196:
121:
433:
460:
296:
24:
380:
The Soviet Union began its development of an analog in the 1940s, while negotiating with
Germany on building an IG Farben plant in Ukraine, basic scientific work was ongoing in 1942. The production only started in 1948 in Klin, after USSR got its hands on the 2000 volumes of IG Farben, and 10,000
472:
Nylon 6 fibres are tough, possessing high tensile strength, elasticity and lustre. They are wrinkleproof and highly resistant to abrasion and chemicals such as acids and alkalis. The fibres can absorb up to 2.4% of water, although this lowers tensile strength. The glass transition temperature of
376:
also succeeded in synthesizing nylon 6.) It was marketed as Perlon, and industrial production with a capacity of 3,500 tons per year was established in Nazi
Germany in 1943, using phenol as a feedstock. At first, the polymer was used to produce coarse fiber for artificial bristle, then the fiber
393:
Nylon 6 can be modified using comonomers or stabilizers during polymerization to introduce new chain end or functional groups, which changes the reactivity and chemical properties. It is often done to change its dyeability or flame retardance. Nylon 6 is synthesized by
530:
At present, polyamide 6 is a significant construction material used in many industries, for instance in the automotive industry, aircraft industry, electronic and electrotechnical industry, clothing industry and medicine. Annual demand for polyamides in
347:
industry. It is sold under numerous trade names including Perlon (Germany), Dederon (former East
Germany), Nylatron, Capron, Ultramid, Akulon, Kapron (former Soviet Union and satellite states), Rugopa (Turkey) and Durethan.
60:
Polycaprolactam, polyamide 6, PA6, poly-ε-caproamide, Perlon, Dederon, Capron, Ultramid, Akulon, Nylatron, Kapron, Alphalon, Tarnamid, Akromid, Frianyl, Schulamid, Durethan, Technyl, Nyorbits ,Winmark
Polymers
522:. Strong interchain interactions from hydrogen bonds between molecular nylon chains is said to be the cause by some sources. However, in 2023 a catalyst that rapidly breaks Nylon 6 down was reported.
622:
Soviet military administration in
Germany 1945-1949: Activities of the SMAG Directorate for studying the achievements of German science and technology in the Soviet zone of occupation of Germany
785:
618:Советская военная администрация в Германии 1945-1949: Деятельность Управления СВАГ по изучению достижений немецкой науки и техники в Советской зоне оккупации Германии
629:
456:
bond reverses at each bond, all nylon 6 amide bonds lie in the same direction (see figure: note the N to C orientation of each amide bond).
476:
As a synthetic fibre, Nylon 6 is generally white but can be dyed in a solution bath prior to production for different color results. Its
604:
722:
760:
518:
fungal strains can also degrade Nylon 6 through oxidation. Compared to aliphatic polyesters, Nylon 6 has been said to have poor
658:
283:
488:
153:
477:
395:
336:
303:
174:
377:
quality was improved, and
Germans started making parachutes, cord for aircraft tires and towing cables for gliders.
838:
833:
736:
809:
The
Promise of Nylon 6: A Case Study in Intelligent Product Design by William McDonough & Michael Braungart
262:
116:
46:
36:
426:
382:
332:
823:
191:
72:
828:
704:
625:
600:
343:. Its competition with nylon 6,6 and the example it set have also shaped the economics of the
546:
495:
g/cm. Its melting point is at 215 °C and can protect heat up to 150 °C on average.
694:
684:
576:
519:
432:
340:
213:
162:
82:
344:
321:
764:
295:
195:
120:
699:
672:
441:
422:
277:
808:
459:
452:
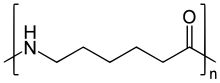
becomes part of the polymer backbone. Unlike nylon 6,6, in which the direction of the
817:
246:
109:
648:", NPTEL (National Programme On Technology Enhanced Learning), retrieved May 9, 2016
564:
357:
142:
786:"Grupa Azoty: Nowa wytwórnia pozwoli zająć pozycję 2. producenta poliamidu w UE"
597:
Synthetic
Socialism: Plastics and Dictatorship in the German Democratic Republic
399:
299:
448:
is broken, with the active groups on each side re-forming two new bonds as the
570:
484:
414:
93:
23:
515:
361:
324:
708:
723:"New Catalyst Completely Breaks Down Durable Plastic Pollution in Minutes"
645:
339:; this makes it a special case in the comparison between condensation and
689:
511:
445:
418:
365:
364:
in late 1930s (first synthesized in 1938) to reproduce the properties of
624:] (in Russian). Moscow: ROSSPEN, Russian State Archive. p. 65.
558:
449:
317:
236:
129:
532:
410:
385:
technical documentation, as a result of victory in the World War II.
369:
276:
Except where otherwise noted, data are given for materials in their
536:
458:
453:
431:
373:
328:
294:
552:
463:
Nylon 6 (above) has a structure similar to Nylon 6,6 (below).
179:
659:
Polyamide Fiber
Physical and Chemical Properties of Nylon 6
372:
on its production. (Around the same time, Kohei
Hoshino at
671:
Tokiwa, Y.; Calabia, B. P.; Ugwu, C. U.; Aiba, S. (2009).
539:. They are produced by all leading chemical companies.
421:for about 4–5 hours, the ring breaks and undergoes
661:”, textilefashionstudy.com, retrieved May 9, 2016.
542:The largest producers of polyamide 6 in Europe:
141:
763:(in Polish). att.grupaazoty.com. Archived from
81:
8:
677:International Journal of Molecular Sciences
646:Synthesis of Modified Polyamides (Nylon 6)
599:. The University of North Carolina Press.
194:
119:
15:
698:
688:
444:, the amide bond within each caprolactam
436:Polymerization of caprolactam to Nylon 6.
425:. Then the molten mass is passed through
406:. When caprolactam is heated at about 533
161:
40:Poly(azepan-2-one); poly(hexano-6-lactam)
588:
514:of Nylon 6, but not polymers. Certain
302:molecule used to synthesize Nylon 6 by
190:
110:
270:434 °C; 813 °F; 707 K
7:
402:. Caprolactam has 6 carbons, hence
132:
742:(in Polish). static.grupaazoty.com
14:
356:Polycaprolactam was developed by
22:
280:(at 25 °C , 100 kPa).
673:"Biodegradability of Plastics"
1:
429:to form fibres of nylon 6.
396:ring-opening polymerization
337:ring-opening polymerization
335:, but instead is formed by
304:ring opening polymerization
855:
788:(in Polish). wyborcza.biz
579:, 100,000 tonnes per year
573:, 100,000 tonnes per year
567:, 125,000 tonnes per year
561:, 170,000 tonnes per year
555:, 240,000 tonnes per year
549:, 260,000 tonnes per year
274:
255:
206:
65:
57:
45:
35:
30:
21:
737:"Segment Tworzywa 2015"
473:Nylon 6 is 47 °C.
616:Zaharov, V.V. (2007).
491:with a density of 1.14
464:
437:
368:without violating the
306:
535:amounts to a million
462:
435:
298:
47:Systematic IUPAC name
690:10.3390/ijms10093722
526:Production in Europe
333:condensation polymer
327:. Unlike most other
510:sp. (NK87) degrade
331:, nylon 6 is not a
18:
725:. 3 December 2023.
595:Rubin, E. (2014),
465:
438:
307:
284:Infobox references
16:
839:German inventions
761:"Alphalon™ (PA6)"
631:978-5-8243-0882-2
341:addition polymers
292:Chemical compound
290:
289:
251:218.3 °C (493 K)
175:CompTox Dashboard
846:
834:Synthetic fibers
797:
796:
794:
793:
782:
776:
775:
773:
772:
757:
751:
750:
748:
747:
741:
733:
727:
726:
719:
713:
712:
702:
692:
668:
662:
655:
649:
642:
636:
635:
613:
607:
593:
520:biodegradability
494:
483:
409:
320:, in particular
214:Chemical formula
199:
198:
183:
181:
165:
145:
134:
123:
112:
85:
26:
19:
854:
853:
849:
848:
847:
845:
844:
843:
814:
813:
805:
800:
791:
789:
784:
783:
779:
770:
768:
759:
758:
754:
745:
743:
739:
735:
734:
730:
721:
720:
716:
670:
669:
665:
656:
652:
643:
639:
632:
615:
614:
610:
594:
590:
586:
528:
501:
492:
481:
470:
407:
391:
354:
345:synthetic fibre
322:semicrystalline
314:polycaprolactam
293:
286:
281:
267:
264:
230:
226:
222:
216:
202:
184:
177:
168:
148:
135:
104:
88:
75:
61:
53:
52:
41:
12:
11:
5:
852:
850:
842:
841:
836:
831:
826:
816:
815:
812:
811:
804:
803:External links
801:
799:
798:
777:
752:
728:
714:
683:(9): 3722–42.
663:
650:
637:
630:
608:
605:978-1469615103
587:
585:
582:
581:
580:
574:
568:
562:
556:
550:
527:
524:
504:Flavobacterium
500:
499:Biodegradation
497:
469:
466:
442:polymerization
423:polymerization
390:
387:
353:
350:
291:
288:
287:
282:
278:standard state
275:
272:
271:
268:
261:
258:
257:
253:
252:
249:
243:
242:
239:
233:
232:
228:
224:
220:
217:
212:
209:
208:
204:
203:
201:
200:
187:
185:
173:
170:
169:
167:
166:
158:
156:
150:
149:
147:
146:
138:
136:
128:
125:
124:
114:
106:
105:
103:
102:
98:
96:
90:
89:
87:
86:
78:
76:
71:
68:
67:
63:
62:
59:
55:
54:
50:
49:
43:
42:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
851:
840:
837:
835:
832:
830:
827:
825:
822:
821:
819:
810:
807:
806:
802:
787:
781:
778:
767:on 2016-04-26
766:
762:
756:
753:
738:
732:
729:
724:
718:
715:
710:
706:
701:
696:
691:
686:
682:
678:
674:
667:
664:
660:
654:
651:
647:
641:
638:
633:
627:
623:
619:
612:
609:
606:
602:
598:
592:
589:
583:
578:
575:
572:
569:
566:
563:
560:
557:
554:
551:
548:
545:
544:
543:
540:
538:
534:
525:
523:
521:
517:
513:
509:
505:
498:
496:
490:
486:
479:
474:
467:
461:
457:
455:
451:
447:
443:
434:
430:
428:
424:
420:
416:
412:
405:
401:
397:
388:
386:
384:
378:
375:
371:
367:
363:
359:
351:
349:
346:
342:
338:
334:
330:
326:
323:
319:
315:
311:
305:
301:
297:
285:
279:
273:
269:
266:
260:
259:
254:
250:
248:
247:Melting point
245:
244:
240:
238:
235:
234:
218:
215:
211:
210:
205:
197:
193:
192:DTXSID6049694
189:
188:
186:
176:
172:
171:
164:
160:
159:
157:
155:
152:
151:
144:
140:
139:
137:
131:
127:
126:
122:
118:
115:
113:
111:ECHA InfoCard
108:
107:
100:
99:
97:
95:
92:
91:
84:
80:
79:
77:
74:
70:
69:
64:
56:
48:
44:
38:
34:
29:
25:
20:
790:. Retrieved
780:
769:. Retrieved
765:the original
755:
744:. Retrieved
731:
717:
680:
676:
666:
653:
640:
621:
617:
611:
596:
591:
541:
529:
507:
503:
502:
475:
471:
439:
413:in an inert
403:
392:
379:
358:Paul Schlack
355:
313:
309:
308:
263:Autoignition
241:1.084 g/mL
66:Identifiers
58:Other names
577:Grupa Azoty
508:Pseudomonas
400:caprolactam
381:volumes of
300:Caprolactam
265:temperature
207:Properties
117:100.124.824
824:Polyamides
818:Categories
792:2016-04-12
771:2016-04-12
746:2016-04-12
584:References
468:Properties
427:spinnerets
415:atmosphere
163:14GUK8I73Z
94:ChemSpider
83:25038-54-4
73:CAS Number
37:IUPAC name
516:white rot
512:oligomers
506:sp. and
389:Synthesis
362:IG Farben
325:polyamide
829:Plastics
709:19865515
480:is 6–8.5
478:tenacity
446:molecule
419:nitrogen
366:Nylon 66
256:Hazards
17:Nylon 6
700:2769161
559:Lanxess
547:Fibrant
450:monomer
440:During
404:Nylon 6
352:History
318:polymer
310:Nylon 6
237:Density
231:
130:PubChem
707:
697:
628:
603:
565:Radici
537:tonnes
533:Europe
493:
482:
408:
370:patent
329:nylons
31:Names
740:(PDF)
620:[
454:amide
374:Toray
316:is a
143:32775
705:PMID
626:ISBN
601:ISBN
571:DOMO
553:BASF
154:UNII
101:None
51:Poly
695:PMC
685:doi
417:of
398:of
383:AEG
360:at
312:or
227:NO)
180:EPA
133:CID
820::
703:.
693:.
681:10
679:.
675:.
485:gf
225:11
219:(C
795:.
774:.
749:.
711:.
687::
657:”
644:"
634:.
489:D
487:/
411:K
229:n
223:H
221:6
182:)
178:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.