1152:
1122:
1121:
1117:
1057:
1056:
1052:
1030:
1029:
1025:
1018:
995:
994:
981:
935:
934:
930:
921:
920:
916:
911:
889:
833:
794:
772:
768:
764:
758:
754:
750:
724:tautomerization
704:
672:
583:
556:vascular stents
530:
497:
490:
485:
484:
483: ?)
474:
470:
466:
462:
435:
429:
402:
401:
400:
399:
392:
385:
378:
374:
366:
343:1.477 @ 632 nm
340:
338:
300:1.19–1.31 g/cm
289:
282:
276:
267:
253:
235:
228:
219:
199:
183:
129:
113:
93:
80:
66:
32:
23:
22:
15:
12:
11:
5:
1607:
1605:
1597:
1596:
1591:
1586:
1581:
1579:Vinyl polymers
1571:
1570:
1567:
1566:
1561:
1559:"Slime" recipe
1556:
1549:
1548:External links
1546:
1543:
1542:
1508:(3): 319–326.
1485:
1472:
1429:
1380:
1341:
1302:
1299:on 2015-03-04.
1279:
1255:
1198:
1191:
1165:
1150:
1115:
1050:
1023:
1017:978-3527306732
1016:
979:
928:
913:
912:
910:
907:
906:
905:
900:
895:
888:
885:
884:
883:
873:
866:
859:
832:
829:
793:
790:
775:
774:
770:
766:
760:
756:
752:
746:
742:with ethanol:
712:polymerization
708:vinyl polymers
703:
700:
671:
668:
646:vascular graft
624:have received
622:microparticles
587:contact lenses
582:
579:
564:contact lenses
526:
495:
492:
491:
486:
464:
463:
459:standard state
456:
453:
452:
447:
440:
439:
436:
427:
425:
422:
421:
414:
413:
410:
404:
403:
393:
386:
379:
364:
363:
362:
361:
359:
350:
349:
345:
344:
341:
336:
328:
325:
324:
321:
312:
311:
308:
302:
301:
298:
292:
291:
287:
280:
274:
268:
263:
260:
259:
255:
254:
252:
251:
238:
236:
224:
221:
220:
218:
217:
209:
207:
201:
200:
198:
197:
193:
191:
185:
184:
182:
181:
173:
171:
165:
164:
158:
150:
149:
139:
131:
130:
128:
127:
123:
121:
115:
114:
112:
111:
103:
101:
95:
94:
92:
91:
83:
81:
76:
73:
72:
68:
67:
64:
60:
59:
55:
54:
46:
45:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1606:
1595:
1592:
1590:
1587:
1585:
1582:
1580:
1577:
1576:
1574:
1565:
1562:
1560:
1557:
1555:
1552:
1551:
1547:
1531:
1527:
1523:
1519:
1515:
1511:
1507:
1503:
1499:
1492:
1490:
1486:
1483:
1476:
1473:
1468:
1464:
1460:
1456:
1452:
1448:
1445:(2): 227–37.
1444:
1440:
1433:
1430:
1425:
1421:
1416:
1411:
1407:
1403:
1399:
1395:
1391:
1384:
1381:
1376:
1372:
1368:
1364:
1360:
1356:
1352:
1345:
1342:
1337:
1333:
1329:
1325:
1321:
1317:
1313:
1306:
1303:
1298:
1294:
1290:
1283:
1280:
1269:
1265:
1259:
1256:
1251:
1247:
1242:
1237:
1233:
1229:
1225:
1221:
1217:
1213:
1209:
1202:
1199:
1194:
1188:
1184:
1180:
1176:
1169:
1166:
1162:
1157:
1155:
1151:
1146:
1142:
1138:
1134:
1131:(5): 1451–7.
1130:
1126:
1119:
1116:
1111:
1107:
1103:
1099:
1094:
1089:
1085:
1081:
1077:
1073:
1069:
1065:
1061:
1054:
1051:
1046:
1042:
1038:
1034:
1027:
1024:
1019:
1013:
1009:
1005:
1001:
1000:
992:
990:
988:
986:
984:
980:
975:
971:
966:
961:
956:
951:
947:
943:
939:
932:
929:
924:
918:
915:
908:
904:
901:
899:
896:
894:
893:Vinyl acetate
891:
890:
886:
882:
878:
874:
871:
867:
864:
860:
857:
853:
852:
851:
849:
845:
841:
837:
830:
828:
826:
822:
818:
814:
810:
805:
803:
802:crystallinity
799:
791:
789:
787:
783:
778:
763:
749:
745:
744:
743:
741:
737:
736:chloroacetate
733:
729:
725:
721:
720:vinyl alcohol
717:
713:
709:
701:
699:
697:
693:
689:
685:
684:Butyraldehyde
681:
677:
669:
667:
665:
661:
660:stool samples
656:
654:
649:
647:
643:
639:
634:
630:
627:
623:
618:
614:
612:
608:
604:
600:
596:
592:
588:
580:
578:
576:
572:
567:
565:
561:
557:
553:
550:
549:biocompatible
546:
542:
538:
534:
529:
524:
521:
517:
513:
509:
505:
501:
489:
482:
477:
460:
454:
451:
450:External MSDS
448:
445:
442:
441:
437:
433:
424:
423:
419:
415:
411:
409:
406:
405:
398:
391:
384:
360:
357:
356:
352:
351:
346:
342:
335:
331:
327:
326:
322:
320:
319:
314:
313:
309:
307:
306:Melting point
304:
303:
299:
297:
294:
293:
285:
279:
273:
269:
266:
262:
261:
256:
248:
244:
243:DTXSID4031930
240:
239:
237:
227:
223:
222:
215:
211:
210:
208:
206:
203:
202:
195:
194:
192:
190:
187:
186:
179:
175:
174:
172:
170:
167:
166:
163:
159:
157:
152:
151:
147:
143:
140:
138:
136:ECHA InfoCard
133:
132:
125:
124:
122:
120:
117:
116:
109:
105:
104:
102:
100:
97:
96:
89:
85:
84:
82:
79:
75:
74:
69:
61:
56:
52:
47:
43:
38:
30:
19:
1533:. Retrieved
1505:
1501:
1475:
1442:
1438:
1432:
1397:
1393:
1383:
1358:
1354:
1344:
1319:
1315:
1305:
1297:the original
1292:
1282:
1271:. Retrieved
1267:
1258:
1218:(1): 12487.
1215:
1211:
1201:
1174:
1168:
1128:
1124:
1118:
1093:10072/417476
1067:
1063:
1053:
1036:
1032:
1026:
997:
945:
941:
931:
917:
842:
838:
834:
806:
795:
779:
776:
761:
747:
728:acetaldehyde
706:Unlike most
705:
688:formaldehyde
673:
657:
653:polyurethane
650:
633:embolisation
619:
615:
584:
568:
527:
511:
507:
503:
499:
498:
417:
354:
333:
317:
189:RTECS number
71:Identifiers
63:Other names
881:clastogenic
825:plasticiser
809:emulsifying
702:Preparation
642:orthopaedic
607:North Korea
545:3D printing
533:papermaking
432:median dose
418:Lethal dose
408:Flash point
258:Properties
142:100.121.648
108:ChEMBL76101
1573:Categories
1535:17 January
1273:2023-08-11
1070:: 110974.
925:. ChemSrc.
909:References
796:PVA is an
694:(PVB) and
674:Polyvinyl
560:cartilages
214:1E30GE2EF7
119:ChemSpider
78:CAS Number
1522:0278-6915
1110:245576810
1102:0014-3057
942:Nanoscale
877:mutagenic
680:aldehydes
664:pathology
655:sponges.
638:cartilage
591:eye drops
552:hydrogels
196:TR8100000
88:9002-89-5
1589:Polymers
1530:12504164
1467:25068302
1459:19590867
1424:10747149
1375:42332293
1336:22514196
1293:Medscape
1250:31462744
1145:22514196
1039:: 7–16.
974:28656183
887:See also
821:humidity
817:solvents
813:adhesive
355:NFPA 704
348:Hazards
155:E number
1241:6713737
1220:Bibcode
1072:Bibcode
798:atactic
782:Kuraray
732:formate
716:monomer
690:afford
676:acetals
603:Vinylon
571:acronym
566:, etc.
520:soluble
514:) is a
481:what is
479: (
296:Density
290:
18:Merocel
1528:
1520:
1465:
1457:
1422:
1412:
1373:
1334:
1248:
1238:
1189:
1143:
1108:
1100:
1014:
972:
848:safety
629:510(k)
589:, and
476:verify
473:
446:(SDS)
323:0.26
178:C00980
160:E1203
99:ChEMBL
58:Names
1463:S2CID
1415:86497
1371:S2CID
1106:S2CID
759:OH →
516:water
510:, or
1554:MSDS
1537:2024
1526:PMID
1518:ISSN
1455:PMID
1420:PMID
1332:PMID
1320:100B
1246:PMID
1187:ISBN
1141:PMID
1098:ISSN
1012:ISBN
970:PMID
870:body
854:The
811:and
784:and
686:and
581:Uses
512:PVAl
504:PVOH
316:log
205:UNII
169:KEGG
126:none
1510:doi
1447:doi
1410:PMC
1402:doi
1363:doi
1324:doi
1236:PMC
1228:doi
1179:doi
1133:doi
1129:100
1088:hdl
1080:doi
1068:164
1041:doi
1004:doi
960:hdl
950:doi
879:or
773:OAc
765:+ C
751:+ C
734:or
726:to
626:FDA
611:oil
508:PVA
231:EPA
1575::
1524:.
1516:.
1506:41
1504:.
1500:.
1488:^
1461:.
1453:.
1443:84
1441:.
1418:.
1408:.
1398:38
1396:.
1392:.
1369:.
1359:18
1357:.
1353:.
1330:.
1318:.
1314:.
1291:.
1266:.
1244:.
1234:.
1226:.
1214:.
1210:.
1185:.
1153:^
1139:.
1127:.
1104:.
1096:.
1086:.
1078:.
1066:.
1062:.
1037:85
1035:.
1010:.
982:^
968:.
958:.
944:.
940:.
682:.
666:.
648:.
640:,
562:,
558:,
535:,
506:,
428:50
426:LD
1539:.
1512::
1469:.
1449::
1426:.
1404::
1377:.
1365::
1338:.
1326::
1276:.
1252:.
1230::
1222::
1216:9
1195:.
1181::
1147:.
1135::
1112:.
1090::
1082::
1074::
1047:.
1043::
1020:.
1006::
976:.
962::
952::
946:9
865:;
771:5
769:H
767:2
762:n
757:5
755:H
753:2
748:n
528:n
518:-
502:(
471:N
434:)
430:(
396:0
389:1
382:0
339:)
337:D
334:n
332:(
318:P
288:x
286:)
284:O
281:4
278:H
275:2
272:C
270:(
233:)
229:(
31:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.