1312:
evaluated in Inter-B-NHL Ritux 2010, a global multicenter, open-label, randomized 1:1 trial of participants six months in age or older with previously untreated, advanced stage, CD20-positive diffuse large B-cell lymphoma, Burkitt lymphoma, Burkitt-like lymphoma, or B-cell acute leukemia. Advanced stage was defined as stage III with elevated lactose dehydrogenase level (lactose dehydrogenase greater than twice the institutional upper limit of normal values) or stage IV B-cell non-Hodgkin's lymphoma or B-cell acute leukemia. Participants were randomized to
Lymphome Malin B chemotherapy that consisted of corticosteroids, vincristine, cyclophosphamide, high-dose methotrexate, cytarabine, doxorubicin, etoposide, and triple drug (methotrexate/cytarabine/corticosteroid) intrathecal therapy alone or in combination with rituximab or non-US licensed rituximab, administered as six infusions of rituximab IV at a dose of 375 mg/m2 as per the Lymphome Malin B scheme.
538:
515:
843:
arthritis (an inflammatory condition of the joints); two inflammatory conditions of blood vessels known as granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA); moderate to severe pemphigus vulgaris, an autoimmune disease characterised by widespread blistering and erosion of the skin and mucous membranes (the linings of internal organs). 'Autoimmune' means that the disease is caused by the immune system (the body's natural defences) attacking the body's own cells.
1124:
1133:
1308:
diseases, in which a person's small blood vessels become inflamed, reducing the amount of blood that can flow through them. This can cause serious problems and damage to organs, most notably the lungs and the kidneys. It also can impact the sinuses and skin. Rituximab was approved by the FDA to treat adults with granulomatosis with polyangiitis and microscopic polyangiitis in 2011.
7420:
3968:
3921:
3872:
2374:
31:
1336:
were approved in the United States, India, the
European Union, Switzerland, Japan, and Australia. The US FDA approved rituximab-abbs (Truxima) in 2018, rituximab-pvvr (Ruxience) in 2019, and rituximab-arrx (Riabni) in 2020. Truxima and Riabni are approximately $ 3600 per 500 mg, wholesale - 10%
4406:
Contreras K, Orozco V, Puche E, González CA, García-Padilla P, Rodríguez MP, et al. (March 2022). "Cost-Effectiveness of
Rituximab (Fixed Schedule vs Tailored Dose) Compared With Azathioprine Maintenance Therapy in Adults With Generalized Antineutrophil Cytoplasm Antibody-Associated Vasculitis
4366:
The pharmaceutical company said Riabni will be marketed at a discount to the reference product of 23.7% below wholesale acquisition cost (WAC), or a WAC of $ 716.80 per 100 mg and $ 3584 per 500 mg single-dose vial. These costs are 15.2% less than the WAC for the biosimilar rituximab
Truxima, Amgen
1307:
In
September 2019, the US FDA approved rituximab injection to treat granulomatosis with polyangiitis and microscopic polyangiitis in children two years of age and older in combination with glucocorticoids (steroid hormones). It is the first approved treatment for children with these rare vasculitis
687:
The most common side effects with intravenous infusions are reactions related to the infusion (such as fever, chills and shivering) while most common serious side effects are infusion reactions, infections and heart-related problems. Similar side effects are seen when it is injected under the skin,
1159:
Rituximab is relatively ineffective in elimination of cells with low CD20 cell-surface levels. It tends to stick to one side of B cells, where CD20 is, forming a cap and drawing proteins over to that side. The presence of the cap changes the effectiveness of natural killer (NK) cells in destroying
1311:
In
December 2021, the US FDA approved rituximab in combination with chemotherapy for children aged 6 months to 18 years with previously untreated, advanced stage, CD20-positive diffuse large B-cell lymphoma, Burkitt lymphoma, Burkitt-like lymphoma, or mature B-cell acute leukemia. Efficacy was
842:
In the
European Union, rituximab is indicated for the treatment of follicular lymphoma and diffuse large B cell non-Hodgkin's lymphoma (two types of non-Hodgkin's lymphoma, a blood cancer); chronic lymphocytic leukemia (CLL, another blood cancer affecting white blood cells); severe rheumatoid
1303:
In June 2017, the US FDA granted regular approval to the combination of rituximab and hyaluronidase human (brand name
Rituxan Hycela) for adults with follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia. The combination is not indicated for the treatment of
1391:
and rituximab treatment had higher risk of severe COVID-19. In persons previously treated with rituximab for multiple sclerosis, 9 of 10 patients who delayed re-dosing until B cell counts passed 40/μL developed protective levels of antibodies after vaccination with the
1127:
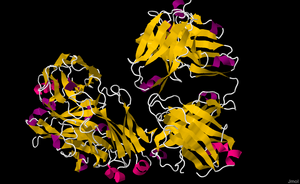
Rituximab mechanisms of action; the three major independent mechanisms are (1) antibody dependent cellular cytotoxicity (ADCC), (2) complement mediated cytotoxicity (CMC), and (3) apoptosis; subset panel illustrates a schematic view of CD20 structure and
3376:
4668:
Fluge Ø, Rekeland IG, Lien K, Thürmer H, Borchgrevink PC, Schäfer C, et al. (May 2019). "B-Lymphocyte
Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial".
3572:
Borsky M, Hrabcakova V, Novotna J, Brychtova Y, Doubek M, Panovska A, et al. (December 2021). "Rituximab induces rapid blood repopulation by CLL cells mediated through their release from immune niches and complement exhaustion".
923:
therapy. In the
European Union, the license is slightly more restrictive: it is licensed for use in combination with methotrexate in patients with severe active RA who have had an inadequate response to one or more anti-TNF therapy.
3720:
2162:
4293:
2359:
2151:"FDA Drug Safety Communication: Boxed Warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab)"
1770:
4210:
1160:
these B cells. When an NK cell latched onto the cap, it had an 80% success rate at killing the cell. In contrast, when the B cell lacked this asymmetric protein cluster, it was killed only 40% of the time.
4442:
Romero G, Ticchioni M, Cohen M, Rosenthal-Allieri MA, Mondot L, Lebrun Frenay C (March 2016). "Neuromyelitis optica: Contribution of therapeutic responses markers monitoring in patients given rituximab".
2253:
3946:"FDA D.I.S.C.O. Burst Edition: FDA approvals of Rituxan (rituximab) plus chemotherapy for pediatric cancer indications, and Keytruda (pembrolizumab) for adjuvant treatment of Stage IIB or IIC melanoma"
3120:
Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, et al. (July 2015). "International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease".
4763:
Bonnan M, Ferrari S, Bertandeau E, Demasles S, Krim E, Miquel M, et al. (2014). "Intrathecal rituximab therapy in multiple sclerosis: review of evidence supporting the need for future trials".
3383:
1220:
Rituximab also induces a release of some chronic lymphocytic leukemia cells from immune niches, which might make them more sensitive to chemotherapy used in combination with an anti-CD20 antibody.
5002:
3801:
272:
3824:
2907:
Shanafelt TD, Madueme HL, Wolf RC, Tefferi A (November 2003). "Rituximab for immune cytopenia in adults: idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia, and Evans syndrome".
2275:
1408:(ME/CFS) symptoms. While initial trials using Rituximab were promising, a phase 3 trial published in 2019 did not find an association between Rituximab treatment and improvements in ME/CFS.
3255:
Molloy ES, Calabrese LH (September 2012). "Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies".
3945:
3400:
Seyfizadeh N, Seyfizadeh N, Hasenkamp J, Huerta-Yepez S (January 2016). "A molecular perspective on rituximab: A monoclonal antibody for B cell non Hodgkin lymphoma and other affections".
3732:
1460:). This strategy for enhancing a monoclonal antibody's ability to induce ADCC takes advantage of the fact that the displayed Fc glycan controls the antibody's affinity for Fc receptors.
2150:
7465:
4712:
Seton KA, Espejo-Oltra JA, Giménez-Orenga K, Haagmans R, Ramadan DJ, Mehlsen J, et al. (European ME Research Group for Early Career Researchers (Young EMERG)) (January 2024).
3849:
1658:
227:
3898:
608:
1224:
The combined effect results in the elimination of B cells (including the cancerous ones) from the body, allowing a new population of healthy B cells to develop from lymphoid
4323:
2490:
1876:
1562:
1493:
1405:
1329:, a class of drugs that are only available through specialty distributors in the US. Because wholesalers discounts and rebates no longer apply, hospitals would pay more.
944:
4289:
6433:
4995:
2351:
919:
for reducing signs and symptoms in adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more anti-
4353:
1760:
4198:
1341:
7445:
3024:
2249:
113:
4798:
2391:
Saini KS, Azim HA, Cocorocchio E, Vanazzi A, Saini ML, Raviele PR, et al. (August 2011). "Rituximab in Hodgkin lymphoma: is the target always a hit?".
4988:
1136:
Rituximab binding to CD20. The CD20 proteins are sticking out of the cell membrane, and rituximab, the Y-shaped antibody, is binding to the CD20 proteins.
4714:"Advancing Research and Treatment: An Overview of Clinical Trials in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Future Perspectives"
3816:
1074:
952:
696:
4564:"Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: A study of the Swedish multiple sclerosis registry"
1189:
Preferential elimination of malignant B cells with high CD20 levels and high BCR signaling propensity, especially in chronic lymphocytic leukemia (CLL).
7450:
45:
6842:
1457:
1172:
1296:
Originally available for intravenous injection (e.g. over 2.5 hrs), in 2016, it gained EU approval in a formulation for subcutaneous injection for
5994:
5637:
1383:
A major concern with continuous rituximab treatment is the difficulty to induce a proper vaccine response. This was brought into focus during the
4266:
2682:
3481:"Rituximab causes a polarization of B cells that augments its therapeutic function in NK-cell-mediated antibody-dependent cellular cytotoxicity"
1744:
4396:
Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
1936:
Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
7440:
7374:
7043:
2226:
The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023)
1290:
729:
1393:
868:
688:
with the exception of reactions around the injections site (pain, swelling and rash), which occur more frequently with the skin injections.
7263:
6426:
5130:
2859:
955:(PML) infection, which is usually fatal; however, only a very small number of cases have been recorded occurring in autoimmune diseases.
1923:
1626:
1594:
971:
967:
661:
2204:
1648:
6900:
6827:
6732:
6108:
826:
in combination with chemotherapy for children (≥ 6 months to < 18 years) with previously untreated, advanced stage, CD20-positive
6757:
2734:
2198:
1176:
4613:"Factors Associated With Serological Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Rituximab"
2056:
Singer O, McCune WJ (May 2017). "Update on maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis".
1404:
In 2009, a patient receiving methotrexate-induced B-cell depletion for cancer treatment, experienced a transient remittal of their
5528:
5054:
3797:
979:
811:
657:
4315:
2482:
1868:
1800:
1554:
1522:
1485:
6419:
1003:
257:
145:
3997:
1304:
non-malignant conditions. The combination was approved based on clinical studies SABRINA/NCT01200758 and MabEase/NCT01649856.
6742:
4478:
Kriston L, Härter M, Hölzel L (March 2009). "Challenges in reporting meta-analyses of diagnostic accuracy studies". Letters.
1168:
4173:
1691:
1356:, for NHL, CLL, rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis and pemphigus vulgaris.
1991:
6847:
4828:
4232:
3440:"Rituximab primarily targets an intra-clonal BCR signaling proficient CLL subpopulation characterized by high CD20 levels"
2604:
McGinley MP, Moss BP, Cohen JA (January 2017). "Safety of monoclonal antibodies for the treatment of multiple sclerosis".
1275:
1027:
880:
827:
7410:
4345:
3028:
6706:
5874:
4238:
4204:
3951:
3904:
3855:
3726:
3681:"IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma"
2740:
2156:
2013:
Tandan R, Hehir MK, Waheed W, Howard DB (August 2017). "Rituximab treatment of myasthenia gravis: A systematic review".
1259:
1112:
940:
904:
864:
792:
649:
434:
2647:
He D, Guo R, Zhang F, Zhang C, Dong S, Zhou H (December 2013). "Rituximab for relapsing-remitting multiple sclerosis".
1380:
infection, suggesting the drug in combination with lymphoma may have weakened the body's immune response to the virus.
7391:
5031:
4515:"Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response"
3165:"Persistence of Autoreactive IgA-Secreting B Cells Despite Multiple Immunosuppressive Medications Including Rituximab"
959:
494:
6055:
2811:"Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura"
1349:
164:
994:—with very encouraging results of approximately 85% rapid recovery in pemphigus, according to a 2006 study), type 1
6485:
6180:
6029:
5778:
5280:
700:
3649:
6950:
5505:
5371:
5084:
4802:
4121:"Treatment of hematologic malignancies associated with circulating tumor cells using chimeric anti-CD20 antibody"
2221:
1918:
1621:
1589:
1345:
1057:
1019:
7460:
5515:
5041:
1156:
influx across plasma membranes, maintaining intracellular Ca concentration and allowing activation of B cells.
1145:
852:
815:
4367:
said. The company added that Riabni's average sales price will be 16.7% below the current average for Rituxan.
1364:
Tailored-dose rituximab is more cost-effective than fixed-dose. It is both more effective and less expensive.
3899:"FDA approves first treatment for children with rare diseases that cause inflammation of small blood vessels"
2512:
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. (June 2004).
1428:
The efficacy and success of rituximab has led to some other anti-CD20 monoclonal antibodies being developed:
7379:
7346:
7287:
7238:
7153:
6777:
5011:
3341:
Burton C, Kaczmarski R, Jan-Mohamed R (June 2003). "Interstitial pneumonitis related to rituximab therapy".
1023:
772:
201:
673:
533:
7317:
7307:
7277:
7068:
7013:
6940:
6930:
6807:
5883:
5366:
5095:
3292:"Peripheral B Cell Deficiency and Predisposition to Viral Infections: The Paradigm of Immune Deficiencies"
745:
483:
3679:
Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. (September 1997).
3025:"Rituximab Treatment of Patients with Severe, Corticosteroid-Resistant Thyroid-Associated Ophthalmopathy"
1946:
1714:"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)"
7455:
7292:
7281:
7243:
7133:
7118:
7048:
7028:
7023:
6802:
6556:
5441:
5381:
5351:
5254:
5090:
4980:
963:
779:
4954:
4262:
2686:
7143:
7003:
6772:
6737:
5296:
5191:
5028:
1741:
1713:
1263:
1062:
1047:
1031:
1015:
900:
798:
786:
681:
653:
645:
350:
208:
108:
rituximab-abbs, rituximab-pvvr, rituximab-arrx, Blitzima Riabni, Rixathon, Riximyo, Ruxience, Truxima
7258:
7228:
7223:
7208:
7038:
6885:
6837:
6797:
6442:
6149:
5416:
5406:
5226:
2809:
Braendstrup P, Bjerrum OW, Nielsen OJ, Jensen BA, Clausen NT, Hansen PB, et al. (April 2005).
1793:"Mabthera 100 mg Concentrate for Solution for Infusion - Summary of Product Characteristics (SmPC)"
1297:
1283:
1279:
1271:
1066:
999:
983:
876:
714:
677:
637:
633:
510:
378:
220:
55:
38:
6496:
4969:
2101:"EBV-Positive Lymphoproliferations of B- T- and NK-Cell Derivation in Non-Immunocompromised Hosts"
1826:"Mabthera 1400 mg Solution for Subcutaneous Injection - Summary of Product Characteristics (SmPC)"
1123:
6980:
6531:
6491:
5804:
5446:
5436:
5421:
5341:
4694:
4650:
4593:
4544:
3145:
2840:
2708:
2629:
2081:
2038:
1388:
936:
891:
to treat previously untreated and previously treated CD20-positive chronic lymphocytic leukemia.
821:
665:
175:
4513:
Oren S, Mandelboim M, Braun-Moscovici Y, Paran D, Ablin J, Litinsky I, et al. (July 2008).
4046:"Chimeric anti-CD20 antibody, rituxan, for use in the treatment of chronic lymphocytic leukemia"
3746:
Scott SD (1998). "Rituximab: a new therapeutic monoclonal antibody for non-Hodgkin's lymphoma".
3479:
Rudnicka D, Oszmiana A, Finch DK, Strickland I, Schofield DJ, Lowe DC, et al. (June 2013).
3438:
Pavlasova G, Borsky M, Svobodova V, Oppelt J, Cerna K, Novotna J, et al. (September 2018).
2882:
1007:
4903:"Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review"
3081:"Immunologic and clinical responses to rituximab in a child with opsoclonus-myoclonus syndrome"
2983:
Jacob A, Weinshenker BG, Violich I, McLinskey N, Krupp L, Fox RJ, et al. (November 2008).
2428:"Nodular lymphocyte-predominant Hodgkin lymphoma: a unique disease deserving unique management"
2276:"FDA Approves Genentech's Rituxan (rituximab) in Children With Two Rare Blood Vessel Disorders"
6867:
6686:
5481:
4932:
4883:
4780:
4745:
4686:
4642:
4585:
4536:
4495:
4460:
4424:
4102:
3763:
3702:
3631:
3590:
3551:
3502:
3461:
3417:
3358:
3323:
3272:
3237:
3186:
3137:
3102:
3006:
2965:
2924:
2832:
2791:
2660:
2621:
2586:
2535:
2457:
2408:
2330:
2194:
2132:
2073:
2030:
1453:
1384:
1108:
1034:. There is some evidence that it is ineffective in treating IgA-mediated autoimmune diseases.
1011:
995:
928:
741:
669:
297:
284:
93:
60:
4379:
1913:
1616:
1584:
1251:
under the name IDEC-C2B8. The US patent for the drug was issued in 1998 and expired in 2015.
5321:
5163:
5158:
4922:
4914:
4901:
Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, et al. (February 2015).
4873:
4863:
4772:
4735:
4725:
4678:
4632:
4624:
4575:
4526:
4491:
4487:
4452:
4416:
4092:
4084:
3755:
3692:
3621:
3582:
3541:
3533:
3492:
3451:
3409:
3350:
3313:
3303:
3264:
3227:
3217:
3176:
3129:
3092:
3059:
2996:
2955:
2916:
2874:
2822:
2781:
2652:
2613:
2576:
2566:
2525:
2447:
2439:
2400:
2320:
2310:
2229:
2188:
2122:
2112:
2065:
2022:
1377:
888:
831:
550:
387:
332:
4199:"FDA approves first biosimilar for treatment of adult patients with non-Hodgkin's lymphoma"
443:
7424:
3850:"FDA approves rituximab plus hyaluronidase combination for Treatment of FL, DLBCL and CLL"
2514:"Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis"
1748:
1207:
835:
340:
3759:
3206:"Infectious Complications of Targeted Therapies in Children with Leukemias and Lymphomas"
2985:"Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients"
2746:
4740:
4713:
4073:"Rituximab: how approval history is reflected by a corresponding patent filing strategy"
537:
514:
7385:
7351:
7018:
6258:
6156:
6104:
5702:
5015:
4927:
4902:
4637:
4628:
4612:
4146:
4097:
4072:
3546:
3521:
3318:
3232:
2581:
2452:
2427:
2325:
2127:
1326:
1255:
1236:
1235:
170–173 and 182–185 on CD20, which are physically close to each other as a result of a
1052:
975:
711:
4878:
4776:
2878:
7434:
7233:
7088:
6641:
6476:
6315:
6205:
6167:
6144:
6076:
6071:
6061:
5837:
5833:
5821:
5628:
5411:
5376:
5196:
5176:
4654:
4597:
3972:
3925:
3876:
3413:
2378:
1825:
1792:
1765:
1653:
872:
802:
757:
526:
310:
137:
4698:
4611:
Tolf A, Wiberg A, Müller M, Nazir FH, Pavlovic I, Laurén I, et al. (May 2022).
4548:
3989:
3149:
3001:
2984:
2844:
2633:
2443:
2085:
2042:
1348:
adopted a positive opinion, recommending the granting of marketing authorization to
7248:
7213:
7198:
7193:
7188:
7183:
7158:
7113:
7108:
7103:
7098:
7083:
6970:
6945:
6925:
6787:
6752:
6691:
6671:
6481:
6466:
6411:
6355:
6340:
6310:
6290:
6172:
6120:
6081:
5969:
5948:
5855:
5817:
5718:
5693:
5689:
5573:
5538:
5491:
5476:
5431:
5306:
5276:
5201:
5019:
4120:
4045:
4019:
2656:
2250:"FDA Approves Rituxan Plus Chemotherapy for the Most Common Type of Adult Leukemia"
1445:
1267:
916:
912:
875:
human, sold under the brand names MabThera SC and Rituxan Hycela, is used to treat
240:
235:
3586:
3163:
He Y, Shimoda M, Ono Y, Villalobos IB, Mitra A, Konia T, et al. (June 2015).
2617:
1332:
Patents on rituximab have expired in the European Union and in the United States.
4456:
3626:
3609:
3497:
3480:
3204:
Kyriakidis I, Mantadakis E, Stiakaki E, Groll AH, Tragiannidis A (October 2022).
3181:
3164:
2069:
1987:
1683:
123:
7356:
7330:
7325:
7297:
7273:
7268:
7203:
7178:
7168:
7138:
7128:
7093:
7078:
7063:
7053:
7033:
7008:
6993:
6965:
6915:
6910:
6905:
6890:
6880:
6857:
6852:
6822:
6767:
6711:
6701:
6676:
6666:
6661:
6656:
6631:
6606:
6596:
6591:
6586:
6576:
6561:
6546:
6541:
6526:
6521:
6511:
6506:
6501:
6471:
6385:
6365:
6360:
6350:
6330:
6305:
6300:
6283:
6231:
6221:
6216:
6211:
6113:
6040:
6010:
6005:
6000:
5984:
5974:
5954:
5829:
5825:
5813:
5809:
5738:
5642:
5588:
5583:
5548:
5486:
5471:
5461:
5456:
5451:
5426:
5391:
5346:
5326:
5316:
5271:
5236:
5216:
5172:
5135:
5123:
5118:
5080:
5064:
3537:
3354:
2944:"Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin"
1449:
1432:
1417:
1373:
1248:
1196:
and adhesion molecules LFA-1 and LFA-3 (lymphocyte function-associated antigen).
1149:
1132:
1084:
884:
768:
692:
131:
4918:
4824:
4420:
3522:"B cell therapy for rheumatoid arthritis: the rituximab (anti-CD20) experience"
2858:
Patel V, Mihatov N, Cooper N, Stasi R, Cunningham-Rundles S, Bussel JB (2007).
2786:
2769:
2571:
2554:
2404:
7302:
7253:
7218:
7148:
7123:
7073:
7058:
6998:
6988:
6960:
6935:
6875:
6817:
6812:
6747:
6727:
6681:
6651:
6646:
6636:
6626:
6621:
6611:
6601:
6571:
6566:
6536:
6516:
6400:
6395:
6370:
6335:
6320:
6295:
6278:
6268:
6226:
6197:
6162:
6128:
6045:
6035:
6015:
5979:
5964:
5959:
5929:
5863:
5859:
5841:
5789:
5768:
5728:
5723:
5662:
5593:
5568:
5543:
5466:
5401:
5361:
5356:
5336:
5331:
5311:
5301:
5291:
5206:
5186:
5168:
5076:
4852:"Monoclonal antibodies targeting cancer: 'magic bullets' or just the trigger?"
4580:
4563:
3456:
3439:
3308:
3291:
2770:"Rituximab in autoimmune thrombotic thrombocytopenic purpura: A success story"
1438:
1333:
1232:
1183:
1102:
991:
908:
807:
589:
418:
215:
103:
3697:
3680:
3052:"Immunomodulators and Rituximab in the Management of Autoimmune Pancreatitis"
2117:
2100:
7173:
7163:
6920:
6895:
6832:
6792:
6782:
6762:
6696:
6616:
6581:
6551:
6390:
6380:
6375:
6345:
6325:
6273:
6263:
6251:
6246:
6192:
6187:
6133:
6066:
5939:
5934:
5919:
5914:
5909:
5899:
5894:
5889:
5794:
5784:
5763:
5758:
5753:
5748:
5743:
5733:
5713:
5677:
5672:
5667:
5657:
5652:
5619:
5614:
5609:
5578:
5558:
5553:
5396:
5386:
5059:
4531:
4514:
3222:
3205:
1225:
1214:
987:
932:
920:
867:, and nodular lymphocyte predominant Hodgkin's lymphoma. This also includes
737:
704:
363:
117:
4936:
4887:
4784:
4749:
4690:
4646:
4589:
4540:
4499:
4464:
4428:
4106:
3635:
3594:
3555:
3506:
3465:
3421:
3362:
3327:
3276:
3241:
3190:
3141:
3106:
3097:
3080:
3010:
2969:
2928:
2836:
2795:
2664:
2625:
2590:
2539:
2461:
2412:
2352:"FDA approves rituximab plus chemotherapy for pediatric cancer indications"
2334:
2315:
2298:
2136:
2077:
2034:
3817:"EU approves second indication for subcutaneous form of Roche's rituximab"
3767:
3706:
1376:
infection in a person with lymphoma. Hepatitis E infection is normally an
1144:. CD20 is widely expressed on B cells, from early pre-B cells to later in
767:. It acts by depleting normal as well as pathogenic B cells while sparing
22:
6451:
6140:
5904:
5708:
5647:
5603:
5563:
5533:
5286:
5150:
5146:
5071:
3064:
3051:
2960:
2943:
2735:"FDA Warns of Safety Concern Regarding Rituxan in New Patient Population"
2530:
2513:
1096:
1078:
860:
856:
398:
159:
4730:
4233:"FDA approves Truxima as biosimilar to Rituxan for non-Hodgkin's lympho"
3657:
2234:
958:
Other autoimmune diseases that have been treated with rituximab include
931:, in a range of other autoimmune diseases, and rituximab is widely used
407:
5244:
4088:
3608:
Binder M, Otto F, Mertelsmann R, Veelken H, Trepel M (September 2006).
3377:"Reports of Bowel Obstruction and Perforation with Rituxan (rituximab)"
2920:
1153:
1042:
Serious adverse events, which can cause death and disability, include:
760:
722:
76:
3268:
3133:
2827:
2810:
2768:
Froissart A, Veyradier A, Hié M, Benhamou Y, Coppo P (November 2015).
2026:
1761:"Health product highlights 2021: Annexes of products approved in 2021"
30:
4682:
1193:
948:
764:
756:
Rituximab is a chimeric monoclonal antibody targeted against CD20, a
733:
641:
474:
4290:"Novartis abandons effort for U.S. approval of biosimilar rituximab"
4868:
4851:
3290:
Grammatikos A, Donati M, Johnston SL, Gompels MM (30 August 2021).
951:. The most dangerous, although among the most rare, side effect is
883:, and chronic lymphocytic leukemia. It is used in combination with
652:(in children and adults, but not recommended in elderly patients),
463:
6124:
5850:
5684:
5633:
5113:
3079:
Pranzatelli MR, Tate ED, Travelstead AL, Longee D (January 2005).
1152:. Although the function of CD20 is unknown, it may play a role in
1131:
1122:
68:
64:
4562:
Spelman T, Forsberg L, McKay K, Glaser A, Hillert J (June 2022).
3971:
This article incorporates text from this source, which is in the
3924:
This article incorporates text from this source, which is in the
3875:
This article incorporates text from this source, which is in the
2483:"Rituxan Hycela- rituximab and hyaluronidase injection, solution"
2377:
This article incorporates text from this source, which is in the
1278:
and many other B-cell lymphomas. In 2010, it was approved by the
6239:
6025:
5523:
5249:
5231:
5221:
5181:
5049:
2683:"Popular Cancer Drug Linked to Often Fatal 'Brain Eating' Virus"
1372:
Rituximab has been reported as a possible cofactor in a chronic
1200:
1141:
718:
454:
319:
291:
81:
6415:
4984:
3798:"Roche Gets EC Nod for Follicular Lymphoma Maintenance Therapy"
304:
154:
2942:
Ahmed AR, Spigelman Z, Cavacini LA, Posner MR (October 2006).
2860:"Long-term responses seen with rituximab in patients with ITP"
2685:. Northwestern University News and Information. Archived from
1721:
2432:
Hematology. American Society of Hematology. Education Program
2299:"Shifting Focus in the Therapeutics of Immunobullous Disease"
728:
Rituximab was approved for medical use in 1997. It is on the
1337:
less than Rituxan. While Ruxience is 24% less than Rituxan.
1095:
Immune toxicity, with depletion of B cells in 70% to 80% of
499:
721:, which is primarily found on the surface of immune system
266:
186:
2099:
Dojcinov SD, Fend F, Quintanilla-Martinez L (March 2018).
927:
There is some evidence for efficacy, but not necessarily
368:
Uncertain: may undergo phagocytosis and catabolism in RES
4955:
Non-Hodgkin's lymphoma: rituximab subcutaneous injection
725:. When it binds to this protein it triggers cell death.
358:
30 to 400 hours (varies by dose and length of treatment)
279:
2709:"Popular Cancer Drug Linked To Often Fatal Brain Virus"
2477:
2475:
2473:
2471:
2193:. Springer Science & Business Media. pp. 1–4.
1291:
World Health Organization's List of Essential Medicines
730:
World Health Organization's List of Essential Medicines
615:
4316:"Teva, Celltrion Maintain Slender Discount to Rituxan"
4265:. Generics and Biosimilars Initiative. 14 April 2017.
911:
rheumatoid disease. In the United States, it has been
7408:
3940:
3938:
3936:
3934:
2555:"Advances in rheumatology: new targeted therapeutics"
1990:. The American Society of Health-System Pharmacists.
1678:
1676:
1282:
for maintenance treatment after initial treatment of
1863:
1861:
1859:
1857:
1855:
1853:
1851:
1849:
1847:
1517:
1515:
1513:
1511:
7339:
7316:
6979:
6866:
6720:
6459:
6450:
6096:
5873:
5514:
5504:
5264:
5145:
5105:
5040:
5027:
1820:
1818:
1480:
1478:
1476:
1474:
1435:, humanized (90%-95% human) B cell-depleting agent.
834:(BL), Burkitt-like lymphoma (BLL), or mature acute
588:
549:
544:
525:
493:
473:
453:
433:
417:
397:
377:
372:
362:
349:
339:
331:
256:
251:
226:
214:
200:
174:
144:
130:
112:
102:
92:
87:
75:
54:
44:
37:
3893:
3891:
3889:
3887:
3885:
1742:Rituximab (rch) (CAS registry number: 174722-31-7)
1549:
1547:
1545:
1543:
1441:(HuMax-CD20) a fully human B cell-depleting agent.
1416:For CNS diseases, rituximab could be administered
1406:myalgic encephalomyelitis/chronic fatigue syndrome
1400:Myalgic encephalomyelitis/chronic fatigue syndrome
707:is safe for the developing fetus or newborn baby.
4174:"Roche Settles With Pfizer Over Rituximab Patent"
2745:(Press release). 18 December 2006. Archived from
1182:Rituximab has a general regulatory effect on the
945:chronic inflammatory demyelinating polyneuropathy
775:, which do not express the CD20 surface antigen.
3382:. Roche Canada. 10 November 2006. Archived from
1908:
1906:
1904:
1902:
1900:
1898:
1896:
1894:
1274:, is superior to CHOP alone in the treatment of
1148:, but it is absent on terminally differentiated
4346:"Amgen Rituximab Biosimilar Gains FDA Approval"
3844:
3842:
3567:
3565:
3433:
3431:
2346:
2344:
1140:The antibody binds to the cell surface protein
871:, a type of NHL. Rituximab in combination with
386:
3990:"Hospitals Furious at Cancer-Drug Price Hikes"
3520:Shaw T, Quan J, Totoritis MC (November 2003).
2190:Drugs Targeting B-Cells in Autoimmune Diseases
2187:Bosch X, Ramos-Casals M, Khamashta MA (2013).
1342:Committee for Medicinal Products for Human Use
7466:World Health Organization essential medicines
6427:
4996:
2278:(Press release). Genentech. 27 September 2019
1649:"Summary Basis of Decision (SBD) for Riximyo"
1523:"Ruxience- rituximab-pvvr injection solution"
1325:In 2014, Genentech reclassified Rituxan as a
8:
3983:
3981:
1486:"Truxima- rituximab-abbs injection solution"
899:Rituximab has been shown to be an effective
691:Severe side effects include reactivation of
163:
21:
2649:The Cochrane Database of Systematic Reviews
2252:(Press release). Biogen. 18 February 2010.
1982:
1980:
1978:
1976:
1974:
1972:
1970:
1968:
1555:"Riabni- rituximab-arrx injection solution"
744:elsewhere except Japan, and co-marketed by
6456:
6434:
6420:
6412:
5511:
5037:
5003:
4989:
4981:
4020:"Recombinant antibodies for human therapy"
3781:Harrison's Principles of Internal Medicine
2297:De A, Ansari A, Sharma N, Sarda A (2017).
2216:
2214:
2182:
2180:
1075:Progressive multifocal leukoencephalopathy
953:progressive multifocal leukoencephalopathy
801:having inadequate response to one or more
697:progressive multifocal leukoencephalopathy
536:
513:
4926:
4877:
4867:
4739:
4729:
4636:
4579:
4530:
4096:
3696:
3625:
3545:
3496:
3455:
3317:
3307:
3231:
3221:
3180:
3096:
3063:
3000:
2959:
2826:
2785:
2580:
2570:
2529:
2451:
2426:Eichenauer DA, Engert A (December 2017).
2324:
2314:
2233:
2126:
2116:
1270:regimens. Rituximab, in combination with
1254:Based on its safety and effectiveness in
703:, and death. It is unclear if use during
442:
4288:Lahiri D, Osterman C (2 November 2018).
3721:"Rituximab Product Approval Information"
1869:"Rituxan- rituximab injection, solution"
1458:antibody-dependent cellular cytotoxicity
1173:antibody-dependent cellular cytotoxicity
7415:
4492:10.7326/0003-4819-150-6-200903170-00025
3402:Critical Reviews in Oncology/Hematology
1470:
1163:The following effects have been found:
509:
406:
136:
4970:"Discovery – Development of Rituximab"
4799:"Genmab.com / HuMax-CD20 (ofatumumab)"
2676:
2674:
684:(injected slowly through an IV line).
527:
20:
4831:from the original on 10 November 2007
4269:from the original on 24 February 2024
4213:from the original on 15 December 2019
3804:from the original on 31 October 2010.
3610:"The epitope recognized by rituximab"
2774:European Journal of Internal Medicine
2228:. Geneva: World Health Organization.
2207:from the original on 5 November 2017.
1926:from the original on 9 September 2021
1629:from the original on 9 September 2021
1597:from the original on 9 September 2021
1420:and this possibility is under study.
482:
122:
7:
7446:Drugs developed by Hoffmann-La Roche
4296:from the original on 3 November 2018
4000:from the original on 20 October 2015
3760:10.1046/j.1523-5394.1998.006003195.x
2362:from the original on 3 December 2021
1951:Union Register of medicinal products
1803:from the original on 21 January 2022
1444:Third-generation anti-CD20s such as
863:, including non-Hodgkin's lymphoma,
239:
4209:(Press release). 28 November 2018.
3343:The New England Journal of Medicine
2948:The New England Journal of Medicine
2518:The New England Journal of Medicine
1694:from the original on 30 August 2019
1258:, rituximab was approved by the US
972:idiopathic thrombocytopenic purpura
968:thrombotic thrombocytopenic purpura
778:In the United States, rituximab is
662:idiopathic thrombocytopenic purpura
462:
4629:10.1001/jamanetworkopen.2022.11497
4356:from the original on 23 April 2021
4326:from the original on 30 March 2024
3909:(Press release). 27 September 2019
3731:. 20 February 2009. Archived from
3050:Hart PA, Chari ST (11 June 2013).
2493:from the original on 27 March 2021
2165:from the original on 28 April 2020
1994:from the original on 27 March 2016
1879:from the original on 4 August 2020
1773:from the original on 25 March 2024
1565:from the original on 25 March 2021
1496:from the original on 25 March 2021
14:
4777:10.2174/1389450115666141029234644
3349:(26): 2690–1, discussion 2690–1.
2356:U.S. Food and Drug Administration
1239:between amino acids 167 and 183.
1177:complement-dependent cytotoxicity
853:cancers of the white blood system
636:medication used to treat certain
7451:Monoclonal antibodies for tumors
7418:
4519:Annals of the Rheumatic Diseases
3966:
3919:
3870:
3827:from the original on 7 June 2016
3526:Annals of the Rheumatic Diseases
3414:10.1016/j.critrevonc.2015.09.001
2559:Arthritis Research & Therapy
2372:
2256:from the original on 9 July 2021
1684:"Rituximab Use During Pregnancy"
1661:from the original on 30 May 2022
1394:Pfizer–BioNTech COVID-19 vaccine
980:granulomatosis with polyangiitis
812:granulomatosis with polyangiitis
658:granulomatosis with polyangiitis
567:
561:
29:
4957:(Report). NICE. September 2014.
4409:Value in Health Regional Issues
3002:10.1001/archneur.65.11.noc80069
2444:10.1182/asheducation-2017.1.324
2058:Current Opinion in Rheumatology
1352:for their rituximab biosimilar
1004:anti-NMDA receptor encephalitis
907:and is now licensed for use in
869:Waldenström's macroglobulinemia
4147:"Methods related to rituximab"
3988:Saporito B (27 October 2014).
2815:American Journal of Hematology
2657:10.1002/14651858.CD009130.pub3
2553:Tak PP, Kalden JR (May 2011).
1452:(Fc) with enhanced binding to
1262:(FDA) in 1997 to treat B-cell
1171:portion of rituximab mediates
695:in those previously infected,
579:
573:
555:
1:
3587:10.1016/j.leukres.2021.106684
2879:10.1016/s1548-5315(11)70061-4
2618:10.1080/14740338.2017.1250881
2606:Expert Opinion on Drug Safety
2303:Indian Journal of Dermatology
1276:diffuse large B-cell lymphoma
1028:Opsoclonus myoclonus syndrome
986:skin disorders (for example,
881:diffuse large B-cell lymphoma
828:diffuse large B-cell lymphoma
7441:Drugs developed by Genentech
4718:Journal of Clinical Medicine
4457:10.1016/j.neurol.2015.12.004
4239:Food and Drug Administration
4205:Food and Drug Administration
3952:Food and Drug Administration
3905:Food and Drug Administration
3856:Food and Drug Administration
3727:Food and Drug Administration
3627:10.1182/blood-2006-04-014639
3498:10.1182/blood-2013-02-482570
3182:10.1001/jamadermatol.2015.59
3122:Arthritis & Rheumatology
2741:Food and Drug Administration
2157:Food and Drug Administration
2070:10.1097/BOR.0000000000000382
1260:Food and Drug Administration
941:systemic lupus erythematosus
935:to treat difficult cases of
915:for use in combination with
905:randomised controlled trials
865:chronic lymphocytic leukemia
793:chronic lymphocytic leukemia
748:and Zenyaku Kogyo in Japan.
732:. Rituxan is co-marketed by
650:chronic lymphocytic leukemia
628:, sold under the brand name
4671:Annals of Internal Medicine
4480:Annals of Internal Medicine
4172:Inserro A (25 March 2019).
3538:10.1136/ard.62.suppl_2.ii55
3355:10.1056/NEJM200306263482619
1424:Other anti-CD20 monoclonals
1247:Rituximab was developed by
960:autoimmune hemolytic anemia
851:Rituximab is used to treat
7482:
5506:Tyrosine kinase inhibitors
4919:10.1016/j.jaut.2014.12.002
4421:10.1016/j.vhri.2021.08.002
4350:The Center For Biosimilars
4263:"Biosimilars of Rituximab"
2787:10.1016/j.ejim.2015.07.021
2572:10.1186/1478-6354-13-S1-S5
2405:10.1016/j.ctrv.2010.11.005
2286:– via Business Wire.
2238:. WHO/MHP/HPS/EML/2023.02.
701:toxic epidermal necrolysis
545:Chemical and physical data
7369:
6951:Mirvetuximab soravtansine
5372:Mirvetuximab soravtansine
4974:National Cancer Institute
4581:10.1177/13524585211026272
4384:European Medicines Agency
3815:Stanton D (31 May 2016).
3457:10.1038/s41375-018-0211-0
3309:10.3389/fimmu.2021.731643
2222:World Health Organization
1919:European Medicines Agency
1622:European Medicines Agency
1590:European Medicines Agency
1346:European Medicines Agency
1058:Cytokine release syndrome
605:
98:Rituxan, Mabthera, others
28:
5516:Receptor tyrosine kinase
5042:Receptor tyrosine kinase
3698:10.1182/blood.V90.6.2188
3257:Arthritis and Rheumatism
2393:Cancer Treatment Reviews
2118:10.3390/pathogens7010028
1747:30 November 2015 at the
816:microscopic polyangiitis
773:hematopoietic stem cells
7347:Depatuxizumab mafodotin
7288:Tucotuzumab celmoleukin
7239:Rovalpituzumab tesirine
7154:Lorvotuzumab mertansine
7044:Clivatuzumab tetraxetan
5106:Others for solid tumors
5012:Targeted cancer therapy
4907:Journal of Autoimmunity
4532:10.1136/ard.2007.077461
3296:Frontiers in Immunology
3223:10.3390/cancers14205022
2909:Mayo Clinic Proceedings
1456:, which increase ADCC (
1448:have a glycoengineered
1199:It elicits shedding of
1024:autoimmune pancreatitis
287: / Schedule D
7308:Vorsetuzumab mafodotin
7264:Tacatuzumab tetraxetan
7069:Denintuzumab mafodotin
7014:Bivatuzumab mertansine
6941:Loncastuximab tesirine
6931:Indatuximab ravtansine
6808:Naptumomab estafenatox
5367:Loncastuximab tesirine
5096:Trastuzumab deruxtecan
4856:Breast Cancer Research
4320:Center for Biosimilars
4178:Center for Biosimilars
3532:(Suppl 2): ii55–ii59.
3098:10.1542/peds.2004-0845
2681:Paul M (20 May 2009).
2316:10.4103/ijd.IJD_199_17
1137:
1129:
1090:Other viral infections
1020:Graves' ophthalmopathy
746:Chugai Pharmaceuticals
680:. It is given by slow
16:Biopharmaceutical drug
7293:Vandortuzumab vedotin
7244:Sacituzumab govitecan
7134:Inotuzumab ozogamicin
7119:Gemtuzumab ozogamicin
7049:Cofetuzumab pelidotin
7029:Cantuzumab ravtansine
7024:Cantuzumab mertansine
6843:Nofetumomab merpentan
6803:Moxetumomab pasudotox
6557:Glembatumumab vedotin
6443:Monoclonal antibodies
5442:Sacituzumab govitecan
5382:Moxetumomab pasudotox
5352:Inotuzumab ozogamicin
5255:Gemtuzumab ozogamicin
5091:Trastuzumab emtansine
5032:monoclonal antibodies
5016:antineoplastic agents
2989:Archives of Neurology
1922:. 17 September 2018.
1387:, where persons with
1264:non-Hodgkin lymphomas
1206:It downregulates the
1135:
1126:
964:pure red cell aplasia
7340:Chimeric + humanized
7144:Lifastuzumab vedotin
7004:Belantamab mafodotin
6773:Ibritumomab tiuxetan
6738:Anatumomab mafenatox
5297:Belantamab mafodotin
4805:on 11 September 2007
4765:Current Drug Targets
4352:. 17 December 2020.
3735:on 10 February 2017.
3065:10.3998/panc.2013.20
2961:10.1056/nejmoa062930
2888:on 29 September 2007
2531:10.1056/NEJMoa032534
2161:. 29 February 2016.
1690:. 16 December 2019.
1249:IDEC Pharmaceuticals
1119:Mechanisms of action
1063:Tumor lysis syndrome
1032:IgG4-related disease
1016:MOG antibody disease
978:, vasculitis (e.g.,
901:rheumatoid arthritis
799:rheumatoid arthritis
787:non-Hodgkin lymphoma
717:against the protein
682:intravenous infusion
678:mucocutaneous ulcers
654:rheumatoid arthritis
646:non-Hodgkin lymphoma
7259:Sofituzumab vedotin
7229:Polatuzumab vedotin
7224:Pinatuzumab vedotin
7209:Oportuzumab monatox
7039:Citatuzumab bogatox
6901:Derlotuximab biotin
6886:Brentuximab vedotin
6838:Taplitumomab paptox
6828:Satumomab pendetide
6798:Nacolomab tafenatox
6733:Altumomab pentetate
6150:Denileukin diftitox
5812:(ALK, ROS1, NTRK),
5417:Polatuzumab vedotin
5407:Oportuzumab monatox
4731:10.3390/jcm13020325
3800:. 29 October 2010.
3785:McGraw Hill Medical
2489:. 3 December 2019.
2358:. 3 December 2021.
1875:. 6 November 2019.
1657:. 23 October 2014.
1316:Society and culture
1298:B-cell CLL/lymphoma
1284:follicular lymphoma
1280:European Commission
1266:resistant to other
1231:Rituximab binds to
1067:acute kidney injury
903:treatment in three
895:Autoimmune diseases
877:follicular lymphoma
820:moderate to severe
715:monoclonal antibody
638:autoimmune diseases
634:monoclonal antibody
632:among others, is a
300:(Prescription only)
275:(Prescription only)
221:Monoclonal antibody
39:Monoclonal antibody
25:
7396:Never to phase III
6758:Capromab pendetide
6532:Enfortumab vedotin
5836:(ROS1, TRK, ALK),
5342:Enfortumab vedotin
4850:Eccles SA (2001).
4568:Multiple Sclerosis
4445:Revue Neurologique
4243:. 28 November 2018
4089:10.4161/mabs.29105
3821:BioPharma-Reporter
2921:10.4065/78.11.1340
2867:Community Oncology
2865:. Correspondence.
2015:Muscle & Nerve
1454:Fc gamma receptors
1389:multiple sclerosis
1350:Reddy Holding GmbH
1340:In July 2024, the
1138:
1130:
937:multiple sclerosis
822:pemphigus vulgaris
674:Epstein–Barr virus
666:pemphigus vulgaris
7406:
7405:
7365:
7364:
6687:Tisotumab vedotin
6409:
6408:
6092:
6091:
5500:
5499:
5482:Tisotumab vedotin
4771:(13): 1205–1214.
4617:JAMA Network Open
4127:. 9 November 1999
4052:. 9 November 1999
3956:. 2 December 2021
3660:on 5 January 2014
3575:Leukemia Research
3491:(23): 4694–4702.
3389:on 27 March 2014.
3269:10.1002/art.34468
3134:10.1002/art.39132
3031:on 22 August 2017
2995:(11): 1443–1448.
2954:(17): 1772–1779.
2915:(11): 1340–1346.
2828:10.1002/ajh.20276
2524:(25): 2572–2581.
2027:10.1002/mus.25597
1799:. 13 March 2021.
1769:. 3 August 2022.
1385:COVID-19 pandemic
1272:CHOP chemotherapy
1109:Bowel obstruction
1048:infusion reaction
1012:Anti-AQP4 disease
996:diabetes mellitus
670:myasthenia gravis
644:. It is used for
623:
622:
495:CompTox Dashboard
323:
308:
295:
282:
270:
190:
157:
7473:
7423:
7422:
7421:
7414:
7318:Rat/mouse hybrid
6457:
6436:
6429:
6422:
6413:
6123:peptide against
5696:(AXL, ALK, LTK))
5512:
5322:Dinutuximab beta
5038:
5005:
4998:
4991:
4982:
4977:
4958:
4941:
4940:
4930:
4898:
4892:
4891:
4881:
4871:
4847:
4841:
4840:
4838:
4836:
4821:
4815:
4814:
4812:
4810:
4801:. Archived from
4795:
4789:
4788:
4760:
4754:
4753:
4743:
4733:
4709:
4703:
4702:
4683:10.7326/M18-1451
4665:
4659:
4658:
4640:
4608:
4602:
4601:
4583:
4574:(7): 1051–1059.
4559:
4553:
4552:
4534:
4510:
4504:
4503:
4475:
4469:
4468:
4439:
4433:
4432:
4403:
4397:
4395:
4393:
4391:
4376:
4370:
4369:
4363:
4361:
4342:
4336:
4335:
4333:
4331:
4312:
4306:
4305:
4303:
4301:
4285:
4279:
4278:
4276:
4274:
4259:
4253:
4252:
4250:
4248:
4229:
4223:
4222:
4220:
4218:
4195:
4189:
4188:
4186:
4184:
4169:
4163:
4162:
4160:
4158:
4143:
4137:
4136:
4134:
4132:
4117:
4111:
4110:
4100:
4071:Storz U (2014).
4068:
4062:
4061:
4059:
4057:
4042:
4036:
4035:
4033:
4031:
4016:
4010:
4009:
4007:
4005:
3985:
3976:
3970:
3969:
3965:
3963:
3961:
3942:
3929:
3923:
3922:
3918:
3916:
3914:
3895:
3880:
3874:
3873:
3869:
3867:
3865:
3846:
3837:
3836:
3834:
3832:
3812:
3806:
3805:
3794:
3788:
3778:
3772:
3771:
3743:
3737:
3736:
3717:
3711:
3710:
3700:
3691:(6): 2188–2195.
3676:
3670:
3669:
3667:
3665:
3656:. Archived from
3646:
3640:
3639:
3629:
3620:(6): 1975–1978.
3605:
3599:
3598:
3569:
3560:
3559:
3549:
3517:
3511:
3510:
3500:
3476:
3470:
3469:
3459:
3450:(9): 2028–2031.
3435:
3426:
3425:
3397:
3391:
3390:
3388:
3381:
3373:
3367:
3366:
3338:
3332:
3331:
3321:
3311:
3287:
3281:
3280:
3263:(9): 3043–3051.
3252:
3246:
3245:
3235:
3225:
3201:
3195:
3194:
3184:
3169:JAMA Dermatology
3160:
3154:
3153:
3128:(7): 1688–1699.
3117:
3111:
3110:
3100:
3091:(1): e115–e119.
3076:
3070:
3069:
3067:
3047:
3041:
3040:
3038:
3036:
3027:. Archived from
3021:
3015:
3014:
3004:
2980:
2974:
2973:
2963:
2939:
2933:
2932:
2904:
2898:
2897:
2895:
2893:
2887:
2881:. Archived from
2864:
2855:
2849:
2848:
2830:
2806:
2800:
2799:
2789:
2765:
2759:
2758:
2756:
2754:
2731:
2725:
2724:
2722:
2720:
2705:
2699:
2698:
2696:
2694:
2678:
2669:
2668:
2651:(12): CD009130.
2644:
2638:
2637:
2601:
2595:
2594:
2584:
2574:
2550:
2544:
2543:
2533:
2509:
2503:
2502:
2500:
2498:
2479:
2466:
2465:
2455:
2423:
2417:
2416:
2388:
2382:
2376:
2375:
2371:
2369:
2367:
2348:
2339:
2338:
2328:
2318:
2294:
2288:
2287:
2285:
2283:
2272:
2266:
2265:
2263:
2261:
2246:
2240:
2239:
2237:
2218:
2209:
2208:
2184:
2175:
2174:
2172:
2170:
2147:
2141:
2140:
2130:
2120:
2096:
2090:
2089:
2053:
2047:
2046:
2010:
2004:
2003:
2001:
1999:
1984:
1963:
1962:
1960:
1958:
1943:
1937:
1935:
1933:
1931:
1910:
1889:
1888:
1886:
1884:
1865:
1842:
1841:
1839:
1837:
1822:
1813:
1812:
1810:
1808:
1789:
1783:
1782:
1780:
1778:
1757:
1751:
1739:
1733:
1732:
1730:
1728:
1718:nctr-crs.fda.gov
1710:
1704:
1703:
1701:
1699:
1680:
1671:
1670:
1668:
1666:
1645:
1639:
1638:
1636:
1634:
1625:. 15 June 2017.
1613:
1607:
1606:
1604:
1602:
1593:. 13 July 2017.
1581:
1575:
1574:
1572:
1570:
1551:
1538:
1537:
1535:
1533:
1519:
1506:
1505:
1503:
1501:
1482:
1077:(PML) caused by
1000:Sjögren syndrome
889:cyclophosphamide
832:Burkitt lymphoma
619:
618:
611:
600:
598:
581:
575:
569:
563:
557:
540:
529:
518:
517:
503:
501:
486:
466:
446:
410:
390:
354:
321:
318:
313:
306:
303:
293:
290:
281:
278:
268:
265:
243:
188:
185:
167:
156:
153:
140:
126:
33:
26:
24:
7481:
7480:
7476:
7475:
7474:
7472:
7471:
7470:
7461:Specialty drugs
7431:
7430:
7429:
7419:
7417:
7409:
7407:
7402:
7401:
7386:Clinical trials
7361:
7335:
7312:
6975:
6862:
6716:
6446:
6440:
6410:
6405:
6259:Pi3K inhibitors
6157:mTOR inhibitors
6088:
5869:
5840:(VEGFR, FGFR),
5496:
5260:
5141:
5101:
5023:
5009:
4976:. 7 March 2014.
4968:
4965:
4953:
4950:
4948:Further reading
4945:
4944:
4900:
4899:
4895:
4849:
4848:
4844:
4834:
4832:
4823:
4822:
4818:
4808:
4806:
4797:
4796:
4792:
4762:
4761:
4757:
4711:
4710:
4706:
4667:
4666:
4662:
4623:(5): e2211497.
4610:
4609:
4605:
4561:
4560:
4556:
4512:
4511:
4507:
4477:
4476:
4472:
4441:
4440:
4436:
4405:
4404:
4400:
4389:
4387:
4380:"Ituxredi EPAR"
4378:
4377:
4373:
4359:
4357:
4344:
4343:
4339:
4329:
4327:
4314:
4313:
4309:
4299:
4297:
4287:
4286:
4282:
4272:
4270:
4261:
4260:
4256:
4246:
4244:
4231:
4230:
4226:
4216:
4214:
4197:
4196:
4192:
4182:
4180:
4171:
4170:
4166:
4156:
4154:
4145:
4144:
4140:
4130:
4128:
4119:
4118:
4114:
4070:
4069:
4065:
4055:
4053:
4044:
4043:
4039:
4029:
4027:
4018:
4017:
4013:
4003:
4001:
3987:
3986:
3979:
3967:
3959:
3957:
3944:
3943:
3932:
3920:
3912:
3910:
3897:
3896:
3883:
3871:
3863:
3861:
3848:
3847:
3840:
3830:
3828:
3814:
3813:
3809:
3796:
3795:
3791:
3783:, Longo et al.
3779:
3775:
3748:Cancer Practice
3745:
3744:
3740:
3719:
3718:
3714:
3678:
3677:
3673:
3663:
3661:
3654:go.drugbank.com
3648:
3647:
3643:
3607:
3606:
3602:
3571:
3570:
3563:
3519:
3518:
3514:
3478:
3477:
3473:
3437:
3436:
3429:
3399:
3398:
3394:
3386:
3379:
3375:
3374:
3370:
3340:
3339:
3335:
3289:
3288:
3284:
3254:
3253:
3249:
3203:
3202:
3198:
3162:
3161:
3157:
3119:
3118:
3114:
3078:
3077:
3073:
3049:
3048:
3044:
3034:
3032:
3023:
3022:
3018:
2982:
2981:
2977:
2941:
2940:
2936:
2906:
2905:
2901:
2891:
2889:
2885:
2862:
2857:
2856:
2852:
2808:
2807:
2803:
2767:
2766:
2762:
2752:
2750:
2733:
2732:
2728:
2718:
2716:
2707:
2706:
2702:
2692:
2690:
2680:
2679:
2672:
2646:
2645:
2641:
2603:
2602:
2598:
2565:(Suppl 1): S5.
2552:
2551:
2547:
2511:
2510:
2506:
2496:
2494:
2481:
2480:
2469:
2425:
2424:
2420:
2390:
2389:
2385:
2373:
2365:
2363:
2350:
2349:
2342:
2296:
2295:
2291:
2281:
2279:
2274:
2273:
2269:
2259:
2257:
2248:
2247:
2243:
2220:
2219:
2212:
2201:
2186:
2185:
2178:
2168:
2166:
2149:
2148:
2144:
2098:
2097:
2093:
2055:
2054:
2050:
2012:
2011:
2007:
1997:
1995:
1986:
1985:
1966:
1956:
1954:
1945:
1944:
1940:
1929:
1927:
1914:"Mabthera EPAR"
1912:
1911:
1892:
1882:
1880:
1867:
1866:
1845:
1835:
1833:
1832:. 13 March 2021
1824:
1823:
1816:
1806:
1804:
1791:
1790:
1786:
1776:
1774:
1759:
1758:
1754:
1749:Wayback Machine
1740:
1736:
1726:
1724:
1712:
1711:
1707:
1697:
1695:
1682:
1681:
1674:
1664:
1662:
1647:
1646:
1642:
1632:
1630:
1617:"Rixathon EPAR"
1615:
1614:
1610:
1600:
1598:
1585:"Blitzima EPAR"
1583:
1582:
1578:
1568:
1566:
1553:
1552:
1541:
1531:
1529:
1521:
1520:
1509:
1499:
1497:
1484:
1483:
1472:
1467:
1426:
1414:
1402:
1370:
1362:
1360:Tailored-dosing
1323:
1318:
1256:clinical trials
1245:
1217:of CD20+ cells.
1208:B cell receptor
1146:differentiation
1121:
1040:
1008:Devic's disease
947:and autoimmune
897:
849:
836:B-cell leukemia
754:
710:Rituximab is a
614:
612:
609:(what is this?)
606:
596:
594:
584:
578:
572:
566:
560:
521:
497:
489:
469:
449:
429:
413:
393:
352:
341:Bioavailability
333:Pharmacokinetic
327:
311:
247:
203:
196:
177:
170:
17:
12:
11:
5:
7479:
7477:
7469:
7468:
7463:
7458:
7453:
7448:
7443:
7433:
7432:
7428:
7427:
7404:
7403:
7400:
7399:
7398:
7397:
7394:
7383:
7377:
7371:
7370:
7367:
7366:
7363:
7362:
7360:
7359:
7354:
7352:Duvortuxizumab
7349:
7343:
7341:
7337:
7336:
7334:
7333:
7328:
7322:
7320:
7314:
7313:
7311:
7310:
7305:
7300:
7295:
7290:
7285:
7271:
7266:
7261:
7256:
7251:
7246:
7241:
7236:
7231:
7226:
7221:
7216:
7211:
7206:
7201:
7196:
7191:
7186:
7181:
7176:
7171:
7166:
7161:
7156:
7151:
7146:
7141:
7136:
7131:
7126:
7121:
7116:
7111:
7106:
7101:
7096:
7091:
7086:
7081:
7076:
7071:
7066:
7061:
7056:
7051:
7046:
7041:
7036:
7031:
7026:
7021:
7019:Brontictuzumab
7016:
7011:
7006:
7001:
6996:
6991:
6985:
6983:
6977:
6976:
6974:
6973:
6968:
6963:
6958:
6953:
6948:
6943:
6938:
6933:
6928:
6923:
6918:
6913:
6908:
6903:
6898:
6893:
6888:
6883:
6878:
6872:
6870:
6864:
6863:
6861:
6860:
6855:
6850:
6845:
6840:
6835:
6830:
6825:
6820:
6815:
6810:
6805:
6800:
6795:
6790:
6785:
6780:
6775:
6770:
6765:
6760:
6755:
6750:
6745:
6740:
6735:
6730:
6724:
6722:
6718:
6717:
6715:
6714:
6709:
6704:
6699:
6694:
6689:
6684:
6679:
6674:
6669:
6664:
6659:
6654:
6649:
6644:
6639:
6634:
6629:
6624:
6619:
6614:
6609:
6604:
6599:
6594:
6589:
6584:
6579:
6574:
6569:
6564:
6559:
6554:
6549:
6544:
6539:
6534:
6529:
6524:
6519:
6514:
6509:
6504:
6499:
6494:
6489:
6486:+hyaluronidase
6479:
6474:
6469:
6463:
6461:
6454:
6448:
6447:
6441:
6439:
6438:
6431:
6424:
6416:
6407:
6406:
6404:
6403:
6398:
6393:
6388:
6383:
6378:
6373:
6368:
6363:
6358:
6353:
6348:
6343:
6338:
6333:
6328:
6323:
6318:
6313:
6308:
6303:
6298:
6293:
6288:
6287:
6286:
6281:
6276:
6271:
6266:
6256:
6255:
6254:
6249:
6236:
6235:
6234:
6229:
6224:
6219:
6214:
6206:CDK inhibitors
6202:
6201:
6200:
6195:
6190:
6177:
6176:
6175:
6170:
6165:
6153:
6137:
6117:
6105:fusion protein
6100:
6098:
6094:
6093:
6090:
6089:
6087:
6086:
6085:
6084:
6079:
6074:
6069:
6064:
6051:
6050:
6049:
6048:
6043:
6038:
6021:
6020:
6019:
6018:
6013:
6008:
6003:
5990:
5989:
5988:
5987:
5982:
5977:
5972:
5967:
5962:
5957:
5944:
5943:
5937:
5925:
5924:
5923:
5922:
5917:
5912:
5907:
5902:
5897:
5892:
5879:
5877:
5871:
5870:
5868:
5867:
5846:
5845:
5844:(VEGFR, EGFR).
5800:
5799:
5798:
5797:
5792:
5787:
5774:
5773:
5772:
5771:
5766:
5761:
5756:
5751:
5746:
5741:
5736:
5731:
5726:
5721:
5716:
5711:
5698:
5697:
5681:
5675:
5670:
5665:
5660:
5655:
5650:
5645:
5625:
5624:
5623:
5622:
5617:
5612:
5602:HER1/EGFR and
5598:
5597:
5591:
5586:
5581:
5576:
5571:
5566:
5561:
5556:
5551:
5546:
5541:
5536:
5520:
5518:
5509:
5502:
5501:
5498:
5497:
5495:
5494:
5489:
5484:
5479:
5474:
5469:
5464:
5459:
5454:
5449:
5444:
5439:
5434:
5429:
5424:
5419:
5414:
5409:
5404:
5399:
5394:
5389:
5384:
5379:
5374:
5369:
5364:
5359:
5354:
5349:
5344:
5339:
5334:
5329:
5324:
5319:
5314:
5309:
5304:
5299:
5294:
5289:
5284:
5281:+hyaluronidase
5274:
5268:
5266:
5262:
5261:
5259:
5258:
5241:
5240:
5214:
5209:
5204:
5199:
5194:
5189:
5155:
5153:
5143:
5142:
5140:
5139:
5127:
5121:
5109:
5107:
5103:
5102:
5100:
5099:
5093:
5088:
5085:+hyaluronidase
5068:
5062:
5046:
5044:
5035:
5025:
5024:
5010:
5008:
5007:
5000:
4993:
4985:
4979:
4978:
4964:
4963:External links
4961:
4960:
4959:
4949:
4946:
4943:
4942:
4893:
4869:10.1186/bcr276
4842:
4825:"Fc-structure"
4816:
4790:
4755:
4704:
4677:(9): 585–593.
4660:
4603:
4554:
4525:(7): 937–941.
4505:
4470:
4451:(3): 220–224.
4434:
4407:in Colombia".
4398:
4386:. 25 July 2024
4371:
4337:
4322:. 4 May 2020.
4307:
4280:
4254:
4224:
4190:
4164:
4151:Google Patents
4138:
4125:Google Patents
4112:
4063:
4050:Google Patents
4037:
4026:. 24 July 1992
4024:Google Patents
4011:
3977:
3930:
3881:
3860:. 22 June 2017
3838:
3807:
3789:
3773:
3754:(3): 195–197.
3738:
3712:
3671:
3641:
3600:
3561:
3512:
3471:
3427:
3392:
3368:
3333:
3282:
3247:
3196:
3175:(6): 646–650.
3155:
3112:
3071:
3042:
3016:
2975:
2934:
2899:
2850:
2821:(4): 275–280.
2801:
2780:(9): 659–665.
2760:
2749:on 13 May 2009
2726:
2700:
2689:on 29 May 2010
2670:
2639:
2596:
2545:
2504:
2467:
2438:(1): 324–328.
2418:
2399:(5): 385–390.
2383:
2340:
2309:(3): 282–290.
2289:
2267:
2241:
2210:
2199:
2176:
2142:
2091:
2064:(3): 248–253.
2048:
2021:(2): 185–196.
2005:
1964:
1938:
1890:
1843:
1814:
1784:
1752:
1734:
1705:
1672:
1640:
1608:
1576:
1539:
1507:
1469:
1468:
1466:
1463:
1462:
1461:
1442:
1436:
1425:
1422:
1413:
1410:
1401:
1398:
1369:
1366:
1361:
1358:
1327:specialty drug
1322:
1319:
1317:
1314:
1244:
1241:
1237:disulfide bond
1222:
1221:
1218:
1211:
1204:
1197:
1190:
1187:
1180:
1120:
1117:
1116:
1115:
1106:
1100:
1093:
1092:
1091:
1088:
1082:
1069:
1060:
1055:
1053:Cardiac arrest
1050:
1039:
1038:Adverse events
1036:
976:Evans syndrome
896:
893:
848:
845:
840:
839:
824:
818:
805:
803:TNF inhibitors
796:
790:
753:
750:
740:in the US, by
621:
620:
603:
602:
592:
586:
585:
582:
576:
570:
564:
558:
553:
547:
546:
542:
541:
531:
523:
522:
520:
519:
506:
504:
491:
490:
488:
487:
479:
477:
471:
470:
468:
467:
459:
457:
451:
450:
448:
447:
439:
437:
431:
430:
428:
427:
423:
421:
415:
414:
412:
411:
403:
401:
395:
394:
392:
391:
383:
381:
375:
374:
370:
369:
366:
360:
359:
356:
347:
346:
343:
337:
336:
329:
328:
326:
325:
316:
301:
288:
276:
262:
260:
254:
253:
249:
248:
246:
245:
232:
230:
224:
223:
218:
212:
211:
206:
204:administration
198:
197:
195:
194:
192:
182:
180:
172:
171:
169:
168:
150:
148:
142:
141:
134:
128:
127:
120:
110:
109:
106:
100:
99:
96:
90:
89:
85:
84:
79:
73:
72:
58:
52:
51:
50:Whole antibody
48:
42:
41:
35:
34:
15:
13:
10:
9:
6:
4:
3:
2:
7478:
7467:
7464:
7462:
7459:
7457:
7454:
7452:
7449:
7447:
7444:
7442:
7439:
7438:
7436:
7426:
7416:
7412:
7395:
7393:
7390:
7389:
7387:
7384:
7381:
7378:
7376:
7373:
7372:
7368:
7358:
7355:
7353:
7350:
7348:
7345:
7344:
7342:
7338:
7332:
7329:
7327:
7324:
7323:
7321:
7319:
7315:
7309:
7306:
7304:
7301:
7299:
7296:
7294:
7291:
7289:
7286:
7283:
7279:
7275:
7272:
7270:
7267:
7265:
7262:
7260:
7257:
7255:
7252:
7250:
7247:
7245:
7242:
7240:
7237:
7235:
7234:Rosmantuzumab
7232:
7230:
7227:
7225:
7222:
7220:
7217:
7215:
7212:
7210:
7207:
7205:
7202:
7200:
7197:
7195:
7192:
7190:
7187:
7185:
7182:
7180:
7177:
7175:
7172:
7170:
7167:
7165:
7162:
7160:
7157:
7155:
7152:
7150:
7147:
7145:
7142:
7140:
7137:
7135:
7132:
7130:
7127:
7125:
7122:
7120:
7117:
7115:
7112:
7110:
7107:
7105:
7102:
7100:
7097:
7095:
7092:
7090:
7089:Enoblituzumab
7087:
7085:
7082:
7080:
7077:
7075:
7072:
7070:
7067:
7065:
7062:
7060:
7057:
7055:
7052:
7050:
7047:
7045:
7042:
7040:
7037:
7035:
7032:
7030:
7027:
7025:
7022:
7020:
7017:
7015:
7012:
7010:
7007:
7005:
7002:
7000:
6997:
6995:
6992:
6990:
6987:
6986:
6984:
6982:
6978:
6972:
6969:
6967:
6964:
6962:
6959:
6957:
6954:
6952:
6949:
6947:
6944:
6942:
6939:
6937:
6934:
6932:
6929:
6927:
6924:
6922:
6919:
6917:
6914:
6912:
6909:
6907:
6904:
6902:
6899:
6897:
6894:
6892:
6889:
6887:
6884:
6882:
6879:
6877:
6874:
6873:
6871:
6869:
6865:
6859:
6856:
6854:
6851:
6849:
6846:
6844:
6841:
6839:
6836:
6834:
6831:
6829:
6826:
6824:
6821:
6819:
6816:
6814:
6811:
6809:
6806:
6804:
6801:
6799:
6796:
6794:
6791:
6789:
6786:
6784:
6781:
6779:
6776:
6774:
6771:
6769:
6766:
6764:
6761:
6759:
6756:
6754:
6751:
6749:
6746:
6744:
6741:
6739:
6736:
6734:
6731:
6729:
6726:
6725:
6723:
6719:
6713:
6710:
6708:
6705:
6703:
6700:
6698:
6695:
6693:
6690:
6688:
6685:
6683:
6680:
6678:
6675:
6673:
6670:
6668:
6665:
6663:
6660:
6658:
6655:
6653:
6650:
6648:
6645:
6643:
6642:Pembrolizumab
6640:
6638:
6635:
6633:
6630:
6628:
6625:
6623:
6620:
6618:
6615:
6613:
6610:
6608:
6605:
6603:
6600:
6598:
6595:
6593:
6590:
6588:
6585:
6583:
6580:
6578:
6575:
6573:
6570:
6568:
6565:
6563:
6560:
6558:
6555:
6553:
6550:
6548:
6545:
6543:
6540:
6538:
6535:
6533:
6530:
6528:
6525:
6523:
6520:
6518:
6515:
6513:
6510:
6508:
6505:
6503:
6500:
6498:
6495:
6493:
6490:
6487:
6483:
6480:
6478:
6477:Ascrinvacumab
6475:
6473:
6470:
6468:
6465:
6464:
6462:
6458:
6455:
6453:
6449:
6444:
6437:
6432:
6430:
6425:
6423:
6418:
6417:
6414:
6402:
6399:
6397:
6394:
6392:
6389:
6387:
6384:
6382:
6379:
6377:
6374:
6372:
6369:
6367:
6364:
6362:
6359:
6357:
6354:
6352:
6349:
6347:
6344:
6342:
6339:
6337:
6334:
6332:
6329:
6327:
6324:
6322:
6319:
6317:
6316:Larotrectinib
6314:
6312:
6309:
6307:
6304:
6302:
6299:
6297:
6294:
6292:
6289:
6285:
6282:
6280:
6277:
6275:
6272:
6270:
6267:
6265:
6262:
6261:
6260:
6257:
6253:
6250:
6248:
6245:
6244:
6243:
6241:
6237:
6233:
6230:
6228:
6225:
6223:
6220:
6218:
6215:
6213:
6210:
6209:
6208:
6207:
6203:
6199:
6196:
6194:
6191:
6189:
6186:
6185:
6184:
6182:
6178:
6174:
6171:
6169:
6168:Ridaforolimus
6166:
6164:
6161:
6160:
6159:
6158:
6154:
6151:
6147:
6146:
6142:
6138:
6135:
6131:
6130:
6126:
6122:
6118:
6115:
6111:
6110:
6106:
6102:
6101:
6099:
6095:
6083:
6080:
6078:
6077:Pirtobrutinib
6075:
6073:
6072:Orelabrutinib
6070:
6068:
6065:
6063:
6062:Acalabrutinib
6060:
6059:
6058:
6057:
6053:
6052:
6047:
6044:
6042:
6039:
6037:
6034:
6033:
6032:
6031:
6027:
6023:
6022:
6017:
6014:
6012:
6009:
6007:
6004:
6002:
5999:
5998:
5997:
5996:
5992:
5991:
5986:
5983:
5981:
5978:
5976:
5973:
5971:
5968:
5966:
5963:
5961:
5958:
5956:
5953:
5952:
5951:
5950:
5946:
5945:
5941:
5938:
5936:
5932:
5931:
5927:
5926:
5921:
5918:
5916:
5913:
5911:
5908:
5906:
5903:
5901:
5898:
5896:
5893:
5891:
5888:
5887:
5886:
5885:
5881:
5880:
5878:
5876:
5872:
5865:
5861:
5857:
5854:
5852:
5848:
5847:
5843:
5839:
5838:Selpercatinib
5835:
5834:Repotrectinib
5831:
5827:
5823:
5822:Larotrectinib
5819:
5815:
5811:
5808:
5806:
5802:
5801:
5796:
5793:
5791:
5788:
5786:
5783:
5782:
5781:
5780:
5776:
5775:
5770:
5767:
5765:
5762:
5760:
5757:
5755:
5752:
5750:
5747:
5745:
5742:
5740:
5737:
5735:
5732:
5730:
5727:
5725:
5722:
5720:
5717:
5715:
5712:
5710:
5707:
5706:
5705:
5704:
5700:
5699:
5695:
5691:
5687:
5686:
5682:
5679:
5676:
5674:
5671:
5669:
5666:
5664:
5661:
5659:
5656:
5654:
5651:
5649:
5646:
5644:
5640:
5639:
5635:
5630:
5629:RTK class III
5627:
5626:
5621:
5618:
5616:
5613:
5611:
5608:
5607:
5606:
5605:
5600:
5599:
5595:
5592:
5590:
5587:
5585:
5582:
5580:
5577:
5575:
5572:
5570:
5567:
5565:
5562:
5560:
5557:
5555:
5552:
5550:
5547:
5545:
5542:
5540:
5537:
5535:
5531:
5530:
5525:
5522:
5521:
5519:
5517:
5513:
5510:
5507:
5503:
5493:
5490:
5488:
5485:
5483:
5480:
5478:
5475:
5473:
5470:
5468:
5465:
5463:
5460:
5458:
5455:
5453:
5450:
5448:
5445:
5443:
5440:
5438:
5435:
5433:
5430:
5428:
5425:
5423:
5420:
5418:
5415:
5413:
5412:Pembrolizumab
5410:
5408:
5405:
5403:
5400:
5398:
5395:
5393:
5390:
5388:
5385:
5383:
5380:
5378:
5377:Mogamulizumab
5375:
5373:
5370:
5368:
5365:
5363:
5360:
5358:
5355:
5353:
5350:
5348:
5345:
5343:
5340:
5338:
5335:
5333:
5330:
5328:
5325:
5323:
5320:
5318:
5315:
5313:
5310:
5308:
5305:
5303:
5300:
5298:
5295:
5293:
5290:
5288:
5285:
5282:
5278:
5275:
5273:
5270:
5269:
5267:
5263:
5256:
5252:
5251:
5246:
5243:
5242:
5238:
5234:
5233:
5228:
5224:
5223:
5218:
5215:
5213:
5210:
5208:
5205:
5203:
5200:
5198:
5197:Mosunetuzumab
5195:
5193:
5190:
5188:
5184:
5183:
5178:
5177:Mosunetuzumab
5174:
5170:
5166:
5165:
5160:
5157:
5156:
5154:
5152:
5148:
5144:
5137:
5133:
5132:
5128:
5125:
5122:
5120:
5116:
5115:
5111:
5110:
5108:
5104:
5097:
5094:
5092:
5089:
5086:
5082:
5078:
5074:
5073:
5069:
5066:
5063:
5061:
5057:
5056:
5051:
5048:
5047:
5045:
5043:
5039:
5036:
5033:
5030:
5026:
5021:
5017:
5013:
5006:
5001:
4999:
4994:
4992:
4987:
4986:
4983:
4975:
4971:
4967:
4966:
4962:
4956:
4952:
4951:
4947:
4938:
4934:
4929:
4924:
4920:
4916:
4912:
4908:
4904:
4897:
4894:
4889:
4885:
4880:
4875:
4870:
4865:
4861:
4857:
4853:
4846:
4843:
4830:
4826:
4820:
4817:
4804:
4800:
4794:
4791:
4786:
4782:
4778:
4774:
4770:
4766:
4759:
4756:
4751:
4747:
4742:
4737:
4732:
4727:
4723:
4719:
4715:
4708:
4705:
4700:
4696:
4692:
4688:
4684:
4680:
4676:
4672:
4664:
4661:
4656:
4652:
4648:
4644:
4639:
4634:
4630:
4626:
4622:
4618:
4614:
4607:
4604:
4599:
4595:
4591:
4587:
4582:
4577:
4573:
4569:
4565:
4558:
4555:
4550:
4546:
4542:
4538:
4533:
4528:
4524:
4520:
4516:
4509:
4506:
4501:
4497:
4493:
4489:
4485:
4481:
4474:
4471:
4466:
4462:
4458:
4454:
4450:
4446:
4438:
4435:
4430:
4426:
4422:
4418:
4414:
4410:
4402:
4399:
4385:
4381:
4375:
4372:
4368:
4355:
4351:
4347:
4341:
4338:
4325:
4321:
4317:
4311:
4308:
4295:
4291:
4284:
4281:
4268:
4264:
4258:
4255:
4242:
4240:
4234:
4228:
4225:
4212:
4208:
4206:
4200:
4194:
4191:
4179:
4175:
4168:
4165:
4153:. 31 May 2013
4152:
4148:
4142:
4139:
4126:
4122:
4116:
4113:
4108:
4104:
4099:
4094:
4090:
4086:
4083:(4): 820–37.
4082:
4078:
4074:
4067:
4064:
4051:
4047:
4041:
4038:
4025:
4021:
4015:
4012:
3999:
3995:
3991:
3984:
3982:
3978:
3974:
3973:public domain
3955:
3953:
3947:
3941:
3939:
3937:
3935:
3931:
3927:
3926:public domain
3908:
3906:
3900:
3894:
3892:
3890:
3888:
3886:
3882:
3878:
3877:public domain
3859:
3857:
3851:
3845:
3843:
3839:
3826:
3822:
3818:
3811:
3808:
3803:
3799:
3793:
3790:
3787:2011 page 931
3786:
3782:
3777:
3774:
3769:
3765:
3761:
3757:
3753:
3749:
3742:
3739:
3734:
3730:
3728:
3722:
3716:
3713:
3708:
3704:
3699:
3694:
3690:
3686:
3682:
3675:
3672:
3659:
3655:
3651:
3645:
3642:
3637:
3633:
3628:
3623:
3619:
3615:
3611:
3604:
3601:
3596:
3592:
3588:
3584:
3580:
3576:
3568:
3566:
3562:
3557:
3553:
3548:
3543:
3539:
3535:
3531:
3527:
3523:
3516:
3513:
3508:
3504:
3499:
3494:
3490:
3486:
3482:
3475:
3472:
3467:
3463:
3458:
3453:
3449:
3445:
3441:
3434:
3432:
3428:
3423:
3419:
3415:
3411:
3407:
3403:
3396:
3393:
3385:
3378:
3372:
3369:
3364:
3360:
3356:
3352:
3348:
3344:
3337:
3334:
3329:
3325:
3320:
3315:
3310:
3305:
3301:
3297:
3293:
3286:
3283:
3278:
3274:
3270:
3266:
3262:
3258:
3251:
3248:
3243:
3239:
3234:
3229:
3224:
3219:
3215:
3211:
3207:
3200:
3197:
3192:
3188:
3183:
3178:
3174:
3170:
3166:
3159:
3156:
3151:
3147:
3143:
3139:
3135:
3131:
3127:
3123:
3116:
3113:
3108:
3104:
3099:
3094:
3090:
3086:
3082:
3075:
3072:
3066:
3061:
3057:
3053:
3046:
3043:
3030:
3026:
3020:
3017:
3012:
3008:
3003:
2998:
2994:
2990:
2986:
2979:
2976:
2971:
2967:
2962:
2957:
2953:
2949:
2945:
2938:
2935:
2930:
2926:
2922:
2918:
2914:
2910:
2903:
2900:
2884:
2880:
2876:
2872:
2868:
2861:
2854:
2851:
2846:
2842:
2838:
2834:
2829:
2824:
2820:
2816:
2812:
2805:
2802:
2797:
2793:
2788:
2783:
2779:
2775:
2771:
2764:
2761:
2748:
2744:
2742:
2736:
2730:
2727:
2714:
2710:
2704:
2701:
2688:
2684:
2677:
2675:
2671:
2666:
2662:
2658:
2654:
2650:
2643:
2640:
2635:
2631:
2627:
2623:
2619:
2615:
2612:(1): 89–100.
2611:
2607:
2600:
2597:
2592:
2588:
2583:
2578:
2573:
2568:
2564:
2560:
2556:
2549:
2546:
2541:
2537:
2532:
2527:
2523:
2519:
2515:
2508:
2505:
2492:
2488:
2484:
2478:
2476:
2474:
2472:
2468:
2463:
2459:
2454:
2449:
2445:
2441:
2437:
2433:
2429:
2422:
2419:
2414:
2410:
2406:
2402:
2398:
2394:
2387:
2384:
2380:
2379:public domain
2361:
2357:
2353:
2347:
2345:
2341:
2336:
2332:
2327:
2322:
2317:
2312:
2308:
2304:
2300:
2293:
2290:
2277:
2271:
2268:
2255:
2251:
2245:
2242:
2236:
2231:
2227:
2223:
2217:
2215:
2211:
2206:
2202:
2200:9783034807067
2196:
2192:
2191:
2183:
2181:
2177:
2164:
2160:
2158:
2152:
2146:
2143:
2138:
2134:
2129:
2124:
2119:
2114:
2110:
2106:
2102:
2095:
2092:
2087:
2083:
2079:
2075:
2071:
2067:
2063:
2059:
2052:
2049:
2044:
2040:
2036:
2032:
2028:
2024:
2020:
2016:
2009:
2006:
1993:
1989:
1983:
1981:
1979:
1977:
1975:
1973:
1971:
1969:
1965:
1953:. 3 June 1998
1952:
1948:
1947:"Mabthera PI"
1942:
1939:
1925:
1921:
1920:
1915:
1909:
1907:
1905:
1903:
1901:
1899:
1897:
1895:
1891:
1878:
1874:
1870:
1864:
1862:
1860:
1858:
1856:
1854:
1852:
1850:
1848:
1844:
1831:
1827:
1821:
1819:
1815:
1802:
1798:
1794:
1788:
1785:
1772:
1768:
1767:
1766:Health Canada
1762:
1756:
1753:
1750:
1746:
1743:
1738:
1735:
1723:
1719:
1715:
1709:
1706:
1693:
1689:
1685:
1679:
1677:
1673:
1660:
1656:
1655:
1654:Health Canada
1650:
1644:
1641:
1628:
1624:
1623:
1618:
1612:
1609:
1596:
1592:
1591:
1586:
1580:
1577:
1564:
1560:
1556:
1550:
1548:
1546:
1544:
1540:
1528:
1524:
1518:
1516:
1514:
1512:
1508:
1495:
1491:
1487:
1481:
1479:
1477:
1475:
1471:
1464:
1459:
1455:
1451:
1447:
1443:
1440:
1437:
1434:
1431:
1430:
1429:
1423:
1421:
1419:
1418:intrathecally
1411:
1409:
1407:
1399:
1397:
1395:
1390:
1386:
1381:
1379:
1375:
1367:
1365:
1359:
1357:
1355:
1351:
1347:
1343:
1338:
1335:
1330:
1328:
1320:
1315:
1313:
1309:
1305:
1301:
1299:
1294:
1292:
1289:It is on the
1287:
1285:
1281:
1277:
1273:
1269:
1265:
1261:
1257:
1252:
1250:
1242:
1240:
1238:
1234:
1229:
1227:
1219:
1216:
1212:
1209:
1205:
1202:
1198:
1195:
1192:It increases
1191:
1188:
1185:
1181:
1178:
1174:
1170:
1166:
1165:
1164:
1161:
1157:
1155:
1151:
1147:
1143:
1134:
1125:
1118:
1114:
1110:
1107:
1104:
1101:
1098:
1094:
1089:
1086:
1083:
1080:
1076:
1073:
1072:
1070:
1068:
1064:
1061:
1059:
1056:
1054:
1051:
1049:
1045:
1044:
1043:
1037:
1035:
1033:
1029:
1025:
1021:
1017:
1013:
1009:
1005:
1001:
997:
993:
989:
985:
981:
977:
973:
969:
965:
961:
956:
954:
950:
946:
942:
938:
934:
930:
925:
922:
918:
914:
910:
906:
902:
894:
892:
890:
886:
882:
878:
874:
873:hyaluronidase
870:
866:
862:
858:
854:
847:Blood cancers
846:
844:
837:
833:
829:
825:
823:
819:
817:
813:
809:
806:
804:
800:
797:
794:
791:
788:
785:
784:
783:
781:
776:
774:
770:
766:
762:
759:
751:
749:
747:
743:
739:
735:
731:
726:
724:
720:
716:
713:
708:
706:
702:
698:
694:
689:
685:
683:
679:
675:
671:
667:
663:
659:
655:
651:
647:
643:
640:and types of
639:
635:
631:
627:
617:
610:
604:
593:
591:
587:
554:
552:
548:
543:
539:
535:
532:
530:
528:ECHA InfoCard
524:
516:
512:
511:DTXSID5040910
508:
507:
505:
496:
492:
485:
484:ChEMBL1201576
481:
480:
478:
476:
472:
465:
461:
460:
458:
456:
452:
445:
441:
440:
438:
436:
432:
425:
424:
422:
420:
416:
409:
405:
404:
402:
400:
396:
389:
385:
384:
382:
380:
376:
371:
367:
365:
361:
357:
355:
348:
344:
342:
338:
334:
330:
324: Rx-only
317:
314:
302:
299:
289:
286:
277:
274:
264:
263:
261:
259:
255:
250:
242:
237:
234:
233:
231:
229:
225:
222:
219:
217:
213:
210:
207:
205:
199:
193:
184:
183:
181:
179:
173:
166:
161:
152:
151:
149:
147:
143:
139:
135:
133:
129:
125:
121:
119:
115:
111:
107:
105:
101:
97:
95:
91:
88:Clinical data
86:
83:
80:
78:
74:
70:
66:
62:
59:
57:
53:
49:
47:
43:
40:
36:
32:
27:
19:
7456:Orphan drugs
7249:Sibrotuzumab
7214:Parsatuzumab
7199:Otlertuzumab
7194:Odronextamab
7189:Ocaratuzumab
7184:Obinutuzumab
7159:Lumretuzumab
7114:Flotetuzumab
7109:Ficlatuzumab
7104:Farletuzumab
7099:Etaracizumab
7084:Emibetuzumab
6971:Zolbetuximab
6955:
6946:Margetuximab
6926:Girentuximab
6788:Minretumomab
6753:Blinatumomab
6692:Teprotumumab
6672:Seribantumab
6497:Botensilimab
6482:Atezolizumab
6467:Adecatumumab
6356:Pexidartinib
6341:Odronextamab
6311:Gilteritinib
6291:Cabozantinib
6238:
6204:
6179:
6173:Temsirolimus
6155:
6139:
6121:proapoptotic
6119:
6103:
6082:Zanubrutinib
6054:
6024:
5993:
5970:Lestaurtinib
5949:Janus kinase
5947:
5928:
5882:
5875:Non-receptor
5856:Cabozantinib
5849:
5818:Infigratinib
5803:
5777:
5719:Fruquintinib
5701:
5694:Gilteritinib
5690:Lestaurtinib
5683:
5632:
5601:
5574:Mobocertinib
5539:Aumolertinib
5527:
5492:Tremelimumab
5477:Tislelizumab
5432:Retifanlimab
5307:Blinatumomab
5277:Atezolizumab
5248:
5230:
5220:
5211:
5202:Obinutuzumab
5180:
5162:
5129:
5112:
5070:
5053:
4973:
4910:
4906:
4896:
4862:(2): 86–90.
4859:
4855:
4845:
4833:. Retrieved
4819:
4807:. Retrieved
4803:the original
4793:
4768:
4764:
4758:
4721:
4717:
4707:
4674:
4670:
4663:
4620:
4616:
4606:
4571:
4567:
4557:
4522:
4518:
4508:
4483:
4479:
4473:
4448:
4444:
4437:
4412:
4408:
4401:
4388:. Retrieved
4383:
4374:
4365:
4358:. Retrieved
4349:
4340:
4328:. Retrieved
4319:
4310:
4298:. Retrieved
4283:
4271:. Retrieved
4257:
4245:. Retrieved
4236:
4227:
4215:. Retrieved
4202:
4193:
4181:. Retrieved
4177:
4167:
4155:. Retrieved
4150:
4141:
4129:. Retrieved
4124:
4115:
4080:
4076:
4066:
4054:. Retrieved
4049:
4040:
4028:. Retrieved
4023:
4014:
4002:. Retrieved
3993:
3958:. Retrieved
3949:
3911:. Retrieved
3902:
3862:. Retrieved
3853:
3829:. Retrieved
3820:
3810:
3792:
3784:
3780:
3776:
3751:
3747:
3741:
3733:the original
3724:
3715:
3688:
3684:
3674:
3662:. Retrieved
3658:the original
3653:
3644:
3617:
3613:
3603:
3578:
3574:
3529:
3525:
3515:
3488:
3484:
3474:
3447:
3443:
3405:
3401:
3395:
3384:the original
3371:
3346:
3342:
3336:
3299:
3295:
3285:
3260:
3256:
3250:
3216:(20): 5022.
3213:
3209:
3199:
3172:
3168:
3158:
3125:
3121:
3115:
3088:
3084:
3074:
3056:Pancreapedia
3055:
3045:
3033:. Retrieved
3029:the original
3019:
2992:
2988:
2978:
2951:
2947:
2937:
2912:
2908:
2902:
2890:. Retrieved
2883:the original
2870:
2866:
2853:
2818:
2814:
2804:
2777:
2773:
2763:
2751:. Retrieved
2747:the original
2738:
2729:
2717:. Retrieved
2715:. 9 May 2009
2713:ScienceDaily
2712:
2703:
2691:. Retrieved
2687:the original
2648:
2642:
2609:
2605:
2599:
2562:
2558:
2548:
2521:
2517:
2507:
2495:. Retrieved
2486:
2435:
2431:
2421:
2396:
2392:
2386:
2364:. Retrieved
2355:
2306:
2302:
2292:
2280:. Retrieved
2270:
2258:. Retrieved
2244:
2235:10665/371090
2225:
2189:
2167:. Retrieved
2154:
2145:
2108:
2104:
2094:
2061:
2057:
2051:
2018:
2014:
2008:
1996:. Retrieved
1955:. Retrieved
1950:
1941:
1928:. Retrieved
1917:
1881:. Retrieved
1872:
1834:. Retrieved
1829:
1805:. Retrieved
1796:
1787:
1775:. Retrieved
1764:
1755:
1737:
1725:. Retrieved
1717:
1708:
1696:. Retrieved
1687:
1663:. Retrieved
1652:
1643:
1631:. Retrieved
1620:
1611:
1599:. Retrieved
1588:
1579:
1567:. Retrieved
1558:
1530:. Retrieved
1526:
1498:. Retrieved
1489:
1446:obinutuzumab
1427:
1415:
1403:
1382:
1371:
1363:
1353:
1339:
1331:
1324:
1310:
1306:
1302:
1295:
1288:
1268:chemotherapy
1253:
1246:
1230:
1223:
1162:
1158:
1150:plasma cells
1139:
1087:reactivation
1081:reactivation
1041:
957:
926:
917:methotrexate
913:FDA approved
898:
850:
841:
808:vasculitides
777:
769:plasma cells
755:
752:Medical uses
727:
709:
690:
686:
629:
625:
624:
613:
607:
351:Elimination
258:Legal status
252:Legal status
146:License data
18:
7382:from market
7357:Ontuxizumab
7331:Ertumaxomab
7326:Catumaxomab
7298:Vanucizumab
7278:+deruxtecan
7274:Trastuzumab
7269:Tigatuzumab
7204:Onartuzumab
7179:Nimotuzumab
7169:Milatuzumab
7139:Labetuzumab
7129:Imgatuzumab
7094:Epcoritamab
7079:Emactuzumab
7064:Dalotuzumab
7054:Dacetuzumab
7034:Cirmtuzumab
7009:Bevacizumab
6994:Alemtuzumab
6966:Ublituximab
6916:Ensituximab
6911:Ecromeximab
6906:Dinutuximab
6891:Carotuximab
6881:Bavituximab
6858:Tositumomab
6853:Tenatumomab
6823:Racotumomab
6768:Edrecolomab
6743:Arcitumomab
6712:Zalutumumab
6702:Vantictumab
6677:Sugemalimab
6667:Robatumumab
6662:Rilotumumab
6657:Ramucirumab
6632:Panitumumab
6607:Necitumumab
6597:Mapatumumab
6592:Lucatumumab
6587:Lexatumumab
6577:Istiratumab
6562:Intetumumab
6547:Flanvotumab
6542:Figitumumab
6527:Dusigitumab
6522:Duligotumab
6512:Daratumumab
6507:Conatumumab
6502:Cixutumumab
6492:Balstilimab
6472:Amivantamab
6386:Tebentafusp
6366:Regorafenib
6361:Quizartinib
6351:Pemigatinib
6331:Midostaurin
6306:Erdafitinib
6301:Entrectinib
6284:Parsaclisib
6232:Trilaciclib
6222:Palbociclib
6217:Dalpiciclib
6212:Abemaciclib
6114:Aflibercept
6041:Entrectinib
6011:Selumetinib
6006:Cobimetinib
6001:Binimetinib
5985:Ruxolitinib
5975:Momelotinib
5955:Baricitinib
5853:inhibitors:
5830:Pralsetinib
5826:Pemigatinib
5814:Futibatinib
5810:Entrectinib
5807:inhibitors:
5739:Regorafenib
5643:Avapritinib
5589:Rociletinib
5584:Osimertinib
5549:Dacomitinib
5487:Toripalimab
5472:Teclistamab
5462:Talquetamab
5457:Tafasitamab
5452:Sugemalimab
5447:Serplulimab
5437:Sabatolimab
5427:Ramucirumab
5422:Prolgolimab
5392:Necitumumab
5347:Epcoritamab
5327:Dostarlimab
5317:Daratumumab
5272:Amivantamab
5237:Alemtuzumab
5227:Brentuximab
5217:Tositumomab
5192:Ibritumomab
5173:Elranatamab
5136:Bevacizumab
5124:Edrecolomab
5119:Catumaxomab
5081:Trastuzumab
5065:Panitumumab
4913:(6): 1–13.
4292:. Reuters.
4217:11 November
3650:"Rituximab"
3408:: 275–290.
1988:"Rituximab"
1930:8 September
1450:Fc fragment
1433:ocrelizumab
1412:Intrathecal
1374:hepatitis E
1334:Biosimilars
1233:amino acids
1213:It induces
1175:(ADCC) and
1113:perforation
1085:Hepatitis B
1071:Infections
1030:(OMS), and
885:fludarabine
763:present on
693:hepatitis B
601: g·mol
534:100.224.382
388:174722-31-7
373:Identifiers
209:Intravenous
132:MedlinePlus
104:Biosimilars
94:Trade names
7435:Categories
7303:Veltuzumab
7282:+emtansine
7254:Simtuzumab
7219:Pertuzumab
7149:Lintuzumab
7124:Glofitamab
7074:Elotuzumab
7059:Demcizumab
6999:Axatilimab
6989:Abituzumab
6961:Siltuximab
6936:Isatuximab
6876:Amatuximab
6848:Pintumomab
6818:Pemtumomab
6813:Oregovomab
6748:Bectumomab
6728:Abagovomab
6682:Tarextumab
6652:Radretumab
6647:Pritumumab
6637:Patritumab
6627:Olaratumab
6622:Ofatumumab
6612:Nesvacumab
6602:Narnatumab
6572:Iratumumab
6567:Ipilimumab
6537:Enoticumab
6517:Drozitumab
6445:for tumors
6401:Venetoclax
6396:Vandetanib
6371:Ripretinib
6336:Nintedanib
6321:Lenvatinib
6296:Capmatinib
6279:Idelalisib
6269:Copanlisib
6242:inhibitors
6227:Ribociclib
6198:Vismodegib
6183:inhibitors
6163:Everolimus
6129:prohibitin
6046:Lorlatinib
6036:Crizotinib
6016:Trametinib
5980:Pacritinib
5965:Filgotinib
5960:Fedratinib
5864:Crizotinib
5860:Capmatinib
5842:Vandetanib
5790:Brigatinib
5769:Vandetanib
5729:Nintedanib
5724:Lenvatinib
5663:Ripretinib
5594:Vandetanib
5569:Lazertinib
5544:Brigatinib
5467:Tarlatamab
5402:Olaratumab
5362:Isatuximab
5357:Ipilimumab
5337:Elotuzumab
5332:Durvalumab
5312:Cemiplimab
5302:Bermekimab
5292:Axatilimab
5207:Ofatumumab
5187:Glofitamab
5169:Glofitamab
5077:Pertuzumab
4835:3 December
4809:3 December
4724:(2): 325.
4486:(6): 430.
4415:: 98–104.
4300:3 November
4004:26 October
3581:: 106684.
3302:: 731643.
3085:Pediatrics
3035:19 October
2873:(2): 107.
2497:2 February
2366:4 December
2169:2 February
1998:8 December
1883:2 February
1727:22 October
1698:2 February
1465:References
1439:ofatumumab
1226:stem cells
1184:cell cycle
1128:rituximab.
1065:, causing
992:pemphigoid
909:refractory
782:to treat:
676:-positive
590:Molar mass
444:4F4X42SYQ6
419:ChemSpider
379:CAS Number
216:Drug class
7392:Phase III
7380:Withdrawn
7174:Naxitamab
7164:Matuzumab
6981:Humanized
6956:Rituximab
6921:Futuximab
6896:Cetuximab
6833:Solitomab
6793:Mitumomab
6783:Lilotomab
6763:Detumomab
6707:Votumumab
6697:Tovetumab
6617:Nivolumab
6582:Icrucumab
6552:Ganitumab
6391:Tepotinib
6381:Sunitinib
6376:Sorafenib
6346:Pazopanib
6326:Masitinib
6274:Duvelisib
6264:Alpelisib
6252:Sotorasib
6247:Adagrasib
6193:Sonidegib
6188:Glasdegib
6134:Adipotide
6067:Ibrutinib
5940:Dasatinib
5935:Bosutinib
5920:Radotinib
5915:Ponatinib
5910:Nilotinib
5900:Dasatinib
5895:Bosutinib
5890:Asciminib
5858:(VEGFR),
5816:(FGFR2),
5795:Ceritinib
5785:Alectinib
5764:Toceranib
5759:Tivozanib
5754:Sunitinib
5749:Sorafenib
5744:Semaxanib
5734:Pazopanib
5714:Cediranib
5678:Toceranib
5673:Sunitinib
5668:Sorafenib
5658:Pazopanib
5653:Masitinib
5620:Tucatinib
5615:Neratinib
5610:Lapatinib
5579:Olmutinib
5559:Gefitinib
5554:Erlotinib
5529:HER1/EGFR
5397:Nivolumab
5387:Naxitamab
5212:Rituximab
5060:Cetuximab
5055:HER1/EGFR
4655:248695128
4598:235710318
2282:27 August
2111:(1): 28.
2105:Pathogens
1688:Drugs.com
1633:7 January
1321:Economics
1215:apoptosis
1103:Pulmonary
988:pemphigus
933:off-label
921:TNF-alpha
861:lymphomas
857:leukemias
830:(DLBCL),
780:indicated
738:Genentech
705:pregnancy
626:Rituximab
364:Excretion
353:half-life
345:100% (IV)
202:Routes of
176:Pregnancy
165:Rituximab
124:Monograph
118:Drugs.com
23:Rituximab
7425:Medicine
6868:Chimeric
6778:Igovomab
6181:hedgehog
6143:against
6141:exotoxin
6107:against
6056:Bruton's
5905:Imatinib
5828:(FGFR),
5824:(NTRK),
5709:Axitinib
5648:Axitinib
5604:HER2/neu
5564:Icotinib
5534:Afatinib
5508:("-nib")
5287:Avelumab
5159:lymphoid
5151:lymphoma
5147:Leukemia
5072:HER2/neu
5034:("-mab")
4937:25578468
4888:11250751
4829:Archived
4785:25355180
4750:38256459
4741:10816159
4699:91186383
4691:30934066
4647:35544139
4590:34212816
4549:21878513
4541:17981914
4500:19293085
4465:26915311
4429:34922060
4360:23 April
4354:Archived
4330:30 March
4324:Archived
4294:Archived
4273:29 April
4267:Archived
4211:Archived
4107:24866199
3998:Archived
3825:Archived
3802:Archived
3664:14 March
3636:16705086
3595:34438120
3556:14532151
3507:23613524
3466:30030508
3444:Leukemia
3422:26443686
3363:12826649
3328:34527001
3277:22422012
3242:36291806
3191:25901938
3150:39750214
3142:25809420
3107:15601813
3011:18779415
2970:17065638
2929:14601692
2892:18 April
2845:42218074
2837:15795920
2796:26293834
2753:29 April
2665:24310855
2634:36762194
2626:27756172
2591:21624184
2540:15201414
2491:Archived
2487:DailyMed
2462:29222274
2413:21183282
2360:Archived
2335:28584371
2254:Archived
2224:(2023).
2205:Archived
2163:Archived
2137:29518976
2086:35805200
2078:28306595
2043:19504332
2035:28164324
1992:Archived
1924:Archived
1877:Archived
1873:DailyMed
1836:26 March
1807:26 March
1801:Archived
1777:25 March
1771:Archived
1745:Archived
1692:Archived
1659:Archived
1627:Archived
1595:Archived
1569:26 March
1563:Archived
1559:DailyMed
1532:26 March
1527:DailyMed
1500:26 March
1494:Archived
1490:DailyMed
1368:Research
1354:Ituxredi
1105:toxicity
1099:patients
1097:lymphoma
1079:JC virus
855:such as
810:such as
712:chimeric
616:(verify)
399:DrugBank
228:ATC code
178:category
160:DailyMed
61:Chimeric
5884:bcr-abl
5245:myeloid
4928:4340844
4638:9096596
4390:27 July
4183:27 July
4157:27 July
4131:27 July
4098:4171018
4056:27 July
4030:27 July
3768:9652253
3707:9310469
3547:1766758
3319:8435594
3233:9599435
3210:Cancers
2582:3123966
2453:6142570
2326:5448263
2260:30 June
2128:5874754
1344:of the
1300:(CLL).
1243:History
1046:Severe
984:bullous
974:(ITP),
970:(TTP),
949:anemias
838:(B-AL).
765:B cells
761:antigen
758:surface
723:B cells
630:Rituxan
551:Formula
408:DB00073
315:Rx-only
312:WARNING
283::
244:)
238: (
236:L01FA01
191: C
162::
138:a607038
7411:Portal
7375:WHO-EM
5131:VEGF-A
4935:
4925:
4886:
4879:138676
4876:
4783:
4748:
4738:
4697:
4689:
4653:
4645:
4635:
4596:
4588:
4547:
4539:
4498:
4463:
4427:
4247:5 July
4105:
4095:
3960:5 July
3913:5 July
3864:5 July
3831:9 June
3766:
3705:
3634:
3593:
3554:
3544:
3505:
3464:
3420:
3361:
3326:
3316:
3275:
3240:
3230:
3189:
3148:
3140:
3105:
3009:
2968:
2927:
2843:
2835:
2794:
2719:5 July
2693:22 May
2663:
2632:
2624:
2589:
2579:
2538:
2460:
2450:
2411:
2333:
2323:
2197:
2135:
2125:
2084:
2076:
2041:
2033:
1957:5 July
1665:29 May
1601:1 July
1194:MHC II
1179:(CDC).
929:safety
734:Biogen
642:cancer
475:ChEMBL
464:D02994
309:
296:
285:℞-only
271:
158:
77:Target
56:Source
6721:Mouse
6460:Human
6452:Tumor
6125:ANXA2
6097:Other
5995:MAP2K
5866:(ALK)
5851:c-MET
5703:VEGFR
5638:PDGFR
5634:C-kit
5265:Other
5114:EpCAM
4695:S2CID
4651:S2CID
4594:S2CID
4545:S2CID
4241:(FDA)
4237:U.S.
4207:(FDA)
4203:U.S.
3954:(FDA)
3950:U.S.
3907:(FDA)
3903:U.S.
3858:(FDA)
3854:U.S.
3729:(FDA)
3725:U.S.
3685:Blood
3614:Blood
3485:Blood
3387:(PDF)
3380:(PDF)
3146:S2CID
2886:(PDF)
2863:(PDF)
2841:S2CID
2743:(FDA)
2739:U.S.
2630:S2CID
2159:(FDA)
2155:U.S.
2082:S2CID
2039:S2CID
1830:(emc)
1797:(emc)
1378:acute
795:(CLL)
789:(NHL)
742:Roche
69:human
65:mouse
6240:KRAS
6145:IL-2
6127:and
6109:VEGF
6026:EML4
5685:FLT3
5636:and
5524:ErbB
5250:CD33
5232:CD52
5222:CD30
5182:CD20
5050:ErbB
4933:PMID
4884:PMID
4837:2007
4811:2007
4781:PMID
4746:PMID
4687:PMID
4643:PMID
4586:PMID
4537:PMID
4496:PMID
4461:PMID
4425:PMID
4392:2024
4362:2021
4332:2024
4302:2018
4275:2017
4249:2024
4219:2019
4185:2024
4159:2024
4133:2024
4103:PMID
4077:mAbs
4058:2024
4032:2024
4006:2015
3994:Time
3962:2024
3915:2024
3866:2024
3833:2016
3764:PMID
3703:PMID
3666:2023
3632:PMID
3591:PMID
3552:PMID
3503:PMID
3462:PMID
3418:PMID
3359:PMID
3324:PMID
3273:PMID
3238:PMID
3187:PMID
3138:PMID
3103:PMID
3037:2011
3007:PMID
2966:PMID
2925:PMID
2894:2007
2833:PMID
2792:PMID
2755:2013
2721:2024
2695:2009
2661:PMID
2622:PMID
2587:PMID
2536:PMID
2499:2020
2458:PMID
2436:2017
2409:PMID
2368:2021
2331:PMID
2284:2024
2262:2021
2195:ISBN
2171:2020
2133:PMID
2074:PMID
2031:PMID
2000:2016
1959:2024
1932:2021
1885:2020
1838:2021
1809:2021
1779:2024
1729:2023
1700:2020
1667:2022
1635:2024
1603:2024
1571:2021
1534:2021
1502:2021
1201:CD23
1167:The
1142:CD20
1111:and
1006:and
887:and
859:and
814:and
771:and
736:and
719:CD20
672:and
577:1987
571:1688
565:9874
559:6416
455:KEGG
435:UNII
426:none
335:data
114:AHFS
82:CD20
46:Type
6030:ALK
5930:Src
5805:RET
5779:ALK
5229:),
5219:),
5179:),
5164:CD3
5020:L01
4923:PMC
4915:doi
4874:PMC
4864:doi
4773:doi
4736:PMC
4726:doi
4679:doi
4675:170
4633:PMC
4625:doi
4576:doi
4527:doi
4488:doi
4484:150
4453:doi
4449:172
4417:doi
4093:PMC
4085:doi
3756:doi
3693:doi
3622:doi
3618:108
3583:doi
3579:111
3542:PMC
3534:doi
3493:doi
3489:121
3452:doi
3410:doi
3351:doi
3347:348
3314:PMC
3304:doi
3265:doi
3228:PMC
3218:doi
3177:doi
3173:151
3130:doi
3093:doi
3089:115
3060:doi
2997:doi
2956:doi
2952:355
2917:doi
2875:doi
2823:doi
2782:doi
2653:doi
2614:doi
2577:PMC
2567:doi
2526:doi
2522:350
2448:PMC
2440:doi
2401:doi
2321:PMC
2311:doi
2230:hdl
2123:PMC
2113:doi
2066:doi
2023:doi
1722:FDA
1018:),
982:),
599:.04
597:860
595:143
500:EPA
298:POM
241:WHO
7437::
7388::
7280:/
5862:,
5832:,
5820:,
5692:,
5631::
5526::
5247::
5175:,
5171:,
5161::
5079:,
5052::
5029:CI
5014:/
4972:.
4931:.
4921:.
4911:57
4909:.
4905:.
4882:.
4872:.
4858:.
4854:.
4827:.
4779:.
4769:15
4767:.
4744:.
4734:.
4722:13
4720:.
4716:.
4693:.
4685:.
4673:.
4649:.
4641:.
4631:.
4619:.
4615:.
4592:.
4584:.
4572:28
4570:.
4566:.
4543:.
4535:.
4523:67
4521:.
4517:.
4494:.
4482:.
4459:.
4447:.
4423:.
4413:28
4411:.
4382:.
4364:.
4348:.
4318:.
4235:.
4201:.
4176:.
4149:.
4123:.
4101:.
4091:.
4079:.
4075:.
4048:.
4022:.
3996:.
3992:.
3980:^
3948:.
3933:^
3901:.
3884:^
3852:.
3841:^
3823:.
3819:.
3762:.
3750:.
3723:.
3701:.
3689:90
3687:.
3683:.
3652:.
3630:.
3616:.
3612:.
3589:.
3577:.
3564:^
3550:.
3540:.
3530:62
3528:.
3524:.
3501:.
3487:.
3483:.
3460:.
3448:32
3446:.
3442:.
3430:^
3416:.
3406:97
3404:.
3357:.
3345:.
3322:.
3312:.
3300:12
3298:.
3294:.
3271:.
3261:64
3259:.
3236:.
3226:.
3214:14
3212:.
3208:.
3185:.
3171:.
3167:.
3144:.
3136:.
3126:67
3124:.
3101:.
3087:.
3083:.
3058:.
3054:.
3005:.
2993:65
2991:.
2987:.
2964:.
2950:.
2946:.
2923:.
2913:78
2911:.
2869:.
2839:.
2831:.
2819:78
2817:.
2813:.
2790:.
2778:26
2776:.
2772:.
2737:.
2711:.
2673:^
2659:.
2628:.
2620:.
2610:16
2608:.
2585:.
2575:.
2563:13
2561:.
2557:.
2534:.
2520:.
2516:.
2485:.
2470:^
2456:.
2446:.
2434:.
2430:.
2407:.
2397:37
2395:.
2354:.
2343:^
2329:.
2319:.
2307:62
2305:.
2301:.
2213:^
2203:.
2179:^
2153:.
2131:.
2121:.
2107:.
2103:.
2080:.
2072:.
2062:29
2060:.
2037:.
2029:.
2019:56
2017:.
1967:^
1949:.
1916:.
1893:^
1871:.
1846:^
1828:.
1817:^
1795:.
1763:.
1720:.
1716:.
1686:.
1675:^
1651:.
1619:.
1587:.
1561:.
1557:.
1542:^
1525:.
1510:^
1492:.
1488:.
1473:^
1396:.
1293:.
1286:.
1228:.
1169:Fc
1154:Ca
1026:,
1022:,
1014:,
1002:,
998:,
990:,
966:,
962:,
943:,
939:,
879:,
699:,
668:,
664:,
660:,
656:,
648:,
583:44
320:EU
305:US
292:UK
280:CA
273:S4
267:AU
187:AU
155:US
7413::
7284:)
7276:(
6488:)
6484:(
6435:e
6428:t
6421:v
6152:)
6148:(
6136:)
6132:(
6116:)
6112:(
6028:-
5942:)
5933:(
5688:(
5680:)
5641:(
5596:)
5532:(
5283:)
5279:(
5257:)
5253:(
5239:)
5235:(
5225:(
5185:(
5167:(
5149:/
5138:)
5134:(
5126:)
5117:(
5098:)
5087:)
5083:(
5075:(
5067:)
5058:(
5022:)
5018:(
5004:e
4997:t
4990:v
4939:.
4917::
4890:.
4866::
4860:3
4839:.
4813:.
4787:.
4775::
4752:.
4728::
4701:.
4681::
4657:.
4627::
4621:5
4600:.
4578::
4551:.
4529::
4502:.
4490::
4467:.
4455::
4431:.
4419::
4394:.
4334:.
4304:.
4277:.
4251:.
4221:.
4187:.
4161:.
4135:.
4109:.
4087::
4081:6
4060:.
4034:.
4008:.
3975:.
3964:.
3928:.
3917:.
3879:.
3868:.
3835:.
3770:.
3758::
3752:6
3709:.
3695::
3668:.
3638:.
3624::
3597:.
3585::
3558:.
3536::
3509:.
3495::
3468:.
3454::
3424:.
3412::
3365:.
3353::
3330:.
3306::
3279:.
3267::
3244:.
3220::
3193:.
3179::
3152:.
3132::
3109:.
3095::
3068:.
3062::
3039:.
3013:.
2999::
2972:.
2958::
2931:.
2919::
2896:.
2877::
2871:4
2847:.
2825::
2798:.
2784::
2757:.
2723:.
2697:.
2667:.
2655::
2636:.
2616::
2593:.
2569::
2542:.
2528::
2501:.
2464:.
2442::
2415:.
2403::
2381:.
2370:.
2337:.
2313::
2264:.
2232::
2173:.
2139:.
2115::
2109:7
2088:.
2068::
2045:.
2025::
2002:.
1961:.
1934:.
1887:.
1840:.
1811:.
1781:.
1731:.
1702:.
1669:.
1637:.
1605:.
1573:.
1536:.
1504:.
1210:.
1203:.
1186:.
1010:(
580:S
574:O
568:N
562:H
556:C
502:)
498:(
322::
307::
294::
269::
189::
116:/
71:)
67:/
63:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.