1493:
1911:, where energy for new particles may come from kinetic energy of other particles, or from one or more photons as part of a system that includes other particles besides a photon. Again, neither the relativistic nor the invariant mass of totally closed (that is, isolated) systems changes when new particles are created. However, different inertial observers will disagree on the value of this conserved mass, if it is the relativistic mass (i.e., relativistic mass is conserved but not invariant). However, all observers agree on the value of the conserved mass if the mass being measured is the invariant mass (i.e., invariant mass is both conserved and invariant).
2490:
1900:, changing the inertial frame of observation for a whole closed system has no effect on the measure of invariant mass of the system, which remains both conserved and invariant (unchanging), even for different observers who view the entire system. Invariant mass is a system combination of energy and momentum, which is invariant for any observer, because in any inertial frame, the energies and momenta of the various particles always add to the same quantity (the momentum may be negative, so the addition amounts to a subtraction). The invariant mass is the relativistic mass of the system when viewed in the
133:
1509:
36:
1786:
1642:
careful experiments were performed in which chemical reactions such as rusting were allowed to take place in sealed glass ampoules; it was found that the chemical reaction did not change the weight of the sealed container and its contents. Weighing of gases using scales was not possible until the invention of the
1889:. Rest masses cannot be summed to derive the total mass of a system because this practice does not take into account other forms of energy, such as kinetic energy, potential energy, and the energy of massless particles such as photons. All forms of energy in a system affect the total mass of the system.
1831:
In special relativity, the conservation of mass does not apply if the system is open and energy escapes. However, it does continue to apply to totally closed (isolated) systems. If energy cannot escape a system, its mass cannot decrease. In relativity theory, so long as any type of energy is retained
1980:
The formula implies that bound systems have an invariant mass (rest mass for the system) less than the sum of their parts, if the binding energy has been allowed to escape the system after the system has been bound. This may happen by converting system potential energy into some other kind of active
1641:
The conservation of mass was obscure for millennia because of the buoyancy effect of the Earth's atmosphere on the weight of gases. For example, a piece of wood weighs less after burning; this seemed to suggest that some of its mass disappears, or is transformed or lost. This was not disproved until
1985:
in bound systems – in other words, the energy needed to break the system apart. The greater the mass defect, the larger the binding energy. The binding energy (which itself has mass) must be released (as light or heat) when the parts combine to form the bound system, and this is the reason the mass
1860:
The change in mass of certain kinds of open systems where atoms or massive particles are not allowed to escape, but other types of energy (such as light or heat) are allowed to enter, escape or be merged, went unnoticed during the 19th century, because the change in mass associated with addition or
1892:
For moving massive particles in a system, examining the rest masses of the various particles also amounts to introducing many different inertial observation frames, which is prohibited if total system energy and momentum are to be conserved. Additionally, in the rest frame of any one particle this
1596:
By the 18th century the principle of conservation of mass during chemical reactions was widely used and was an important assumption during experiments, even before a definition was widely established, though an expression of the law can be dated back to Hero of
Alexandria’s time, as can be seen in
1181:
The interpretation of the continuity equation for mass is the following: For a given closed surface in the system, the change, over any time interval, of the mass enclosed by the surface is equal to the mass that traverses the surface during that time interval: positive if the matter goes in and
1665:
supported the consistency of this law in chemical reactions, even though they were carried out with other intentions. His research indicated that in certain reactions the loss or gain could not have been more than 2 to 4 parts in 100,000. The difference in the accuracy aimed at and attained by
1695:
pointed out, a change in mass as a result of extraction or addition of chemical energy, as predicted by
Einstein's theory, is so small that it could not be measured with the available instruments and could not be presented as a test of special relativity. Einstein speculated that the energies
1843:
be perfectly conserved in isolated systems, even though mass is always conserved in such systems. However, matter is so nearly conserved in chemistry that violations of matter conservation were not measured until the nuclear age, and the assumption of matter conservation remains an important
1653:
to modern chemistry. Once early chemists realized that chemical substances never disappeared but were only transformed into other substances with the same weight, these scientists could for the first time embark on quantitative studies of the transformations of substances. The idea of mass
1700:
were significant enough, compared with the mass of systems producing them, to enable their change of mass to be measured, once the energy of the reaction had been removed from the system. This later indeed proved to be possible, although it was eventually to be the first artificial
1621:
The universal law was formulated by
Lomonosov on the basis of general philosophical materialistic considerations, it was never questioned or tested by him, but on the contrary, served him as a solid starting position in all research throughout his life.
1285:
1976:
This form of the equation in terms of changes was the form in which it was originally presented by
Einstein. In this sense, mass changes in any system are explained if the mass of the energy added or removed from the system is taken into account.
1877:
implies the viewpoint of a single observer (or the view from a single inertial frame) since changing inertial frames may result in a change of the total energy (relativistic energy) for systems, and this quantity determines the relativistic mass.
1686:
in 1905, he suggested an equivalence between mass and energy. This theory implied several assertions, like the idea that internal energy of a system could contribute to the mass of the whole system, or that mass could be converted into
1573:", so that what exists now has always existed: no new matter can come into existence where there was none before. An explicit statement of this, along with the further principle that nothing can pass away into nothing, is found in
1079:
902:
has to be taken into account; thus mass–energy conservation becomes a more complex concept, subject to different definitions, and neither mass nor energy is as strictly and simply conserved as is the case in special relativity.
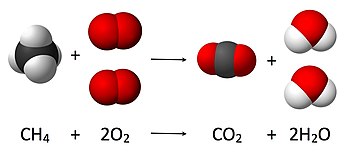
1346:, is founded on the principle of conservation of mass. The principle implies that during a chemical reaction the total mass of the reactants is equal to the total mass of the products. For example, in the following reaction
1762:. Special relativity also redefines the concept of mass and energy, which can be used interchangeably and are defined relative to the frame of reference. Several quantities had to be defined for consistency, such as the
1926:
will escape also. In this case, the mass–energy equivalence formula predicts that the change in mass of a system is associated with the change in its energy due to energy being added or subtracted:
1205:
1658:, as well as the idea that all chemical processes and transformations (such as burning and metabolic reactions) are reactions between invariant amounts or weights of these chemical elements.
1986:
of the bound system decreases when the energy leaves the system. The total invariant mass is actually conserved, when the mass of the binding energy that has escaped, is taken into account.
1473:
atoms, 4 oxygen atoms and one carbon atom are present (as well as in the final state); thus the number water molecules produced must be exactly two per molecule of carbon dioxide produced.
205:
1974:
802:
The law implies that mass can neither be created nor destroyed, although it may be rearranged in space, or the entities associated with it may be changed in form. For example, in
140:. Where 4 atoms of hydrogen, 4 atoms of oxygen, and 1 of carbon are present before and after the reaction. The total mass after the reaction is the same as before the reaction.
1015:
1865:
in chemical reactions is very small. (In theory, mass would not change at all for experiments conducted in isolated systems where heat and work were not allowed in or out.)
1581:
4th century BCE): "For it is impossible for anything to come to be from what is not, and it cannot be brought about or heard of that what is should be utterly destroyed."
1146:
1172:
1759:
1310:
806:, the mass of the chemical components before the reaction is equal to the mass of the components after the reaction. Thus, during any chemical reaction and low-energy
943:
1654:
conservation plus a surmise that certain "elemental substances" also could not be transformed into others by chemical reactions, in turn led to an understanding of
860:, which states that energy and mass form one conserved quantity. For very energetic systems the conservation of mass only is shown not to hold, as is the case in
1099:
987:
963:
754:
1588:
around the 3rd century BCE, who wrote in describing the nature of the
Universe that "the totality of things was always such as it is now, and always will be".
1200:
1123:
1630:
who expressed his conclusion in 1773 and popularized the principle of conservation of mass. The demonstrations of the principle disproved the then popular
2008:
of such an expansion. The conservation of both mass and energy therefore depends on various corrections made to energy in the theory, due to the changing
1981:
energy, such as kinetic energy or photons, which easily escape a bound system. The difference in system masses, called a mass defect, is a measure of the
2403:
2287:
2262:
2218:
2176:
2146:
2112:
53:
2183:
1492:
2104:
1328:
2600:
2357:
1469:). The number of molecules resulting from the reaction can be derived from the principle of conservation of mass, as initially four
747:
119:
2472:Закон сохранения массы при химических реакциях и физические воззрения Ломоносова // Ломоносов М.В. Сборник статей и материалов, T.5
2419:
Pomper, Philip (October 1962). "Lomonosov and the
Discovery of the Law of the Conservation of Matter in Chemical Transformations".
1893:
procedure ignores the momenta of other particles, which affect the system mass if the other particles are in motion in this frame.
100:
2153:
1324:
72:
2347:
2119:
829:. Historically, mass conservation in chemical reactions was primarily demonstrated in the 17th century and finally confirmed by
1613:
in 1756. He may have demonstrated it by experiments and certainly had discussed the principle in 1748 in correspondence with
720:
57:
421:
258:
79:
1531:
branched from the discoveries of
Antoine Lavoisier. Lavoisier's quantitative experiments revealed that combustion involved
2378:
1313:
876:
740:
461:
347:
2061:
1717:
857:
416:
2041:
2036:
1548:
1520:
325:
86:
2031:
208:
1562:(2nd century CE) states that a substance is permanent, but its modes are characterised by creation and destruction.
2622:
2254:
1995:
1826:
1706:
844:
In reality, the conservation of mass only holds approximately and is considered part of a series of assumptions in
2085:
1844:
practical concept in most systems in chemistry and other studies that do not involve the high energies typical of
46:
1688:
1480:
problems are solved by following the mass distribution of a given system over time; this methodology is known as
892:
332:
161:
68:
2455:
1901:
1566:
627:
622:
237:
1929:
411:
404:
1280:{\displaystyle {\frac {{\text{d}}M}{{\text{d}}t}}={\frac {\text{d}}{{\text{d}}t}}\int \rho \,{\text{d}}V=0,}
690:
685:
354:
1617:, though his claim on the subject is sometimes challenged. According to the Soviet physicist Yakov Dorfman:
1182:
negative if the matter goes out. For the whole isolated system, this condition implies that the total mass
1713:
1679:
242:
1907:
The conservation of both relativistic and invariant mass applies even to systems of particles created by
2280:
The
Hellenistic Philosophers. Vol 1: Translations of the principal sources with philosophical commentary
1702:
1009:
807:
665:
283:
2312:
2009:
1606:
879:. Such is the case when any energy or matter is allowed into, or out of, the system. However, unless
833:
in the late 18th century. The formulation of this law was of crucial importance in the progress from
503:
320:
300:
288:
1678:
The law of conservation of mass was challenged with the advent of special relativity. In one of the
2021:
1709:, that proved the first successful test of Einstein's theory regarding mass loss with energy gain.
1555:, stated that the universe and its constituents such as matter cannot be destroyed or created. The
1339:
1005:
1001:
912:
845:
705:
553:
446:
152:
1904:. It is the minimum mass which a system may exhibit, as viewed from all possible inertial frames.
132:
93:
2451:
1886:
1570:
966:
899:
853:
725:
359:
315:
310:
1128:
2596:
2399:
2353:
2328:
2283:
2258:
2214:
2172:
2142:
2108:
1919:
1874:
1728:
1631:
1627:
1610:
1536:
1515:'s discovery of the law of conservation of mass led to many new findings in the 19th century.
1512:
1501:
1497:
1155:
883:
or nuclear reactions are involved, the amount of energy entering or escaping such systems (as
880:
849:
830:
803:
342:
293:
2393:
2200:
1290:
2428:
2320:
2138:
2026:
1849:
1655:
918:
869:
861:
680:
655:
568:
543:
538:
493:
2205:. book series, Library of Religious Beliefs and Practices, edited by John R. Hinnels &
1508:
1915:
1908:
1721:
1683:
1602:
1559:
1544:
997:
838:
670:
594:
508:
439:
373:
275:
1202:, the sum of the masses of all components in the system, does not change over time, i.e.
558:
428:
2576:
2558:
2470:
2316:
1074:{\displaystyle {\frac {\partial \rho }{\partial t}}+\nabla \cdot (\rho \mathbf {v} )=0,}
2001:
1982:
1923:
1897:
1881:
The principle that the mass of a system of particles must be equal to the sum of their
1862:
1764:
1614:
1437:
1084:
990:
972:
948:
888:
826:
675:
533:
498:
399:
305:
1649:
Once understood, the conservation of mass was of great importance in progressing from
915:, in which the energy scales associated with an isolated system are much smaller than
2617:
2611:
2238:
Udaipur:Sri Tarak Guru Jain Gran. p.57. Also see
Tattvarthasutra verses 5.29 and 5.37
1845:
1697:
1667:
1528:
1516:
1343:
1175:
784:
715:
548:
2303:
Whitaker, Robert D. (1975-10-01). "An historical note on the conservation of mass".
1500:
formulated the law of mass conservation in 1756 and came to the conclusion that the
2580:: an account of its method and historical development, with illustrative quotations
2206:
1598:
1481:
1185:
1108:
865:
700:
695:
660:
392:
2278:
Long, A. A.; D. N. Sedley (1987). "Epicureanism: The principals of conservation".
2542:
2515:
2166:
2132:
2098:
17:
2196:
1643:
1524:
1477:
710:
613:
35:
2572:
2432:
1692:
1635:
1574:
1149:
632:
528:
2332:
895:) is usually too small to be measured as a change in the mass of the system.
2210:
2005:
1882:
1662:
1556:
822:
818:
772:
604:
599:
433:
2171:(illustrated ed.). Springer Science & Business Media. p. 29.
1856:
The mass associated with chemical amounts of energy is too small to measure
1661:
Following the pioneering work of
Lavoisier, the exhaustive experiments of
1585:
1552:
1470:
1401:
1331:
describe the conservation and flow of mass and matter in a given system.
1317:
583:
488:
468:
454:
1914:
The mass–energy equivalence formula gives a different prediction in non-
1650:
1405:
1335:
1102:
834:
817:
The concept of mass conservation is widely used in many fields such as
811:
768:
337:
137:
2324:
2004:
of photons in an expanding volume of space will decrease, due to the
1836:
1772:(in another frame). The latter term is usually less frequently used.
1532:
1421:
792:
788:
478:
814:, or starting materials, must be equal to the mass of the products.
2373:
1507:
1491:
1453:
382:
1004:, where the conservation of mass is usually expressed using the
884:
796:
1768:
of a particle (mass in the rest frame of the particle) and the
1626:
A more refined series of experiments were later carried out by
965:
is the mass of a typical object in the system, measured in the
1779:
518:
29:
2395:
The Swings of Science: From Complexity to Simplicity and Back
1716:
were finally generalized and unified into the principle of
27:
Scientific law that a closed system's mass remains constant
996:
The law can be formulated mathematically in the fields of
911:
The law of conservation of mass can only be formulated in
2282:. Cambridge: Cambridge University Press. pp. 25–26.
2234:
Devendra (Muni.), T. G. Kalghatgi, T. S. Devadoss (1983)
1712:
The law of conservation of mass and the analogous law of
848:. The law has to be modified to comply with the laws of
2530:
Nouv. Recherches sur les lois des proportions chimiques
2448:
Mikhail Vasil'evich Lomonosov on the Corpuscular Theory
1869:
Mass conservation remains correct if energy is not lost
1800:
1726:
1918:, since if energy is allowed to escape a system, both
1293:
1188:
1158:
1131:
1111:
1087:
1932:
1731:
1208:
1018:
975:
951:
921:
898:
For systems that include large gravitational fields,
164:
2249:
Kirk, G. S.; J. E. Raven; Malcolm Schofield (1983).
1885:, though true in classical physics, may be false in
1323:
The continuity equation for the mass is part of the
2491:"3.7 Conservation of Mass - There is No New Matter"
60:. Unsourced material may be challenged and removed.
2060:Sterner, R. W.; Small, G. E.; Hood, J. M. (2011).
1968:
1753:
1584:A further principle of conservation was stated by
1304:
1279:
1194:
1166:
1140:
1117:
1093:
1073:
981:
957:
937:
199:
1609:. One of the first to outline the principle was
2168:Early Russian Organic Chemists and Their Legacy
1634:that said that mass could be gained or lost in
1334:In chemistry, the calculation of the amount of
2374:An Historical Note on the Conservation of Mass
1535:rather than what was previously thought to be
799:of the system must remain constant over time.
810:in an isolated system, the total mass of the
748:
8:
1832:within a system, this energy exhibits mass.
2475:. М.-Л.: Издательство АН СССР. p. 193.
200:{\displaystyle J=-D{\frac {d\varphi }{dx}}}
2541:"Conservation of Mass in Chemical Changes"
755:
741:
588:
378:
221:
143:
2548:, Part 2 Chemical Society (Great Britain)
1957:
1948:
1931:
1745:
1730:
1294:
1292:
1260:
1259:
1242:
1236:
1222:
1212:
1209:
1207:
1187:
1159:
1157:
1130:
1110:
1086:
1054:
1019:
1017:
974:
950:
929:
920:
177:
163:
120:Learn how and when to remove this message
2194:Mahavira is dated 598 BC - 526 BC. See:
1969:{\displaystyle \Delta m=\Delta E/c^{2}.}
875:Mass is also not generally conserved in
131:
2446:Lomonosov, Mikhail Vasil’evich (1970).
2052:
1861:loss of small quantities of thermal or
1835:Also, mass must be differentiated from
612:
567:
517:
477:
381:
250:
224:
151:
2484:
2482:
2105:Springer Science & Business Media
1320:over the whole volume of the system.
7:
2489:Agnew, Henry; Alviar-Agnew, Marisa.
2134:Energy and Mass in Relativity Theory
1896:For the special type of mass called
1670:and Stas on the other, is enormous.
58:adding citations to reliable sources
2349:Rheology: An Historical Perspective
2346:Tanner, R. I.; Walters, K. (1998).
1436:are converted into one molecule of
2544:Journal - Chemical Society, London
1942:
1933:
1705:reaction in 1932, demonstrated by
1666:Lavoisier on the one hand, and by
1132:
1042:
1030:
1022:
25:
2578:The study of Chemical Composition
2514:Matthew Moncrieff Pattison Muir,
2236:A source-book in Jaina philosophy
2000:In general relativity, the total
1696:associated with newly discovered
969:where the object is at rest, and
2097:Volkenstein, Mikhail V. (2009).
1784:
1160:
1055:
34:
45:needs additional citations for
2247:Fr. 12; see pp.291–2 of
2010:gravitational potential energy
1329:convection–diffusion equations
1327:of fluid dynamics. Many other
1059:
1048:
781:principle of mass conservation
1:
2560:A Course in General Chemistry
2379:Journal of Chemical Education
2305:Journal of Chemical Education
2454:(trans.). Cambridge, Mass.:
2251:The Presocratic Philosophers
2131:Okuň, Lev Borisovič (2009).
2557:William Edwards Henderson,
2458:. Introduction, p. 25.
2042:Law of multiple proportions
2037:Law of definite proportions
1795:may have misleading content
1521:law of definite proportions
1342:in a chemical reaction, or
777:law of conservation of mass
136:The Combustion reaction of
2639:
2255:Cambridge University Press
2062:"The Conservation of Mass"
1996:Mass in general relativity
1993:
1827:Mass in special relativity
1824:
1571:Nothing comes from nothing
1551:based on the teachings of
1549:non-creationist philosophy
1141:{\textstyle \nabla \cdot }
864:and particle-antiparticle
2517:The Elements of Chemistry
2433:10.1179/amb.1962.10.3.119
2382:, 52, 10, 658-659, Oct 75
2253:(2 ed.). Cambridge:
1689:electromagnetic radiation
1167:{\textstyle \mathbf {v} }
893:electromagnetic radiation
2456:Harvard University Press
2398:. Springer. p. 41.
2103:(illustrated ed.).
2032:Fick's laws of diffusion
1902:center of momentum frame
1754:{\displaystyle E=mc^{2}}
1592:Discoveries in chemistry
1567:ancient Greek philosophy
1305:{\textstyle {\text{d}}V}
1105:(mass per unit volume),
907:Formulation and examples
259:Clausius–Duhem (entropy)
209:Fick's laws of diffusion
2100:Entropy and Information
1718:mass–energy equivalence
858:mass–energy equivalence
856:under the principle of
808:thermodynamic processes
417:Navier–Stokes equations
355:Material failure theory
2593:Astrophysical Formulae
2469:Дорфман, Яков (1961).
1970:
1755:
1714:conservation of energy
1680:Annus Mirabilis papers
1624:
1540:
1505:
1306:
1281:
1196:
1168:
1142:
1119:
1095:
1075:
983:
959:
939:
938:{\displaystyle mc^{2}}
201:
141:
69:"Conservation of mass"
2495:LibreTexts™ Chemistry
2372:Robert D. Whitaker, "
2165:Lewis, David (2012).
1971:
1756:
1703:nuclear transmutation
1646:in the 17th century.
1619:
1565:An important idea in
1543:As early as 520 BCE,
1511:
1495:
1307:
1282:
1197:
1169:
1143:
1120:
1096:
1076:
984:
960:
940:
412:Bernoulli's principle
405:Archimedes' principle
202:
135:
2532:(1865) 152, 171, 189
2392:Pismen, Len (2018).
1930:
1873:The conservation of
1729:
1707:Cockcroft and Walton
1638:and heat processes.
1291:
1206:
1186:
1156:
1129:
1109:
1085:
1016:
973:
949:
919:
787:to all transfers of
783:states that for any
504:Cohesion (chemistry)
326:Infinitesimal strain
162:
54:improve this article
2595:, Springer (1999),
2317:1975JChEd..52..658W
2154:Extract of page 253
2022:Charge conservation
1839:, since matter may
1801:clarify the content
1006:continuity equation
1002:continuum mechanics
913:classical mechanics
846:classical mechanics
422:Poiseuille equation
153:Continuum mechanics
147:Part of a series on
2452:Henry M. Leicester
2184:Extract of page 29
2120:Extract of page 20
2086:Lavoisier's Method
1990:General relativity
1966:
1887:special relativity
1821:Special relativity
1751:
1541:
1506:
1496:Russian scientist
1302:
1277:
1192:
1164:
1138:
1115:
1094:{\textstyle \rho }
1091:
1071:
979:
967:frame of reference
955:
935:
900:general relativity
854:special relativity
804:chemical reactions
628:Magnetorheological
623:Electrorheological
360:Fracture mechanics
197:
142:
2623:Conservation laws
2591:Kenneth R. Lang,
2405:978-3-319-99777-3
2325:10.1021/ed052p658
2289:978-0-521-27556-9
2264:978-0-521-27455-5
2220:978-0-415-26606-2
2178:978-3-642-28219-5
2148:978-981-281-412-8
2114:978-3-0346-0078-1
2012:of such systems.
1920:relativistic mass
1875:relativistic mass
1850:nuclear reactions
1818:
1817:
1770:relativistic mass
1656:chemical elements
1632:phlogiston theory
1628:Antoine Lavoisier
1611:Mikhail Lomonosov
1513:Antoine Lavoisier
1502:phlogiston theory
1498:Mikhail Lomonosov
1316:that defines the
1297:
1263:
1251:
1245:
1240:
1231:
1225:
1215:
1037:
1010:differential form
982:{\displaystyle c}
958:{\displaystyle m}
862:nuclear reactions
850:quantum mechanics
831:Antoine Lavoisier
765:
764:
640:
639:
574:
573:
343:Contact mechanics
266:
265:
195:
130:
129:
122:
104:
18:Mass conservation
16:(Redirected from
2630:
2603:
2589:
2583:
2570:
2564:
2555:
2549:
2539:
2533:
2527:
2521:
2512:
2506:
2505:
2503:
2501:
2486:
2477:
2476:
2466:
2460:
2459:
2443:
2437:
2436:
2416:
2410:
2409:
2389:
2383:
2370:
2364:
2363:
2343:
2337:
2336:
2300:
2294:
2293:
2275:
2269:
2268:
2245:
2239:
2232:
2226:
2224:
2192:
2186:
2182:
2162:
2156:
2152:
2139:World Scientific
2128:
2122:
2118:
2094:
2088:
2083:
2077:
2076:
2074:
2072:
2057:
2027:Conservation law
1975:
1973:
1972:
1967:
1962:
1961:
1952:
1916:isolated systems
1813:
1810:
1804:
1788:
1787:
1780:
1761:
1758:
1757:
1752:
1750:
1749:
1580:
1468:
1466:
1465:
1451:
1450:
1449:
1435:
1434:
1433:
1419:
1418:
1417:
1396:
1394:
1393:
1383:
1382:
1381:
1371:
1370:
1369:
1359:
1358:
1357:
1311:
1309:
1308:
1303:
1298:
1295:
1286:
1284:
1283:
1278:
1264:
1261:
1252:
1250:
1246:
1243:
1238:
1237:
1232:
1230:
1226:
1223:
1220:
1216:
1213:
1210:
1201:
1199:
1198:
1193:
1173:
1171:
1170:
1165:
1163:
1147:
1145:
1144:
1139:
1124:
1122:
1121:
1116:
1100:
1098:
1097:
1092:
1080:
1078:
1077:
1072:
1058:
1038:
1036:
1028:
1020:
988:
986:
985:
980:
964:
962:
961:
956:
944:
942:
941:
936:
934:
933:
870:particle physics
757:
750:
743:
589:
554:Gay-Lussac's law
544:Combined gas law
494:Capillary action
379:
222:
206:
204:
203:
198:
196:
194:
186:
178:
144:
125:
118:
114:
111:
105:
103:
62:
38:
30:
21:
2638:
2637:
2633:
2632:
2631:
2629:
2628:
2627:
2608:
2607:
2606:
2590:
2586:
2571:
2567:
2556:
2552:
2540:
2536:
2528:
2524:
2513:
2509:
2499:
2497:
2488:
2487:
2480:
2468:
2467:
2463:
2445:
2444:
2440:
2418:
2417:
2413:
2406:
2391:
2390:
2386:
2371:
2367:
2360:
2345:
2344:
2340:
2302:
2301:
2297:
2290:
2277:
2276:
2272:
2265:
2248:
2246:
2242:
2233:
2229:
2221:
2195:
2193:
2189:
2179:
2164:
2163:
2159:
2149:
2141:. p. 253.
2130:
2129:
2125:
2115:
2096:
2095:
2091:
2084:
2080:
2070:
2068:
2059:
2058:
2054:
2050:
2018:
1998:
1992:
1953:
1928:
1927:
1909:pair production
1871:
1858:
1829:
1823:
1814:
1808:
1805:
1798:
1789:
1785:
1778:
1741:
1727:
1725:
1722:Albert Einstein
1720:, described by
1684:Albert Einstein
1676:
1603:Henry Cavendish
1594:
1578:
1560:Tattvarthasutra
1545:Jain philosophy
1490:
1464:
1461:
1460:
1459:
1457:
1448:
1445:
1444:
1443:
1441:
1432:
1429:
1428:
1427:
1425:
1416:
1413:
1412:
1411:
1409:
1398:
1392:
1389:
1388:
1387:
1385:
1380:
1377:
1376:
1375:
1373:
1368:
1365:
1364:
1363:
1361:
1356:
1353:
1352:
1351:
1349:
1325:Euler equations
1289:
1288:
1241:
1221:
1211:
1204:
1203:
1184:
1183:
1154:
1153:
1127:
1126:
1107:
1106:
1083:
1082:
1029:
1021:
1014:
1013:
998:fluid mechanics
971:
970:
947:
946:
925:
917:
916:
909:
889:mechanical work
839:natural science
761:
732:
731:
730:
650:
642:
641:
595:Viscoelasticity
586:
576:
575:
563:
513:
509:Surface tension
473:
376:
374:Fluid mechanics
366:
365:
364:
278:
276:Solid mechanics
268:
267:
219:
211:
187:
179:
160:
159:
126:
115:
109:
106:
63:
61:
51:
39:
28:
23:
22:
15:
12:
11:
5:
2636:
2634:
2626:
2625:
2620:
2610:
2609:
2605:
2604:
2584:
2565:
2550:
2534:
2522:
2507:
2478:
2461:
2438:
2427:(3): 119–127.
2411:
2404:
2384:
2365:
2358:
2338:
2295:
2288:
2270:
2263:
2240:
2227:
2219:
2187:
2177:
2157:
2147:
2123:
2113:
2107:. p. 20.
2089:
2078:
2051:
2049:
2046:
2045:
2044:
2039:
2034:
2029:
2024:
2017:
2014:
2002:invariant mass
1994:Main article:
1991:
1988:
1983:binding energy
1965:
1960:
1956:
1951:
1947:
1944:
1941:
1938:
1935:
1924:invariant mass
1898:invariant mass
1870:
1867:
1863:radiant energy
1857:
1854:
1825:Main article:
1822:
1819:
1816:
1815:
1792:
1790:
1783:
1777:
1776:Generalization
1774:
1748:
1744:
1740:
1737:
1734:
1691:. However, as
1675:
1674:Modern physics
1672:
1615:Leonhard Euler
1593:
1590:
1489:
1486:
1462:
1446:
1438:carbon dioxide
1430:
1414:
1390:
1378:
1366:
1354:
1348:
1301:
1276:
1273:
1270:
1267:
1258:
1255:
1249:
1235:
1229:
1219:
1195:{\textstyle M}
1191:
1162:
1137:
1134:
1118:{\textstyle t}
1114:
1090:
1070:
1067:
1064:
1061:
1057:
1053:
1050:
1047:
1044:
1041:
1035:
1032:
1027:
1024:
991:speed of light
978:
954:
932:
928:
924:
908:
905:
841:of chemistry.
837:to the modern
827:fluid dynamics
763:
762:
760:
759:
752:
745:
737:
734:
733:
729:
728:
723:
718:
713:
708:
703:
698:
693:
688:
683:
678:
673:
668:
663:
658:
652:
651:
648:
647:
644:
643:
638:
637:
636:
635:
630:
625:
617:
616:
610:
609:
608:
607:
602:
597:
587:
582:
581:
578:
577:
572:
571:
565:
564:
562:
561:
556:
551:
546:
541:
536:
531:
525:
522:
521:
515:
514:
512:
511:
506:
501:
499:Chromatography
496:
491:
485:
482:
481:
475:
474:
472:
471:
452:
451:
450:
431:
419:
414:
402:
389:
386:
385:
377:
372:
371:
368:
367:
363:
362:
357:
352:
351:
350:
340:
335:
330:
329:
328:
323:
313:
308:
303:
298:
297:
296:
286:
280:
279:
274:
273:
270:
269:
264:
263:
262:
261:
253:
252:
248:
247:
246:
245:
240:
235:
227:
226:
220:
217:
216:
213:
212:
207:
193:
190:
185:
182:
176:
173:
170:
167:
156:
155:
149:
148:
128:
127:
42:
40:
33:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2635:
2624:
2621:
2619:
2616:
2615:
2613:
2602:
2601:3-540-29692-1
2598:
2594:
2588:
2585:
2581:
2579:
2574:
2569:
2566:
2562:
2561:
2554:
2551:
2547:
2545:
2538:
2535:
2531:
2526:
2523:
2519:
2518:
2511:
2508:
2496:
2492:
2485:
2483:
2479:
2474:
2473:
2465:
2462:
2457:
2453:
2449:
2442:
2439:
2434:
2430:
2426:
2422:
2415:
2412:
2407:
2401:
2397:
2396:
2388:
2385:
2381:
2380:
2375:
2369:
2366:
2361:
2359:9780444829467
2355:
2351:
2350:
2342:
2339:
2334:
2330:
2326:
2322:
2318:
2314:
2310:
2306:
2299:
2296:
2291:
2285:
2281:
2274:
2271:
2266:
2260:
2256:
2252:
2244:
2241:
2237:
2231:
2228:
2222:
2216:
2212:
2208:
2204:
2203:
2198:
2191:
2188:
2185:
2180:
2174:
2170:
2169:
2161:
2158:
2155:
2150:
2144:
2140:
2136:
2135:
2127:
2124:
2121:
2116:
2110:
2106:
2102:
2101:
2093:
2090:
2087:
2082:
2079:
2067:
2063:
2056:
2053:
2047:
2043:
2040:
2038:
2035:
2033:
2030:
2028:
2025:
2023:
2020:
2019:
2015:
2013:
2011:
2007:
2003:
1997:
1989:
1987:
1984:
1978:
1963:
1958:
1954:
1949:
1945:
1939:
1936:
1925:
1921:
1917:
1912:
1910:
1905:
1903:
1899:
1894:
1890:
1888:
1884:
1879:
1876:
1868:
1866:
1864:
1855:
1853:
1851:
1847:
1846:radioactivity
1842:
1838:
1833:
1828:
1820:
1812:
1809:December 2017
1802:
1796:
1793:This article
1791:
1782:
1781:
1775:
1773:
1771:
1767:
1766:
1760:
1746:
1742:
1738:
1735:
1732:
1723:
1719:
1715:
1710:
1708:
1704:
1699:
1698:radioactivity
1694:
1690:
1685:
1681:
1673:
1671:
1669:
1664:
1659:
1657:
1652:
1647:
1645:
1639:
1637:
1633:
1629:
1623:
1618:
1616:
1612:
1608:
1604:
1600:
1597:the works of
1591:
1589:
1587:
1582:
1576:
1572:
1568:
1563:
1561:
1558:
1554:
1550:
1546:
1538:
1534:
1530:
1529:atomic theory
1526:
1522:
1518:
1517:Joseph Proust
1514:
1510:
1504:is incorrect.
1503:
1499:
1494:
1487:
1485:
1483:
1479:
1474:
1472:
1455:
1452:) and two of
1439:
1423:
1407:
1403:
1347:
1345:
1344:stoichiometry
1341:
1337:
1332:
1330:
1326:
1321:
1319:
1315:
1299:
1274:
1271:
1268:
1265:
1256:
1253:
1247:
1233:
1227:
1217:
1189:
1179:
1177:
1176:flow velocity
1151:
1135:
1125:is the time,
1112:
1104:
1088:
1068:
1065:
1062:
1051:
1045:
1039:
1033:
1025:
1011:
1007:
1003:
999:
994:
992:
976:
968:
952:
930:
926:
922:
914:
906:
904:
901:
896:
894:
890:
886:
882:
881:radioactivity
878:
873:
871:
867:
863:
859:
855:
851:
847:
842:
840:
836:
832:
828:
824:
820:
815:
813:
809:
805:
800:
798:
794:
790:
786:
785:system closed
782:
778:
774:
770:
758:
753:
751:
746:
744:
739:
738:
736:
735:
727:
724:
722:
719:
717:
714:
712:
709:
707:
704:
702:
699:
697:
694:
692:
689:
687:
684:
682:
679:
677:
674:
672:
669:
667:
664:
662:
659:
657:
654:
653:
646:
645:
634:
631:
629:
626:
624:
621:
620:
619:
618:
615:
611:
606:
603:
601:
598:
596:
593:
592:
591:
590:
585:
580:
579:
570:
566:
560:
557:
555:
552:
550:
547:
545:
542:
540:
539:Charles's law
537:
535:
532:
530:
527:
526:
524:
523:
520:
516:
510:
507:
505:
502:
500:
497:
495:
492:
490:
487:
486:
484:
483:
480:
476:
470:
467:
463:
460:
456:
453:
448:
447:non-Newtonian
445:
441:
437:
436:
435:
432:
430:
427:
423:
420:
418:
415:
413:
410:
406:
403:
401:
398:
394:
391:
390:
388:
387:
384:
380:
375:
370:
369:
361:
358:
356:
353:
349:
346:
345:
344:
341:
339:
336:
334:
333:Compatibility
331:
327:
324:
322:
321:Finite strain
319:
318:
317:
314:
312:
309:
307:
304:
302:
299:
295:
292:
291:
290:
287:
285:
282:
281:
277:
272:
271:
260:
257:
256:
255:
254:
249:
244:
241:
239:
236:
234:
231:
230:
229:
228:
225:Conservations
223:
215:
214:
210:
191:
188:
183:
180:
174:
171:
168:
165:
158:
157:
154:
150:
146:
145:
139:
134:
124:
121:
113:
102:
99:
95:
92:
88:
85:
81:
78:
74:
71: –
70:
66:
65:Find sources:
59:
55:
49:
48:
43:This article
41:
37:
32:
31:
19:
2592:
2587:
2577:
2568:
2559:
2553:
2543:
2537:
2529:
2525:
2516:
2510:
2498:. Retrieved
2494:
2471:
2464:
2447:
2441:
2424:
2420:
2414:
2394:
2387:
2377:
2368:
2352:. Elsevier.
2348:
2341:
2308:
2304:
2298:
2279:
2273:
2250:
2243:
2235:
2230:
2207:Ninian Smart
2201:
2197:Dundas, Paul
2190:
2167:
2160:
2133:
2126:
2099:
2092:
2081:
2069:. Retrieved
2065:
2055:
1999:
1979:
1913:
1906:
1895:
1891:
1880:
1872:
1859:
1840:
1834:
1830:
1806:
1799:Please help
1794:
1769:
1763:
1724:'s equation
1711:
1677:
1660:
1648:
1640:
1625:
1620:
1599:Joseph Black
1595:
1583:
1564:
1542:
1482:mass balance
1475:
1399:
1333:
1322:
1314:differential
1180:
995:
910:
897:
877:open systems
874:
866:annihilation
843:
816:
801:
780:
776:
766:
614:Smart fluids
559:Graham's law
465:
458:
443:
429:Pascal's law
425:
408:
396:
251:Inequalities
232:
116:
107:
97:
90:
83:
76:
64:
52:Please help
47:verification
44:
2311:(10): 658.
1883:rest masses
1644:vacuum pump
1525:John Dalton
1478:engineering
1008:, given in
633:Ferrofluids
534:Boyle's law
306:Hooke's law
284:Deformation
2612:Categories
2573:Ida Freund
2500:10 January
2209:. London:
2071:21 October
2048:References
1693:Max Planck
1636:combustion
1575:Empedocles
1569:was that "
1537:phlogiston
1424:molecules
1420:) and two
1400:where one
1150:divergence
686:Gay-Lussac
649:Scientists
549:Fick's law
529:Atmosphere
348:frictional
301:Plasticity
289:Elasticity
80:newspapers
2333:0021-9584
2211:Routledge
2202:The Jains
2006:red shift
1943:Δ
1934:Δ
1765:rest mass
1663:Jean Stas
1557:Jain text
1384:+ 2
1360:+ 2
1257:ρ
1254:∫
1136:⋅
1133:∇
1089:ρ
1052:ρ
1046:⋅
1043:∇
1031:∂
1026:ρ
1023:∂
823:mechanics
819:chemistry
812:reactants
773:chemistry
726:Truesdell
656:Bernoulli
605:Rheometer
600:Rheometry
440:Newtonian
434:Viscosity
184:φ
172:−
2546:, Vol.64
2199:(2002).
2016:See also
1607:Jean Rey
1586:Epicurus
1553:Mahavira
1471:hydrogen
1402:molecule
1340:products
1336:reactant
1318:integral
945:, where
584:Rheology
489:Adhesion
469:Pressure
455:Buoyancy
400:Dynamics
238:Momentum
110:May 2020
2313:Bibcode
1651:alchemy
1488:History
1406:methane
1312:is the
1178:field.
1174:is the
1148:is the
1103:density
1101:is the
989:is the
835:alchemy
769:physics
671:Charles
479:Liquids
393:Statics
338:Bending
138:methane
94:scholar
2599:
2582:(1904)
2563:(1921)
2520:(1904)
2402:
2356:
2331:
2286:
2261:
2217:
2175:
2145:
2111:
2066:Nature
1837:matter
1668:Morley
1605:, and
1579:
1533:oxygen
1422:oxygen
1287:where
1152:, and
1081:where
825:, and
795:, the
793:energy
789:matter
775:, the
721:Stokes
716:Pascal
706:Navier
701:Newton
691:Graham
666:Cauchy
569:Plasma
464:
462:Mixing
457:
442:
424:
407:
395:
383:Fluids
316:Strain
311:Stress
294:linear
243:Energy
96:
89:
82:
75:
67:
2421:Ambix
2225:p. 24
1476:Many
1454:water
891:, or
696:Hooke
676:Euler
661:Boyle
519:Gases
101:JSTOR
87:books
2618:Mass
2597:ISBN
2502:2024
2400:ISBN
2354:ISBN
2329:ISSN
2284:ISBN
2259:ISBN
2215:ISBN
2173:ISBN
2143:ISBN
2109:ISBN
2073:2022
1922:and
1848:and
1547:, a
1523:and
1338:and
1000:and
885:heat
852:and
797:mass
791:and
771:and
711:Noll
681:Fick
233:Mass
218:Laws
73:news
2429:doi
2376:",
2321:doi
1841:not
1682:of
1577:(c.
1527:'s
1519:'s
1404:of
1012:as
868:in
779:or
767:In
56:by
2614::
2575:,
2493:.
2481:^
2450:.
2425:10
2423:.
2327:.
2319:.
2309:52
2307:.
2257:.
2213:.
2137:.
2064:.
1852:.
1601:,
1484:.
1442:CO
1410:CH
1374:CO
1372:→
1350:CH
993:.
887:,
872:.
821:,
2504:.
2435:.
2431::
2408:.
2362:.
2335:.
2323::
2315::
2292:.
2267:.
2223:.
2181:.
2151:.
2117:.
2075:.
1964:.
1959:2
1955:c
1950:/
1946:E
1940:=
1937:m
1811:)
1807:(
1803:.
1797:.
1747:2
1743:c
1739:m
1736:=
1733:E
1539:.
1467:O
1463:2
1458:H
1456:(
1447:2
1440:(
1431:2
1426:O
1415:4
1408:(
1397:,
1395:O
1391:2
1386:H
1379:2
1367:2
1362:O
1355:4
1300:V
1296:d
1275:,
1272:0
1269:=
1266:V
1262:d
1248:t
1244:d
1239:d
1234:=
1228:t
1224:d
1218:M
1214:d
1190:M
1161:v
1113:t
1069:,
1066:0
1063:=
1060:)
1056:v
1049:(
1040:+
1034:t
977:c
953:m
931:2
927:c
923:m
756:e
749:t
742:v
466:·
459:·
449:)
444:·
438:(
426:·
409:·
397:·
192:x
189:d
181:d
175:D
169:=
166:J
123:)
117:(
112:)
108:(
98:·
91:·
84:·
77:·
50:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.