232:, signaling that it has been targeted, after which the particle is processed and consequently destroyed by recruited immune cells. Neutralizing antibodies on the other hand can neutralize the biological effects of the antigen without a need for immune cells. In some cases, non-neutralizing antibodies, or an insufficient amount of neutralizing antibodies binding to viral particles, can be utilized by some species of virus to facilitate uptake into their host cells. This mechanism is known as
483:. By understanding the binding sites and structure of neutralizing antibodies in a natural immune response a vaccine can be rationally designed such that it stimulates the immune system to produce neutralizing antibodies and not binding antibodies. Introducing a weakened form of a virus through vaccination allows for the production of neutralizing antibodies by
166:
40:
551:
specificity to the old strain can no longer bind to the new virus strain. This immune evasion strategy prevents the immune system from developing immunological memory against the pathogen. Broadly neutralizing antibodies (bNAbs), on the other hand, have the special ability to bind and neutralize multiple strains of a virus species.
182:. Neutralizing antibodies can inhibit infectivity by binding to the pathogen and blocking the molecules needed for cell entry. This can be due to the antibodies statically interfering with the pathogens, or toxins attaching to host cell receptors. In case of a viral infection, NAbs can bind to glycoproteins of
2118:
Simek, MD; Rida, W; Priddy, FH; Pung, P; Carrow, E; Laufer, DS; Lehrman, JK; Boaz, M; Tarragona-Fiol, T; Miiro, G; Birungi, J; Pozniak, A; McPhee, DA; Manigart, O; Karita, E; Inwoley, A; Jaoko, W; Dehovitz, J; Bekker, LG; Pitisuttithum, P; Paris, R; Walker, LM; Poignard, P; Wrin, T; Fast, PE; Burton,
388:
against the infectious agent. This showed that antibodies could be used as an effective treatment for viral infections and toxins. Antiserum is a very crude therapy, because antibodies in the plasma are not purified or standardized and the blood plasma could be rejected by the donor. As it relies on
1529:
Shen, Chenguang; Wang, Zhaoqin; Zhao, Fang; Yang, Yang; Li, Jinxiu; Yuan, Jing; Wang, Fuxiang; Li, Delin; Yang, Minghui; Xing, Li; Wei, Jinli; Xiao, Haixia; Yang, Yan; Qu, Jiuxin; Qing, Ling; Chen, Li; Xu, Zhixiang; Peng, Ling; Li, Yanjie; Zheng, Haixia; Chen, Feng; Huang, Kun; Jiang, Yujing; Liu,
1451:
Hung, I. F.; To, K. K.; Lee, C.-K.; Lee, K.-L.; Chan, K.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y.; Koo, C.-K.; Buckley, T.; Chow, F.-L.; Wong, K.-K.; Chan, H.-S.; Ching, C.-K.; Tang, B. S.; Lau, C. C.; Li, I. W.; Liu, S.-H.; Chan, K.-H.; Lin, C.-K.; Yuen, K.-Y. (19 January 2011).
491:
that produce antibodies specific to the virus. An effective vaccine induces the production of antibodies that are able to neutralize the majority of variants of a virus, although virus mutation resulting in antibody evasion may require vaccines to be updated in response. Some viruses evolve faster
227:
Not all antibodies that bind to a pathogenic particle are neutralizing. Non-neutralizing antibodies, or binding antibodies, bind specifically to the pathogen, but do not interfere with their infectivity. That might be because they do not bind to the right region. Non-neutralizing antibodies can be
558:
screening study showed that only 1% of all patients develop bNAbs against HIV. bNABs can neutralize a wide range of virus strains by binding to conserved regions of the virus surface proteins that are unable to mutate because they are functionally essential for the virus replication. Most binding
346:
during the course of an immune response, thereby improving recognition of viral particles. Conserved parts of viral proteins that play a central role in viral function are less likely to evolve over time, and therefore are more vulnerable to antibody binding. However, viruses have evolved certain
550:
Most of the neutralizing antibodies produced by the immune system are very specific for a single virus strain due to affinity maturation by B cells. Some pathogens with high genetic variability, such as HIV, constantly change their surface structure such that neutralizing antibodies with high
628:
Mike Recher; Karl S Lang; Lukas
Hunziker; Stefan Freigang; Bruno Eschli; Nicola L Harris; Alexander Navarini; Beatrice M Senn; Katja Fink; Marius Lötscher; Lars Hangartner; Raphaël Zellweger; Martin Hersberger; Alexandre Theocharides; Hans Hengartner; Rolf M Zinkernagel (8 August 2004).
286:. A strong diversity in the antibody repertoire allows the immune system to recognize a plethora of pathogens which can come in all different forms and sizes. During an infection only antibodies that bind to the pathogenic antigen with high affinity are produced. This is achieved by
428:. Polyclonal antibodies are obtained from human donors or animals that have been exposed to the antigen. The antigen injected into the animal donors can be designed in such a way to preferably produce neutralizing antibodies. Polyclonal antibodies have been used as treatment for
594:
Preliminary research is conducted to identify and test bNAbs against HIV-1. bNAbs are used in research to rationally design vaccines to stimulate production of bNAbs and immunity against viruses. No antigen that triggers bNAb production in animal models or humans is known.
389:
the donation from recovered patients it cannot be easily scaled up. However, serum therapy is today still used as the first line of defence during an outbreak as it can relatively quickly obtained. Serum therapy was shown to reduce mortality in patients during the
949:
Dejnirattisai, Wanwisa; Supasa, Piyada; Wongwiwat, Wiyada; Rouvinski, Alexander; Barba-Spaeth, Giovanna; Duangchinda, Thaneeya; Sakuntabhai, Anavaj; Cao-Lormeau, Van-Mai; Malasit, Prida; Rey, Felix A; Mongkolsapaya, Juthathip; Screaton, Gavin R (23 June 2016).
456:. By treating with antibodies binding multiple epitopes, the treatment is still effective even if the virus mutates and one of the epitopes changes in structure. However, because of the nature of the production, treatment with polyclonal antibodies has
195:
of viral proteins that mediate the membrane fusion needed for entry into the host cell. In some cases, the virus is unable to infect even after the antibody dissociates. The pathogen-antibody complex is eventually taken up and degraded by macrophages.
1700:
Multi-National PREVAIL II Study Team, Davey RT Jr, Dodd L, Proschan MA, Neaton J, Neuhaus
Nordwall J, Koopmeiners JS, Beigel J, Tierney J, Lane HC, Fauci AS, Massaquoi MBF, Sahr F, Malvy D, et al. (PREVAIL II Writing Group) (13 October 2016).
468:, which allows the production of mAbs in large quantities. mAbs against infections stop working when virus mutates the epitope that is targeted by the mAbs or multiple strain are circulating. Example of drugs that use monoclonal antibodies include
351:
access of an antibody to these regions, making binding difficult. Viruses with a low density of surface structural proteins are more difficult for antibodies to bind to. Some viral glycoproteins are heavily glycosylated by N- and O- linked
2335:
Durham, ND; Agrawal, A; Waltari, E; Croote, D; Zanini, F; Fouch, M; Davidson, E; Smith, O; Carabajal, E; Pak, JE; Doranz, BJ; Robinson, M; Sanz, AM; Albornoz, LL; Rosso, F; Einav, S; Quake, SR; McCutcheon, KM; Goo, L (10 December 2019).
511:
administered to the body which would otherwise treat multiple sclerosis. Recombinant protein drugs, especially those derived from animals, are commonly targeted by neutralizing antibodies. A few examples are Rebif, Betaseron and Avonex.
2121:"Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm"
190:
of non-enveloped viruses. Furthermore, neutralizing antibodies can act by preventing particles from undergoing structural changes often needed for successful cell entry. For example, neutralizing antibodies can prevent
169:
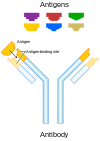
Covering a pathogen's antigen in antibodies make the pathogen less infectious and less pathogenic. In the image on the right, virus entry to the cell is prevented by neutralizing antibodies binding to the
1400:
Schmidt, Rebecca; Beltzig, Lea C.; Sawatsky, Bevan; Dolnik, Olga; Dietzel, Erik; Krähling, Verena; Volz, Asisa; Sutter, Gerd; Becker, Stephan; von
Messling, Veronika (5 October 2018).
207:. Neutralizing antibodies are not effective against extracellular bacteria, as the binding of antibodies does not prevent bacteria from replicating. Here, the immune system uses other
129:
or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the
1635:
Bregenholt, S; Jensen, A; Lantto, J; Hyldig, S; Haurum, JS (2006). "Recombinant human polyclonal antibodies: A new class of therapeutic antibodies against viral infections".
2387:
Goo, L; Debbink, K; Kose, N; Sapparapu, G; Doyle, MP; Wessel, AW; Richner, JM; Burgomaster, KE; Larman, BC; Dowd, KA; Diamond, MS; Crowe JE, Jr; Pierson, TC (January 2019).
302:
on their cell surface, which is just the antibody anchored to the cell membrane. When the B-cell receptor binds to its cognate antigen with high affinity, an intracellular
96:
322:. Plasma cells then secrete the antigen-specific antibody in large quantities. After a first encounter of the antigen by vaccination or natural infection,
2284:
Colbert, MD; Flyak, AI; Ogega, CO; Kinchen, VJ; Massaccesi, G; Hernandez, M; Davidson, E; Doranz, BJ; Cox, AL; Crowe JE, Jr; Bailey, JR (15 July 2019).
174:
In order to enter cells, pathogens, such as circulating viral particles or extracellular bacteria, use molecules on their surfaces to interact with the
342:
that allow viruses to evade a neutralizing antibody will be selected for, and hence prevail. Conversely, antibodies also simultaneously evolve by
376:, and can be used for patients even if they do not have a healthy immune system. In the early 20th century, infected patients were injected with
1051:
863:
812:
545:
521:
394:
199:
Neutralizing antibodies are also important in neutralizing the toxic effects of bacterial toxins. An example of a neutralizing antibody is
524:(which compares counts of virus plaques in control wells with those in inoculated cultures), microneutralization (which is performed in
1678:
356:, creating a so-called glycan shield, which may decrease antibody binding affinity and facilitate evasion of neutralizing antibodies.
788:
318:
response of the immune system against the pathogen. Once a B cell is fully activated, it rapidly proliferates and differentiates into
879:
Schmaljohn, AL (July 2013). "Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts".
1947:
568:
520:
Neutralization assays are capable of being performed and measured in different ways, including the use of techniques such as
233:
492:
than others, which can require the need for vaccines to be updated in response. A well known example is the vaccine for the
464:. Monoclonal antibodies, on the other hand, all bind the same epitope with high specificity. They can be produced with the
89:
1454:"Convalescent Plasma Treatment Reduced Mortality in Patients With Severe Pandemic Influenza A (H1N1) 2009 Virus Infection"
424:(mAb) can be used. Polyclonal antibodies are collection of antibodies that target the same pathogen but bind to different
2389:"A protective human monoclonal antibody targeting the West Nile virus E protein preferentially recognizes mature virions"
445:
82:
1504:
559:
sites of bNAbs against HIV are on HIV's exposed surface antigen, the envelope (Env) protein (a trimer composed of
571:
HIV Databases is a comprehensive resource that has a wealth of information about HIV sequences, bNAbs, and more.
1778:"Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity"
1078:"Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity"
1752:
279:
1505:"WHO | Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease"
390:
402:
315:
134:
2338:"Broadly neutralizing human antibodies against dengue virus identified by single B cell transcriptomics"
533:
421:
417:
385:
326:
allows for a more rapid production of neutralizing antibodies following the next exposure to the virus.
267:
192:
175:
952:"Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus"
2084:
829:
465:
449:
373:
323:
295:
200:
487:. After a second exposure, the neutralizing antibody response is more rapid due to the existence of
153:) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its
2498:
508:
343:
271:
142:
496:
virus, which must be updated annually to account for the recent circulating strains of the virus.
1918:
704:
658:
500:
2286:"Broadly Neutralizing Antibodies Targeting New Sites of Vulnerability in Hepatitis C Virus E1E2"
2475:
2457:
2418:
2369:
2317:
2266:
2203:
2152:
2100:
2057:
2003:
1943:
1910:
1902:
1864:
1809:
1734:
1652:
1614:
1563:
1485:
1433:
1382:
1331:
1287:
1231:
1213:
1172:
1154:
1115:
1097:
1047:
1022:
981:
931:
914:
Tirado, SM; Yoon, KJ (2003). "Antibody-dependent enhancement of virus infection and disease".
896:
859:
808:
784:
759:
650:
604:
433:
410:
216:
179:
1937:
2465:
2449:
2408:
2400:
2359:
2349:
2307:
2297:
2256:
2248:
2193:
2183:
2142:
2132:
2092:
2047:
2037:
1993:
1983:
1894:
1854:
1844:
1799:
1789:
1724:
1714:
1670:
1644:
1604:
1594:
1553:
1543:
1475:
1465:
1423:
1413:
1372:
1362:
1321:
1277:
1267:
1221:
1203:
1162:
1146:
1105:
1089:
1012:
971:
963:
923:
888:
837:
749:
739:
642:
609:
525:
453:
406:
287:
204:
130:
1828:
1150:
583:
429:
357:
299:
187:
146:
2235:
Corti, D; Cameroni, E; Guarino, B; Kallewaard, NL; Zhu, Q; Lanzavecchia, A (June 2017).
2088:
2470:
2437:
2413:
2388:
2364:
2337:
2312:
2285:
2261:
2236:
2198:
2171:
2147:
2120:
2052:
2025:
1998:
1971:
1859:
1832:
1804:
1777:
1729:
1702:
1609:
1582:
1558:
1531:
1480:
1453:
1428:
1401:
1377:
1350:
1282:
1255:
1226:
1191:
1167:
1134:
1110:
1077:
976:
951:
754:
727:
461:
348:
303:
183:
154:
122:
17:
1017:
1000:
2492:
728:"Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives"
529:
488:
441:
311:
306:
is triggered. In addition to binding to an antigen, B cells need to be stimulated by
290:
of a single B cell clone: B cells are recruited to the site of infection by sensing
1922:
682:
662:
631:"Deliberate removal of T cell help improves virus-neutralizing antibody production"
457:
437:
237:
229:
212:
2438:"Broadly neutralizing antibodies as treatment: effects on virus and immune system"
2096:
554:
bNAbs have been initially found in HIV patients. However, they are quite rare: an
282:. Therefore, every B cell produces antibodies that bind specifically to different
274:), which results in every mature B cell producing antibodies that differ in their
2252:
2188:
892:
334:
Viruses use a variety of mechanisms to evade neutralizing antibodies. Viral
1849:
1833:"Rational Design of Vaccines to Elicit Broadly Neutralizing Antibodies to HIV-1"
683:"Neutralizing Antibodies and Disease-Modifying Therapies for Multiple Sclerosis"
575:
567:
subunits). These site include the CD4 binding site or the gp41-gp120 interface.
480:
473:
381:
319:
263:
1648:
1532:"Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma"
1351:"Treatment of Ebola Virus Infection with Antibodies from Reconvalescent Donors"
1254:
Salazar, Georgina; Zhang, Ningyan; Fu, Tong-Ming; An, Zhiqiang (10 July 2017).
927:
405:, which uses a mixture of antibodies obtained from healthy people, is given to
2453:
2404:
1418:
1272:
504:
291:
275:
241:
2461:
2042:
1906:
1217:
1158:
1101:
841:
493:
377:
2479:
2422:
2373:
2321:
2270:
2207:
2156:
2104:
2061:
2007:
1914:
1868:
1813:
1776:
VanBlargan, Laura A.; Goo, Leslie; Pierson, Theodore C. (26 October 2016).
1738:
1656:
1618:
1567:
1548:
1489:
1437:
1386:
1367:
1335:
1291:
1235:
1176:
1119:
1026:
985:
935:
900:
763:
654:
630:
574:
Additionally, bNAbs have been found for other viruses including influenza,
536:(which depend on biomarkers indicating metabolic inhibition of the virus).
2170:
Haynes, Barton F.; Burton, Dennis R.; Mascola, John R. (30 October 2019).
1988:
1794:
1719:
1208:
1093:
744:
2302:
2137:
1470:
1256:"Antibody therapies for the prevention and treatment of viral infections"
1192:"Innate Immune Evasion Strategies by Human Immunodeficiency Virus Type 1"
425:
398:
361:
339:
307:
208:
178:
of their target cell which allows them to enter the cell and start their
126:
118:
2354:
2221:
476:
against RSV. Many mABs against other infections are in clinical trials.
165:
1326:
1309:
283:
150:
1599:
579:
484:
353:
335:
259:
253:
967:
39:
2026:"Broadly neutralizing antibodies in HIV-1 treatment and prevention"
2024:
Kumar, R; Qureshi, H; Deshpande, S; Bhattacharya, J (August 2018).
1898:
1703:"A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection"
646:
516:
Methods for detection and quantification of neutralizing antibodies
479:
Neutralizing antibodies also play a role in active immunisation by
1402:"Generation of therapeutic antisera for emerging viral infections"
560:
469:
164:
138:
223:
Difference between neutralizing antibodies and binding antibodies
1530:
Dongjing; Zhang, Zheng; Liu, Yingxia; Liu, Lei (27 March 2020).
1076:
VanBlargan, Laura A.; Goo, Leslie; Pierson, Theodore C. (2016).
564:
1885:
Burton, Dennis R. (2002). "Antibodies, viruses and vaccines".
1133:
Crispin, Max; Ward, Andrew B.; Wilson, Ian A. (20 May 2018).
1936:
Kaslow, R. A.; Stanberry, L.R.; Le Duc, J. W., eds. (2014).
1308:
Casadevall, A; Dadachova, E; Pirofski, LA (September 2004).
1135:"Structure and Immune Recognition of the HIV Glycan Shield"
1581:
Casadevall, Arturo; Pirofski, Liise-anne (13 March 2020).
805:
Principles of
Virology, Volume 2: Pathogenesis and Control
503:. Although this type of antibody has the ability to fight
384:
of a previously infected and recovered patient containing
2237:"Tackling influenza with broadly neutralizing antibodies"
1071:
1069:
1067:
1065:
1063:
1046:(8th ed.). Garland Science. 2012. pp. 389–404.
499:
Neutralizing antibodies may also assist the treatment of
2172:"Multiple roles for HIV broadly neutralizing antibodies"
1753:"Label - Palivizumab (Synagis), Medimmune, Incorporated"
294:
that are released by the infected cells as part of the
1583:"The convalescent sera option for containing COVID-19"
266:, the genes that encode the antibodies undergo random
2436:
Bhiman, Jinal N.; Lynch, Rebecca M. (27 March 2017).
1972:"HIV-1 Genetic Variability and Clinical Implications"
1939:
Viral
Infections of Humans: Epidemiology and Control
858:(8th ed.). Garland Science. 2012. p. 388.
397:. It is also being tested as possible treatment for
781:
Principles of
Virology, Volume 1: Molecular Biology
416:For a more specific and robust treatment, purified
70:
62:
54:
49:
2030:Therapeutic Advances in Vaccines and Immunotherapy
1310:"Passive antibody therapy for infectious diseases"
1630:
1628:
1303:
1301:
203:, which can neutralize the biological effects of
149:. By binding specifically to surface structures (
1038:
1036:
1880:
1878:
1677:. Centers for Disease Control and Prevention.
1249:
1247:
1245:
999:Jung, David; Alt, Frederick W (January 2004).
807:(4th ed.). ASM Press. 2015. p. 125.
783:(4th ed.). ASM Press. 2015. p. 31.
90:
8:
2019:
2017:
676:
674:
672:
32:
1837:Cold Spring Harbor Perspectives in Medicine
452:contains polyclonal antibodies against the
1782:Microbiology and Molecular Biology Reviews
1082:Microbiology and Molecular Biology Reviews
97:
83:
2469:
2412:
2363:
2353:
2311:
2301:
2260:
2197:
2187:
2146:
2136:
2051:
2041:
1997:
1987:
1858:
1848:
1803:
1793:
1728:
1718:
1608:
1598:
1557:
1547:
1479:
1469:
1427:
1417:
1376:
1366:
1325:
1281:
1271:
1225:
1207:
1190:Guha, Debjani; Ayyavoo, Velpandi (2013).
1166:
1109:
1016:
975:
753:
743:
330:Virus evasion of neutralizing antibodies
258:Antibodies are produced and secreted by
620:
368:Medical uses of neutralizing antibodies
1942:(5th ed.). Springer. p. 56.
775:
773:
31:
2075:Cohen, J. (2013). "Bound for Glory".
1681:from the original on 16 December 2016
1151:10.1146/annurev-biophys-060414-034156
546:Broadly neutralizing HIV-1 antibodies
507:infections, in some cases it attacks
372:Neutralizing antibodies are used for
7:
681:Stachowiak, Julie (15 August 2008).
395:Western African Ebola virus epidemic
262:. When B cells are produced in the
1831:; Nabel, G. J. (1 September 2011).
228:important to flag the particle for
726:Klasse, P. J. (9 September 2014).
413:patients to fight off infections.
219:activation, to kill the bacteria.
25:
2224:. Los Alamos National Laboratory.
1587:Journal of Clinical Investigation
364:, uses both of these mechanisms.
1675:Infectious Diseases Laboratories
1001:"Unraveling V(D)J Recombination"
569:Los Alamos National Laboratory's
44:Standard antibody representation
38:
1970:Santoro, MM; Perno, CF (2013).
1707:New England Journal of Medicine
1349:Kreil, Thomas R. (March 2015).
540:Broadly neutralizing antibodies
2176:Science Translational Medicine
234:antibody-dependent enhancement
1:
2097:10.1126/science.341.6151.1168
1637:Current Pharmaceutical Design
1018:10.1016/S0092-8674(04)00039-X
528:filled with small amounts of
157:it might infect and destroy.
2253:10.1016/j.coviro.2017.03.002
2189:10.1126/scitranslmed.aaz2686
1458:Clinical Infectious Diseases
1355:Emerging Infectious Diseases
1314:Nature Reviews. Microbiology
893:10.2174/1570162x113116660057
2241:Current Opinion in Virology
1850:10.1101/cshperspect.a007278
1503:World Health Organization.
1139:Annual Review of Biophysics
828:Treffers, Henry P. (2014).
446:respiratory syncytial virus
236:. It has been observed for
2515:
2119:DR; Koff, WC (July 2009).
1649:10.2174/138161206777442173
928:10.1089/088282403763635465
543:
251:
66:Neutralization of antigens
2454:10.1007/s11904-017-0352-1
2405:10.1038/s41564-018-0283-7
1887:Nature Reviews Immunology
1419:10.1038/s41541-018-0082-4
1273:10.1038/s41541-017-0019-3
78:
37:
2442:Current HIV/AIDS Reports
2043:10.1177/2515135518800689
842:10.1036/1097-8542.450600
458:batch to batch variation
1044:Janeway's immunobiology
856:Janeway's immunobiology
830:"Neutralizing antibody"
705:"Neutralizing antibody"
391:2009 swine flu pandemic
338:mutate at a high rate.
33:Neutralizing antibodies
18:Neutralising antibodies
1549:10.1001/jama.2020.4783
1368:10.3201/eid2103.141838
707:. Biology-Online. 2008
403:Immunoglobulin therapy
296:innate immune response
280:antigen-binding region
193:conformational changes
176:cell surface receptors
171:
135:adaptive immune system
1795:10.1128/MMBR.00024-15
1720:10.1056/NEJMoa1604330
1094:10.1128/MMBR.00024-15
422:monoclonal antibodies
386:polyclonal antibodies
360:, the cause of human
347:mechanisms to hinder
268:genetic recombination
168:
111:neutralizing antibody
2303:10.1128/JVI.02070-18
2138:10.1128/JVI.00110-09
881:Current HIV Research
466:Hybridoma technology
450:Diphtheria antitoxin
374:passive immunisation
324:immunological memory
211:of antibodies, like
201:diphtheria antitoxin
2393:Nature Microbiology
2355:10.7554/eLife.52384
2290:Journal of Virology
2125:Journal of Virology
2089:2013Sci...341.1168C
2083:(6151): 1168–1171.
1989:10.1155/2013/481314
1209:10.1155/2013/954806
745:10.1155/2014/157895
732:Advances in Biology
534:colorimetric assays
344:affinity maturation
272:V(D)J recombination
34:
1471:10.1093/cid/ciq106
1327:10.1038/nrmicro974
501:multiple sclerosis
472:against Ebola and
304:signalling cascade
298:. B cells display
172:
2182:(516): eaaz2686.
1976:ISRN Microbiology
1713:(15): 1448–1456.
1600:10.1172/JCI138003
1542:(16): 1582–1589.
1053:978-0-8153-4243-4
956:Nature Immunology
865:978-0-8153-4243-4
814:978-1-555-81951-4
635:Nature Immunology
605:Blocking antibody
526:microtiter plates
434:hepatitis b virus
184:enveloped viruses
180:replication cycle
107:
106:
16:(Redirected from
2506:
2484:
2483:
2473:
2433:
2427:
2426:
2416:
2384:
2378:
2377:
2367:
2357:
2332:
2326:
2325:
2315:
2305:
2281:
2275:
2274:
2264:
2232:
2226:
2225:
2218:
2212:
2211:
2201:
2191:
2167:
2161:
2160:
2150:
2140:
2115:
2109:
2108:
2072:
2066:
2065:
2055:
2045:
2021:
2012:
2011:
2001:
1991:
1967:
1961:
1960:
1958:
1956:
1933:
1927:
1926:
1882:
1873:
1872:
1862:
1852:
1824:
1818:
1817:
1807:
1797:
1773:
1767:
1766:
1764:
1762:
1757:
1749:
1743:
1742:
1732:
1722:
1697:
1691:
1690:
1688:
1686:
1667:
1661:
1660:
1632:
1623:
1622:
1612:
1602:
1593:(4): 1545–1548.
1578:
1572:
1571:
1561:
1551:
1526:
1520:
1519:
1517:
1515:
1500:
1494:
1493:
1483:
1473:
1448:
1442:
1441:
1431:
1421:
1397:
1391:
1390:
1380:
1370:
1346:
1340:
1339:
1329:
1305:
1296:
1295:
1285:
1275:
1251:
1240:
1239:
1229:
1211:
1187:
1181:
1180:
1170:
1130:
1124:
1123:
1113:
1073:
1058:
1057:
1040:
1031:
1030:
1020:
996:
990:
989:
979:
962:(9): 1102–1108.
946:
940:
939:
916:Viral Immunology
911:
905:
904:
876:
870:
869:
852:
846:
845:
825:
819:
818:
801:
795:
794:
777:
768:
767:
757:
747:
723:
717:
716:
714:
712:
701:
695:
694:
692:
690:
678:
667:
666:
625:
610:Humoral immunity
522:plaque reduction
454:diphtheria toxin
411:immunosuppressed
300:B-cell receptors
288:clonal selection
278:sequence in the
205:diphtheria toxin
133:response of the
99:
92:
85:
42:
35:
27:Type of antibody
21:
2514:
2513:
2509:
2508:
2507:
2505:
2504:
2503:
2489:
2488:
2487:
2435:
2434:
2430:
2386:
2385:
2381:
2334:
2333:
2329:
2283:
2282:
2278:
2234:
2233:
2229:
2222:"HIV Databases"
2220:
2219:
2215:
2169:
2168:
2164:
2131:(14): 7337–48.
2117:
2116:
2112:
2074:
2073:
2069:
2023:
2022:
2015:
1969:
1968:
1964:
1954:
1952:
1950:
1935:
1934:
1930:
1884:
1883:
1876:
1826:
1825:
1821:
1788:(4): 989–1010.
1775:
1774:
1770:
1760:
1758:
1755:
1751:
1750:
1746:
1699:
1698:
1694:
1684:
1682:
1671:"Our Formulary"
1669:
1668:
1664:
1643:(16): 2007–15.
1634:
1633:
1626:
1580:
1579:
1575:
1528:
1527:
1523:
1513:
1511:
1502:
1501:
1497:
1450:
1449:
1445:
1399:
1398:
1394:
1348:
1347:
1343:
1307:
1306:
1299:
1253:
1252:
1243:
1189:
1188:
1184:
1132:
1131:
1127:
1088:(4): 989–1010.
1075:
1074:
1061:
1054:
1042:
1041:
1034:
998:
997:
993:
968:10.1038/ni.3515
948:
947:
943:
913:
912:
908:
878:
877:
873:
866:
854:
853:
849:
836:. McGraw-Hill.
827:
826:
822:
815:
803:
802:
798:
791:
779:
778:
771:
725:
724:
720:
710:
708:
703:
702:
698:
688:
686:
680:
679:
670:
627:
626:
622:
618:
601:
592:
584:West Nile virus
548:
542:
518:
509:pharmaceuticals
462:antibody titers
430:cytomegalovirus
407:immunodeficient
380:, which is the
370:
332:
314:as part of the
256:
250:
225:
188:capsid proteins
163:
147:microbial toxin
121:that defends a
103:
45:
28:
23:
22:
15:
12:
11:
5:
2512:
2510:
2502:
2501:
2491:
2490:
2486:
2485:
2428:
2379:
2327:
2276:
2227:
2213:
2162:
2110:
2067:
2013:
1962:
1948:
1928:
1899:10.1038/nri891
1893:(9): 706–713.
1874:
1843:(1): a007278.
1829:Mascola, J. R.
1827:Kwong, P. D.;
1819:
1768:
1744:
1692:
1662:
1624:
1573:
1521:
1495:
1464:(4): 447–456.
1443:
1392:
1361:(3): 521–523.
1341:
1320:(9): 695–703.
1297:
1241:
1182:
1145:(1): 499–523.
1125:
1059:
1052:
1032:
1011:(2): 299–311.
991:
941:
906:
871:
864:
847:
820:
813:
796:
790:978-1555819330
789:
769:
718:
696:
668:
647:10.1038/ni1102
641:(9): 934–942.
619:
617:
614:
613:
612:
607:
600:
597:
591:
588:
541:
538:
517:
514:
489:memory B cells
369:
366:
331:
328:
312:T helper cells
249:
246:
224:
221:
162:
159:
105:
104:
102:
101:
94:
87:
79:
76:
75:
72:
68:
67:
64:
60:
59:
56:
52:
51:
47:
46:
43:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2511:
2500:
2497:
2496:
2494:
2481:
2477:
2472:
2467:
2463:
2459:
2455:
2451:
2447:
2443:
2439:
2432:
2429:
2424:
2420:
2415:
2410:
2406:
2402:
2398:
2394:
2390:
2383:
2380:
2375:
2371:
2366:
2361:
2356:
2351:
2347:
2343:
2339:
2331:
2328:
2323:
2319:
2314:
2309:
2304:
2299:
2295:
2291:
2287:
2280:
2277:
2272:
2268:
2263:
2258:
2254:
2250:
2246:
2242:
2238:
2231:
2228:
2223:
2217:
2214:
2209:
2205:
2200:
2195:
2190:
2185:
2181:
2177:
2173:
2166:
2163:
2158:
2154:
2149:
2144:
2139:
2134:
2130:
2126:
2122:
2114:
2111:
2106:
2102:
2098:
2094:
2090:
2086:
2082:
2078:
2071:
2068:
2063:
2059:
2054:
2049:
2044:
2039:
2035:
2031:
2027:
2020:
2018:
2014:
2009:
2005:
2000:
1995:
1990:
1985:
1981:
1977:
1973:
1966:
1963:
1951:
1949:9781489974488
1945:
1941:
1940:
1932:
1929:
1924:
1920:
1916:
1912:
1908:
1904:
1900:
1896:
1892:
1888:
1881:
1879:
1875:
1870:
1866:
1861:
1856:
1851:
1846:
1842:
1838:
1834:
1830:
1823:
1820:
1815:
1811:
1806:
1801:
1796:
1791:
1787:
1783:
1779:
1772:
1769:
1754:
1748:
1745:
1740:
1736:
1731:
1726:
1721:
1716:
1712:
1708:
1704:
1696:
1693:
1680:
1676:
1672:
1666:
1663:
1658:
1654:
1650:
1646:
1642:
1638:
1631:
1629:
1625:
1620:
1616:
1611:
1606:
1601:
1596:
1592:
1588:
1584:
1577:
1574:
1569:
1565:
1560:
1555:
1550:
1545:
1541:
1537:
1533:
1525:
1522:
1510:
1506:
1499:
1496:
1491:
1487:
1482:
1477:
1472:
1467:
1463:
1459:
1455:
1447:
1444:
1439:
1435:
1430:
1425:
1420:
1415:
1411:
1407:
1403:
1396:
1393:
1388:
1384:
1379:
1374:
1369:
1364:
1360:
1356:
1352:
1345:
1342:
1337:
1333:
1328:
1323:
1319:
1315:
1311:
1304:
1302:
1298:
1293:
1289:
1284:
1279:
1274:
1269:
1265:
1261:
1257:
1250:
1248:
1246:
1242:
1237:
1233:
1228:
1223:
1219:
1215:
1210:
1205:
1201:
1197:
1193:
1186:
1183:
1178:
1174:
1169:
1164:
1160:
1156:
1152:
1148:
1144:
1140:
1136:
1129:
1126:
1121:
1117:
1112:
1107:
1103:
1099:
1095:
1091:
1087:
1083:
1079:
1072:
1070:
1068:
1066:
1064:
1060:
1055:
1049:
1045:
1039:
1037:
1033:
1028:
1024:
1019:
1014:
1010:
1006:
1002:
995:
992:
987:
983:
978:
973:
969:
965:
961:
957:
953:
945:
942:
937:
933:
929:
925:
921:
917:
910:
907:
902:
898:
894:
890:
887:(5): 345–53.
886:
882:
875:
872:
867:
861:
857:
851:
848:
843:
839:
835:
834:AccessScience
831:
824:
821:
816:
810:
806:
800:
797:
792:
786:
782:
776:
774:
770:
765:
761:
756:
751:
746:
741:
737:
733:
729:
722:
719:
706:
700:
697:
684:
677:
675:
673:
669:
664:
660:
656:
652:
648:
644:
640:
636:
632:
624:
621:
615:
611:
608:
606:
603:
602:
598:
596:
589:
587:
585:
581:
577:
572:
570:
566:
562:
557:
552:
547:
539:
537:
535:
531:
527:
523:
515:
513:
510:
506:
502:
497:
495:
490:
486:
482:
477:
475:
471:
467:
463:
459:
455:
451:
447:
443:
442:measles virus
439:
435:
431:
427:
423:
419:
414:
412:
408:
404:
400:
396:
392:
387:
383:
379:
375:
367:
365:
363:
359:
355:
350:
345:
341:
337:
329:
327:
325:
321:
317:
313:
309:
305:
301:
297:
293:
289:
285:
281:
277:
273:
269:
265:
261:
255:
247:
245:
243:
239:
235:
231:
222:
220:
218:
214:
210:
206:
202:
197:
194:
189:
185:
181:
177:
167:
160:
158:
156:
152:
148:
144:
140:
136:
132:
128:
124:
120:
116:
112:
100:
95:
93:
88:
86:
81:
80:
77:
73:
69:
65:
61:
57:
53:
48:
41:
36:
30:
19:
2448:(2): 54–62.
2445:
2441:
2431:
2399:(1): 71–77.
2396:
2392:
2382:
2345:
2341:
2330:
2293:
2289:
2279:
2244:
2240:
2230:
2216:
2179:
2175:
2165:
2128:
2124:
2113:
2080:
2076:
2070:
2036:(4): 61–68.
2033:
2029:
1979:
1975:
1965:
1953:. Retrieved
1938:
1931:
1890:
1886:
1840:
1836:
1822:
1785:
1781:
1771:
1759:. Retrieved
1747:
1710:
1706:
1695:
1683:. Retrieved
1674:
1665:
1640:
1636:
1590:
1586:
1576:
1539:
1535:
1524:
1512:. Retrieved
1508:
1498:
1461:
1457:
1446:
1409:
1406:npj Vaccines
1405:
1395:
1358:
1354:
1344:
1317:
1313:
1263:
1260:npj Vaccines
1259:
1199:
1195:
1185:
1142:
1138:
1128:
1085:
1081:
1043:
1008:
1004:
994:
959:
955:
944:
922:(1): 69–86.
919:
915:
909:
884:
880:
874:
855:
850:
833:
823:
804:
799:
780:
735:
731:
721:
709:. Retrieved
699:
687:. Retrieved
638:
634:
623:
593:
573:
555:
553:
549:
519:
498:
478:
438:rabies virus
415:
371:
333:
320:plasma cells
310:produced by
257:
238:Dengue virus
230:immune cells
226:
213:opsonisation
198:
173:
114:
110:
108:
58:Immunoglobin
55:Protein Type
29:
685:. About.com
576:hepatitis C
481:vaccination
474:Palivizumab
382:blood serum
292:interferons
264:bone marrow
2499:Antibodies
1982:: 481314.
1761:4 February
1685:9 December
1202:: 954806.
616:References
544:See also:
505:retroviral
418:polyclonal
276:amino acid
252:See also:
248:Production
242:Zika virus
217:complement
155:host cells
71:Production
50:Properties
2462:1548-3568
2247:: 60–69.
1907:1474-1733
1412:(1): 42.
1266:(1): 19.
1218:2090-939X
1196:ISRN AIDS
1159:1936-122X
1102:1092-2172
494:influenza
378:antiserum
340:Mutations
308:cytokines
209:functions
161:Mechanism
2493:Category
2480:28349376
2423:30455471
2374:31820734
2322:31068427
2271:28527859
2208:31666399
2157:19439467
2105:24030996
2062:30345419
2008:23844315
1915:12209139
1869:22229123
1814:27784796
1739:27732819
1679:Archived
1657:16787244
1619:32167489
1568:32219428
1490:21248066
1438:30323953
1387:25695274
1336:15372080
1292:29263875
1236:24052891
1177:29595997
1120:27784796
1027:14744439
986:27339099
936:12725690
901:24191933
764:27099867
738:: 1–24.
655:15300247
599:See also
590:Research
460:and low
426:epitopes
399:COVID-19
393:and the
316:cellular
284:antigens
143:bacteria
137:against
127:pathogen
119:antibody
117:) is an
63:Function
2471:5401706
2414:6435290
2365:6927745
2313:6600205
2262:7102826
2199:7171597
2148:2704778
2085:Bibcode
2077:Science
2053:6187420
1999:3703378
1955:4 April
1923:9376285
1860:3234457
1805:5116878
1730:5086427
1610:7108922
1559:7101507
1514:5 April
1481:7531589
1429:6173733
1378:4344290
1283:5627241
1227:3767209
1168:6163090
1111:5116878
977:4994874
755:4835181
689:13 June
663:1351951
556:in situ
532:), and
485:B cells
448:(RSV).
436:(HBV),
432:(CMV),
354:glycans
336:genomes
260:B cells
151:antigen
139:viruses
131:humoral
125:from a
74:B cells
2478:
2468:
2460:
2421:
2411:
2372:
2362:
2320:
2310:
2296:(14).
2269:
2259:
2206:
2196:
2155:
2145:
2103:
2060:
2050:
2006:
1996:
1946:
1921:
1913:
1905:
1867:
1857:
1812:
1802:
1737:
1727:
1655:
1617:
1607:
1566:
1556:
1488:
1478:
1436:
1426:
1385:
1375:
1334:
1290:
1280:
1234:
1224:
1216:
1175:
1165:
1157:
1118:
1108:
1100:
1050:
1025:
984:
974:
934:
899:
862:
811:
787:
762:
752:
711:4 July
661:
653:
580:dengue
444:, and
349:steric
254:B cell
170:virus.
2342:eLife
1919:S2CID
1756:(PDF)
659:S2CID
561:gp120
470:ZMapp
358:HIV-1
2476:PMID
2458:ISSN
2419:PMID
2370:PMID
2318:PMID
2267:PMID
2204:PMID
2153:PMID
2101:PMID
2058:PMID
2004:PMID
1980:2013
1957:2020
1944:ISBN
1911:PMID
1903:ISSN
1865:PMID
1810:PMID
1763:2020
1735:PMID
1687:2016
1653:PMID
1615:PMID
1564:PMID
1536:JAMA
1516:2020
1486:PMID
1434:PMID
1383:PMID
1332:PMID
1288:PMID
1232:PMID
1214:ISSN
1200:2013
1173:PMID
1155:ISSN
1116:PMID
1098:ISSN
1048:ISBN
1023:PMID
1005:Cell
982:PMID
932:PMID
897:PMID
860:ISBN
809:ISBN
785:ISBN
760:PMID
736:2014
713:2009
691:2009
651:PMID
582:and
565:gp41
563:and
530:sera
362:AIDS
240:and
215:and
145:and
123:cell
2466:PMC
2450:doi
2409:PMC
2401:doi
2360:PMC
2350:doi
2308:PMC
2298:doi
2257:PMC
2249:doi
2194:PMC
2184:doi
2143:PMC
2133:doi
2093:doi
2081:341
2048:PMC
2038:doi
1994:PMC
1984:doi
1895:doi
1855:PMC
1845:doi
1800:PMC
1790:doi
1725:PMC
1715:doi
1711:375
1645:doi
1605:PMC
1595:doi
1591:130
1554:PMC
1544:doi
1540:323
1509:WHO
1476:PMC
1466:doi
1424:PMC
1414:doi
1373:PMC
1363:doi
1322:doi
1278:PMC
1268:doi
1222:PMC
1204:doi
1163:PMC
1147:doi
1106:PMC
1090:doi
1013:doi
1009:116
972:PMC
964:doi
924:doi
889:doi
838:doi
750:PMC
740:doi
643:doi
420:or
409:or
186:or
115:NAb
2495::
2474:.
2464:.
2456:.
2446:14
2444:.
2440:.
2417:.
2407:.
2395:.
2391:.
2368:.
2358:.
2348:.
2344:.
2340:.
2316:.
2306:.
2294:93
2292:.
2288:.
2265:.
2255:.
2245:24
2243:.
2239:.
2202:.
2192:.
2180:11
2178:.
2174:.
2151:.
2141:.
2129:83
2127:.
2123:.
2099:.
2091:.
2079:.
2056:.
2046:.
2032:.
2028:.
2016:^
2002:.
1992:.
1978:.
1974:.
1917:.
1909:.
1901:.
1889:.
1877:^
1863:.
1853:.
1839:.
1835:.
1808:.
1798:.
1786:80
1784:.
1780:.
1733:.
1723:.
1709:.
1705:.
1673:.
1651:.
1641:12
1639:.
1627:^
1613:.
1603:.
1589:.
1585:.
1562:.
1552:.
1538:.
1534:.
1507:.
1484:.
1474:.
1462:52
1460:.
1456:.
1432:.
1422:.
1408:.
1404:.
1381:.
1371:.
1359:21
1357:.
1353:.
1330:.
1316:.
1312:.
1300:^
1286:.
1276:.
1262:.
1258:.
1244:^
1230:.
1220:.
1212:.
1198:.
1194:.
1171:.
1161:.
1153:.
1143:47
1141:.
1137:.
1114:.
1104:.
1096:.
1086:80
1084:.
1080:.
1062:^
1035:^
1021:.
1007:.
1003:.
980:.
970:.
960:17
958:.
954:.
930:.
920:16
918:.
895:.
885:11
883:.
832:.
772:^
758:.
748:.
734:.
730:.
671:^
657:.
649:.
637:.
633:.
586:.
578:,
440:,
401:.
244:.
141:,
109:A
2482:.
2452::
2425:.
2403::
2397:4
2376:.
2352::
2346:8
2324:.
2300::
2273:.
2251::
2210:.
2186::
2159:.
2135::
2107:.
2095::
2087::
2064:.
2040::
2034:6
2010:.
1986::
1959:.
1925:.
1897::
1891:2
1871:.
1847::
1841:1
1816:.
1792::
1765:.
1741:.
1717::
1689:.
1659:.
1647::
1621:.
1597::
1570:.
1546::
1518:.
1492:.
1468::
1440:.
1416::
1410:3
1389:.
1365::
1338:.
1324::
1318:2
1294:.
1270::
1264:2
1238:.
1206::
1179:.
1149::
1122:.
1092::
1056:.
1029:.
1015::
988:.
966::
938:.
926::
903:.
891::
868:.
844:.
840::
817:.
793:.
766:.
742::
715:.
693:.
665:.
645::
639:5
270:(
113:(
98:e
91:t
84:v
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.