5487: plane, and it could only take a few discrete values. This contradicted the obvious fact that an atom could be turned this way and that relative to the coordinates without restriction. The Sommerfeld quantization can be performed in different canonical coordinates and sometimes gives different answers. The incorporation of radiation corrections was difficult, because it required finding action-angle coordinates for a combined radiation/atom system, which is difficult when the radiation is allowed to escape. The whole theory did not extend to non-integrable motions, which meant that many systems could not be treated even in principle. In the end, the model was replaced by the modern quantum-mechanical treatment of the
5210:
experimentally obtained screening term should be replaced by 0.4). Notwithstanding its restricted validity, Moseley's law not only established the objective meaning of atomic number, but as Bohr noted, it also did more than the
Rydberg derivation to establish the validity of the Rutherford/Van den Broek/Bohr nuclear model of the atom, with atomic number (place on the periodic table) standing for whole units of nuclear charge. Van den Broek had published his model in January 1913 showing the periodic table was arranged according to charge while Bohr's atomic model was not published until July 1913.
4878:
which ... only a certain number of electrons—namely, eight in our case—should be arranged. As soon as one ring or shell is completed, a new one has to be started for the next element; the number of electrons, which are most easily accessible, and lie at the outermost periphery, increases again from element to element and, therefore, in the formation of each new shell the chemical periodicity is repeated." Later, chemist
Langmuir realized that the effect was caused by charge screening, with an inner shell containing only 2 electrons. In his 1919 paper,
4784: = 3 minus the screening effect of the other, which crudely reduces the nuclear charge by 1 unit. This means that the innermost electrons orbit at approximately 1/2 the Bohr radius. The outermost electron in lithium orbits at roughly the Bohr radius, since the two inner electrons reduce the nuclear charge by 2. This outer electron should be at nearly one Bohr radius from the nucleus. Because the electrons strongly repel each other, the effective charge description is very approximate; the effective charge
306:
354:, was the best available. Thomson proposed a model with electrons rotating in coplanar rings within an atomic-sized, positively-charged, spherical volume. Thomson showed that this model was mechanically stable by lengthy calculations and electrodynamically stable under his original assumption of thousands of electrons per atom. Moreover he suggested that the particularly stable configurations of electrons in rings was connected to chemical properties of the atoms and he developed a formula for the scattering of
4808:
filled when it has two electrons, which explains why helium is inert. The second orbit allows eight electrons, and when it is full the atom is neon, again inert. The third orbital contains eight again, except that in the more correct
Sommerfeld treatment (reproduced in modern quantum mechanics) there are extra "d" electrons. The third orbit may hold an extra 10 d electrons, but these positions are not filled until a few more orbitals from the next level are filled (filling the n=3 d orbitals produces the 10
1575:
5593:
5254:"around" the nucleus at all, but merely to go tightly around it in an ellipse with zero area (this may be pictured as "back and forth", without striking or interacting with the nucleus). This is only reproduced in a more sophisticated semiclassical treatment like Sommerfeld's. Still, even the most sophisticated semiclassical model fails to explain the fact that the lowest energy state is spherically symmetric – it doesn't point in any particular direction.
3549:; the orbit energy begins to be comparable to rest energy. Sufficiently large nuclei, if they were stable, would reduce their charge by creating a bound electron from the vacuum, ejecting the positron to infinity. This is the theoretical phenomenon of electromagnetic charge screening which predicts a maximum nuclear charge. Emission of such positrons has been observed in the collisions of heavy ions to create temporary super-heavy nuclei.
4532:< 8." For smaller atoms, the electron shells would be filled as follows: "rings of electrons will only join together if they contain equal numbers of electrons; and that accordingly the numbers of electrons on inner rings will only be 2, 4, 8". However, in larger atoms the innermost shell would contain eight electrons, "on the other hand, the periodic system of the elements strongly suggests that already in neon
5343:
7785:
7795:
49:
5271:
coincidental agreements are found between the semiclassical vs. full quantum mechanical treatment of the atom; these include identical energy levels in the hydrogen atom and the derivation of a fine-structure constant, which arises from the relativistic Bohr–Sommerfeld model (see below) and which happens to be equal to an entirely different concept, in full modern quantum mechanics).
5523:. At higher-order perturbations, however, the Bohr model and quantum mechanics differ, and measurements of the Stark effect under high field strengths helped confirm the correctness of quantum mechanics over the Bohr model. The prevailing theory behind this difference lies in the shapes of the orbitals of the electrons, which vary according to the energy state of the electron.
3714:
convincing
Rutherford of the importance of Bohr's model, for it explained the fact that the frequencies of lines in the spectra for singly ionized helium do not differ from those of hydrogen by a factor of exactly 4, but rather by 4 times the ratio of the reduced mass for the hydrogen vs. the helium systems, which was much closer to the experimental ratio than exactly 4.
477:. To avoid immediate collapse of this system he required that electrons come in pairs so the rotational acceleration of each electron was matched across the orbit. By 1913 Bohr had already shown from the analysis of alpha particle energy loss that hydrogen had only a single electron. Thus Bohr's atomic model would abandon classical electrodynamics.
250:. While the Rydberg formula had been known experimentally, it did not gain a theoretical basis until the Bohr model was introduced. Not only did the Bohr model explain the reasons for the structure of the Rydberg formula, it also provided a justification for the fundamental physical constants that make up the formula's empirical results.
3034:
5263:, the proper deformation (careful full extension) of the semi-classical result adjusts the angular momentum value to the correct effective one. As a consequence, the physical ground state expression is obtained through a shift of the vanishing quantum angular momentum expression, which corresponds to spherical symmetry.
3708:
4885:
In the
Moseley experiment, one of the innermost electrons in the atom is knocked out, leaving a vacancy in the lowest Bohr orbit, which contains a single remaining electron. This vacancy is then filled by an electron from the next orbit, which has n=2. But the n=2 electrons see an effective charge of
457:
published a model of the atom which would influence Bohr's model. Nicholson developed his model based on the analysis of astrophysical spectroscopy. He connected the observed spectral line frequencies with the orbits of electrons in his atoms. The connection he adopted associated the atomic electron
4799:
of gases and density of pure crystalline solids. Atoms tend to get smaller toward the right in the periodic table, and become much larger at the next line of the table. Atoms to the right of the table tend to gain electrons, while atoms to the left tend to lose them. Every element on the last column
410:
By the early twentieth century, it was expected that the atom would account for the spectral lines. In 1897, Lord
Rayleigh analyzed the problem. By 1906, Rayleigh said, "The frequencies observed in the spectrum may not be frequencies of disturbance or of oscillation in the ordinary sense at all, but
3713:
However, these numbers are very nearly the same, due to the much larger mass of the proton, about 1836.1 times the mass of the electron, so that the reduced mass in the system is the mass of the electron multiplied by the constant 1836.1/(1+1836.1) = 0.99946. This fact was historically important in
440:
The discussions outlined the need for the quantum theory to be included in the atom and the difficulties in atomic theory. Planck explicitly mentions the failings of classical mechanics. Bohr's first paper on his atomic model cites the Solvay proceedings saying: "Whatever the alteration in the laws
358:
that seemed to match experimental results. However
Thomson himself later showed that the atom had a factor of a thousand fewer electrons, challenging the stability argument and forcing the poorly understood positive sphere to have most of the atom's mass. Thomson was also unable to explain the many
5253:
for the ground state orbital angular momentum: The angular momentum in the true ground state is known to be zero from experiment. Although mental pictures fail somewhat at these levels of scale, an electron in the lowest modern "orbital" with no orbital momentum, may be thought of as not to rotate
3717:
For positronium, the formula uses the reduced mass also, but in this case, it is exactly the electron mass divided by 2. For any value of the radius, the electron and the positron are each moving at half the speed around their common center of mass, and each has only one fourth the kinetic energy.
4877:
in 1914 and in 1916 who explained that in the periodic table new elements would be created as electrons were added to the outer shell. In Kossel's paper, he writes: "This leads to the conclusion that the electrons, which are added further, should be put into concentric rings or shells, on each of
4807:
In the shell model, this phenomenon is explained by shell-filling. Successive atoms become smaller because they are filling orbits of the same size, until the orbit is full, at which point the next atom in the table has a loosely bound outer electron, causing it to expand. The first Bohr orbit is
4516:
Bohr's original three papers in 1913 described mainly the electron configuration in lighter elements. Bohr called his electron shells, "rings" in 1913. Atomic orbitals within shells did not exist at the time of his planetary model. Bohr explains in Part 3 of his famous 1913 paper that the maximum
465:
The other critical influence of
Nicholson work was his detailed analysis of spectra. Before Nicholson's work Bohr thought the spectral data was not useful for understanding atoms. In comparing his work to Nicholson's, Bohr came to understand the spectral data and their value. When he then learned
4869:
Moseley wrote to Bohr, puzzled about his results, but Bohr was not able to help. At that time, he thought that the postulated innermost "K" shell of electrons should have at least four electrons, not the two which would have neatly explained the result. So
Moseley published his results without a
2037:
However, in quantum mechanics, the quantization of angular momentum leads to discrete energy levels of the orbits, and the emitted frequencies are quantized according to the energy differences between these levels. This discrete nature of energy levels introduces a fundamental departure from the
5270:
that grows denser near the nucleus. The rate-constant of probability-decay in hydrogen is equal to the inverse of the Bohr radius, but since Bohr worked with circular orbits, not zero area ellipses, the fact that these two numbers exactly agree is considered a "coincidence". (However, many such
462:. Whereas Planck focused on a quantum of energy, Nicholson's angular momentum quantum relates to orbital frequency. This new concept gave Planck constant an atomic meaning for the first time. In his 1913 paper Bohr cites Nicholson as finding quantized angular momentum important for the atom.
2033:
In classical mechanics, if an electron is orbiting around an atom with period T, and if its coupling to the electromagnetic field is weak, so that the orbit doesn't decay very much in one cycle, it will emit electromagnetic radiation in a pattern repeating at every period, so that the
Fourier
5526:
The Bohr–Sommerfeld quantization conditions lead to questions in modern mathematics. Consistent semiclassical quantization condition requires a certain type of structure on the phase space, which places topological limitations on the types of symplectic manifolds which can be quantized. In
4185:
5209:
is the Rydberg constant, in terms of frequency equal to 3.28 x 10 Hz. For values of Z between 11 and 31 this latter relationship had been empirically derived by Moseley, in a simple (linear) plot of the square root of X-ray frequency against atomic number (however, for silver, Z = 47, the
4756:
of 1904, although Kossel had already predicted a maximum of eight per shell in 1916. Heavier atoms have more protons in the nucleus, and more electrons to cancel the charge. Bohr took from these chemists the idea that each discrete orbit could only hold a certain number of electrons. Per
444:
Rutherford could have outlined these points to Bohr or given him a copy of the proceedings since he quoted from them and used them as a reference. In a later interview, Bohr said he read the Solvay reports and that Rutherford's remarks about the Solvay Congress were very interesting.
2809:
5286:
group) can also be approximately predicted. Also, if the empiric electron–nuclear screening factors for many atoms are known, many other spectral lines can be deduced from the information, in similar atoms of differing elements, via the Ritz–Rydberg combination principles (see
6414:
Well, yes," says Bohr. "But I can hardly imagine it will involve light quanta. Look, even if Einstein had found an unassailable proof of their existence and would want to inform me by telegram, this telegram would only reach me because of the existence and reality of radio
2041:
Bohr considered circular orbits. Classically, these orbits must decay to smaller circles when photons are emitted. The energy level spacing between circular orbits can be calculated with the correspondence formula. For a hydrogen atom, the classical orbits have a period
4738:, Bohr extended the model of hydrogen to give an approximate model for heavier atoms. This gave a physical picture that reproduced many known atomic properties for the first time although these properties were proposed contemporarily with the identical work of chemist
332:. As electrons in orbit are continuously accelerating, they would be mechanically unstable. Larmor noted that electromagnetic effect of multiple electrons suitable arranged would cancel each other, a restriction applied in the subsequent classical atomic models.
5182:
5360:, which suggested that electrons travel in elliptical orbits around a nucleus instead of the Bohr model's circular orbits. This model supplemented the quantized angular momentum condition of the Bohr model with an additional radial quantization condition, the
1537:
divided by the electron momentum. In 1913, however, Bohr justified his rule by appealing to the correspondence principle, without providing any sort of wave interpretation. In 1913, the wave behavior of matter particles such as the electron was not suspected.
2374:
686:, of 0.0529 nm for hydrogen. Once an electron is in this lowest orbit, it can get no closer to the nucleus. Starting from the angular momentum quantum rule as Bohr admits is previously given by Nicholson in his 1912 paper, Bohr was able to calculate the
2824:
5579:
of the molecular system is achieved through the balance of forces between the forces of attraction of nuclei to the plane of the ring of electrons and the forces of mutual repulsion of the nuclei. The Bohr model of the chemical bond took into account the
5037:
5514:
However, this is not to say that the Bohr–Sommerfeld model was without its successes. Calculations based on the Bohr–Sommerfeld model were able to accurately explain a number of more complex atomic spectral effects. For example, up to first-order
4726:
In Bohr's third 1913 paper Part III called "Systems Containing Several Nuclei", he says that two atoms form molecules on a symmetrical plane and he reverts to describing hydrogen. The 1913 Bohr model did not discuss higher elements in detail and
3562:
3186:
4413:
3809:
developed increasingly accurate empirical formula matching measured atomic spectral lines. Critical for Bohr's later work, Rydberg expressed his formula in terms of wave-number, equivalent to frequency. These formula contained a constant,
480:
Nicholson's model of radiation was quantum but was attached to the orbits of the electrons. Bohr would adopt Nicholson's interpretation of frequency quantization, but associate it with differences in energy levels of his model of hydrogen.
7586:: Elektronenbahnen, Quantensprünge und Spektren, in: Charlotte Bigg & Jochen Hennig (eds.) Atombilder. Ikonografien des Atoms in Wissenschaft und Öffentlichkeit des 20. Jahrhunderts, Göttingen: Wallstein-Verlag 2009, pp. 51–61
4025:
1734:
2550:
3313:
2658:
441:
of motion of the electrons may be, it seems necessary to introduce in the laws in question a quantity foreign to the classical electrodynamics, i.e. the Planck constant, or as it often is called the elementary quantum of action."
4820:
Niels Bohr said in 1962: "You see actually the Rutherford work was not taken seriously. We cannot understand today, but it was not taken seriously at all. There was no mention of it any place. The great change came from Moseley."
4187:
Therefore Bohr's theory gives the Rydberg formula and moreover the numerical value the Rydberg constant for hydrogen in terms of more fundamental constants of nature, including the electron's charge, the electron's mass, and the
1561:
independently, and by different reasoning. Schrödinger employed de Broglie's matter waves, but sought wave solutions of a three-dimensional wave equation describing electrons that were constrained to move about the nucleus of a
389:
developed a new scattering model, showing that the observed large angle scattering could be explained by a compact, highly charged mass at the center of the atom. Rutherford scattering did not involve the electrons and thus his
3399:
4812:). The irregular filling pattern is an effect of interactions between electrons, which are not taken into account in either the Bohr or Sommerfeld models and which are difficult to calculate even in the modern treatment.
1850:
4894:, and lower it by −1 (due to the electron's negative charge screening the nuclear positive charge). The energy gained by an electron dropping from the second shell to the first gives Moseley's law for K-alpha lines,
3908:
4768:
This model is even more approximate than the model of hydrogen, because it treats the electrons in each shell as non-interacting. But the repulsions of electrons are taken into account somewhat by the phenomenon of
4773:. The electrons in outer orbits do not only orbit the nucleus, but they also move around the inner electrons, so the effective charge Z that they feel is reduced by the number of the electrons in the inner orbit.
3917:
between orbital energy levels is able to explain these formula. For the hydrogen atom Bohr starts with his derived formula for the energy released as a free electron moves into a stable circular orbit indexed by
2684:
2134:
4020:
5605:
has been widely used as a symbol for atoms and even for "atomic" energy (even though this is more properly considered nuclear energy). Examples of its use over the past century include but are not limited to:
2227:
3499:
3419:
is the atomic number. This will now give us energy levels for hydrogenic (hydrogen-like) atoms, which can serve as a rough order-of-magnitude approximation of the actual energy levels. So for nuclei with
5600:
Although Bohr's atomic model was superseded by quantum models in the 1920's, the visual image of electrons orbiting a nucleus has remained the popular concept of atoms. The concept of an atom as a tiny
5294:
The relative intensities of spectral lines; although in some simple cases, Bohr's formula or modifications of it, was able to provide reasonable estimates (for example, calculations by Kramers for the
4268:
3780:
1917:
1582:
The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the
7472:
p. 434. (provides an elegant reformulation of the Bohr–Sommerfeld quantization conditions, as well as an important insight into the quantization of non-integrable (chaotic) dynamical systems.)
316:
Until the second decade of the 20th century, atomic models were generally speculative and even the concept of atoms let alone atoms with internal structure faced opposition from some scientists.
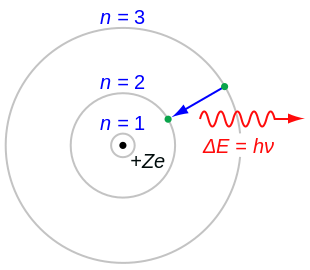
520:
suggests. These stable orbits are called stationary orbits and are attained at certain discrete distances from the nucleus. The electron cannot have any other orbit in between the discrete ones.
4846:
in terms of models as these had been published before Moseley's work and Moseley's 1913 paper was published the same month as the first Bohr model paper). The two additional assumptions that
5437:
2443:
5048:
3029:{\displaystyle E=-{\frac {Zk_{\mathrm {e} }e^{2}}{2r_{n}}}=-{\frac {Z^{2}(k_{\mathrm {e} }e^{2})^{2}m_{\mathrm {e} }}{2\hbar ^{2}n^{2}}}\approx {\frac {-13.6Z^{2}}{n^{2}}}~\mathrm {eV} .}
2265:
2021:
437:. Lorentz explained that the Planck constant could be taken as determining the size of atoms, or that the size of atoms could be taken to determine the Planck constant as Haas had done.
1443:
781:
569:
4314:
1229:
1345:
1287:
4900:
324:
In the late 1800's speculations on the possible structure of the atom included planetary models with orbiting charged electrons. These models faced a significant constraint. In 1897,
3068:, these are also the binding energies of a highly excited atom with one electron in a large circular orbit around the rest of the atom. The hydrogen formula also coincides with the
3235:
1774:. It is assumed here that the mass of the nucleus is much larger than the electron mass (which is a good assumption). This equation determines the electron's speed at any radius:
269:. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to
4536:= 10 an inner ring of eight electrons will occur". Bohr wrote "From the above we are led to the following possible scheme for the arrangement of the electrons in light atoms:"
1174:
826:
of the electromagnetic field. Quantization of the electromagnetic field was explained by the discreteness of the atomic energy levels; Bohr did not believe in the existence of
3703:{\displaystyle m_{\text{red}}={\frac {m_{\mathrm {e} }m_{\mathrm {p} }}{m_{\mathrm {e} }+m_{\mathrm {p} }}}=m_{\mathrm {e} }{\frac {1}{1+m_{\mathrm {e} }/m_{\mathrm {p} }}}.}
660:
1515:
698:. In these orbits, the electron's acceleration does not result in radiation and energy loss. The Bohr model of an atom was based upon Planck's quantum theory of radiation.
7479:
La théorie du rayonnement et les quanta : rapports et discussions de la réunion tenue à Bruxelles, du 30 octobre au 3 novembre 1911, sous les auspices de M.E. Solvay
3088:
2168:
619:
4325:
5575:, the electrons of the atoms of the molecule form a rotating ring whose plane is perpendicular to the axis of the molecule and equidistant from the atomic nuclei. The
2257:
938:
5330:
Doublets and triplets appear in the spectra of some atoms as very close pairs of lines. Bohr's model cannot say why some energy levels should be very close together.
1125:
4890: − 1, which is the value appropriate for the charge of the nucleus, when a single electron remains in the lowest Bohr orbit to screen the nuclear charge +
3936:
1394:
1044:), the two orbits involved in the emission process have nearly the same rotation frequency, so that the classical orbital frequency is not ambiguous. But for small
905:
871:
851:
719:
1477:
1374:
1644:
3828:
2465:
1535:
1082:
1062:
1038:
1018:
998:
978:
958:
801:
680:
3837:
3246:
2565:
3405:
Since this derivation is with the assumption that the nucleus is orbited by one electron, we can generalize this result by letting the nucleus have a charge
6427:
6836:
4752:
is credited with the first viable arrangement of electrons in shells with only two in the first shell and going up to eight in the next according to the
3941:
433:
in the discussion of Planck's lecture raised the question of the composition of the atom based on Thomson's model with the quantum modification added by
3324:
701:
Electrons can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency
5278:
Much of the spectra of larger atoms. At best, it can make predictions about the K-alpha and some L-alpha X-ray emission spectra for larger atoms, if
4418:
Bohr's derivation of the Rydberg constant, as well as the concomitant agreement of Bohr's formula with experimentally observed spectral lines of the
4180:{\displaystyle h\nu =W_{\tau _{2}}-W_{\tau _{1}}={\frac {2\pi ^{2}me^{4}}{h^{2}}}\left({\frac {1}{\tau _{2}^{2}}}-{\frac {1}{\tau _{1}^{2}}}\right)}
1779:
394:
was incomplete. Bohr begins his first paper on his atomic model by describing Rutherford's atom as consisting of a small, dense, positively charged
7001:
Dahl, Jens Peder; Springborg, Michael (10 December 1982). "Wigner's phase space function and atomic structure: I. The hydrogen atom ground state".
4791:
The shell model was able to qualitatively explain many of the mysterious properties of atoms which became codified in the late 19th century in the
7680:
5611:
523:
The stationary orbits are attained at distances for which the angular momentum of the revolving electron is an integer multiple of the reduced
4195:
7833:
7599:
7228:
6565:
6372:
On the Constitution of Atoms and Molecules ... Papers of 1913 reprinted from the Philosophical Magazine, with an introduction by L. Rosenfeld
6307:
6204:
5994:
5922:
5884:
2804:{\displaystyle r_{1}={\frac {\hbar ^{2}}{k_{\mathrm {e} }e^{2}m_{\mathrm {e} }}}\approx 5.29\times 10^{-11}~\mathrm {m} =52.9~\mathrm {pm} .}
4457:=3) series, and successful theoretical prediction of other lines not yet observed, was one reason that his model was immediately accepted.
1135:
condition: the electron is described by a wave and a whole number of wavelengths must fit along the circumference of the electron's orbit:
5309:
in spectral lines, which are known to be due to a variety of relativistic and subtle effects, as well as complications from electron spin.
2064:
2176:
4882:
postulated the existence of "cells" which could each only contain two electrons each, and these were arranged in "equidistant layers".
4731:
was one of the first to prove in 1914 that it couldn't work for lithium, but was an attractive theory for hydrogen and ionized helium.
7119:
5622:
4838:. Moseley's empiric formula was found to be derivable from Rydberg's formula and later Bohr's formula (Moseley actually mentions only
7574:
7555:
7525:
5727:
5361:
4502:
and the later discussion of the "Shell Model of the Atom" below). This was established empirically before Bohr presented his model.
3430:
368:
281:
in 1910 but was rejected until the 1911 Solvay Congress where it was thoroughly discussed. The quantum theory of the period between
466:
from a friend about Balmer's compact formula for the spectral line data, Bohr quickly realized his model would match it in detail.
5549:
Bohr also updated his model in 1922, assuming that certain numbers of electrons (for example, 2, 8, and 18) correspond to stable "
4828:
found an empirical relationship between the strongest X-ray line emitted by atoms under electron bombardment (then known as the
7477:
5568:
5562:
1100:
469:
Nicholson's model was based on classical electrodynamics with negative electron orbiting a positive nucleus along the lines of
3724:
4792:
1861:
4748:
who corrected Bohr's work to show that electrons interacted through the outer rings, and Kossel called the rings: "shells".
2139:
It is possible to determine the energy level spacings by recursively stepping down orbit by orbit, but there is a shortcut.
31:
516:
The electron is able to revolve in certain stable orbits around the nucleus without radiating any energy, contrary to what
1931:, the energy is zero, corresponding to a motionless electron infinitely far from the proton. The total energy is half the
7219:
Schirrmacher, Arne (2009). "Bohr's Atomic Model". In Greenberger, Daniel M.; Hentschel, Klaus; Weinert, Friedel (eds.).
6894:(Interview). Interviewed by Thomas S. Kuhn; Leon Rosenfeld; Aage Petersen; Erik Rudinger. American Institute of Physics.
6232:(Interview). Interviewed by Thomas S. Kuhn; Leon Rosenfeld; Aage Petersen; Erik Rudinger. American Institute of Physics.
5714:
5320:; these are also due to more complicated quantum principles interacting with electron spin and orbital magnetic fields.
309:
Bohr model in 1921 after Sommerfeld expansion of 1913 model showing maximum electrons per shell with shells labeled in
7848:
7673:
6930:
5327:
in that it considers electrons to have known orbits and locations, two things which cannot be measured simultaneously.
5177:{\displaystyle f=\nu =R_{\mathrm {v} }\left({\frac {3}{4}}\right)(Z-1)^{2}=(2.46\times 10^{15}~{\text{Hz}})(Z-1)^{2}.}
277:
before moving on to the more accurate, but more complex, valence shell atom. A related quantum model was proposed by
7655:—An interactive simulation to intuitively explain the quantization condition of standing waves in Bohr's atomic mode
7609:
Kragh, Helge (November 2011). "Conceptual objections to the Bohr atomic theory — do electrons have a 'free will'?".
6977:
5374:
5282:
additional ad hoc assumptions are made. Emission spectra for atoms with a single outer-shell electron (atoms in the
2393:
2369:{\displaystyle \Delta E\propto {\frac {1}{(L+\Delta L)^{2}}}-{\frac {1}{L^{2}}}\approx -{\frac {2\Delta L}{L^{3}}}.}
880:
at integer multiples of this frequency. This result is obtained from the Bohr model for jumps between energy levels
265:
of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an
5677:
3914:
517:
266:
6623:
Bohr, N. (1913). "On the Constitution of Atoms and Molecules, Part II. Systems containing only a Single Nucleus".
5708:
5351:
489:
Next, Bohr was told by his friend, Hans Hansen, that the Balmer series is calculated using the Balmer formula, an
411:
rather form an essential part of the original constitution of the atom as determined by conditions of stability."
7838:
7711:
7652:
6982:
1979:
6891:
6805:
6229:
5903:. RePoSS: Research Publications on Science Studies 10. Aarhus: Centre for Science Studies, University of Aarhus.
5032:{\displaystyle E=h\nu =E_{i}-E_{f}=R_{\mathrm {E} }(Z-1)^{2}\left({\frac {1}{1^{2}}}-{\frac {1}{2^{2}}}\right),}
2379:
This is as desired for equally spaced angular momenta. If one kept track of the constants, the spacing would be
1402:
728:
530:
427:'s oscillators, their energy quanta and the relationship between a theory of radiation and material particles.
7823:
7689:
5532:
5469:
5465:
4278:
1193:
1085:
622:
300:
247:
99:
1298:
1240:
1184:
5333:
Multi-electron atoms do not have energy levels predicted by the model. It does not work for (neutral) helium.
1927:. This means that it takes energy to pull the orbiting electron away from the proton. For infinite values of
235:
interpretation introduced by Haas and Nicholson, but forsaking any attempt to explain radiation according to
7828:
5504:
5480:
5258:
3316:
1935:, the difference being the kinetic energy of the electron. This is also true for noncircular orbits by the
1554:
262:
3201:
328:
showed that in classical electrodynamics an accelerating charge would radiate power, a result known as the
7666:
7083:
6973:
6906:
5550:
5543:
4843:
4762:
4728:
1096:
823:
454:
305:
228:
195:
In the history of atomic physics, it followed, and ultimately replaced, several earlier models, including
1088:, requiring quantum theory to agree with the classical theory only in the limit of large quantum numbers.
231:'s nuclear quantum model (1912). The improvement over the 1911 Rutherford model mainly concerned the new
5661:
5633:
5519:, the Bohr model and quantum mechanics make the same predictions for the spectral line splitting in the
5324:
694:
atoms and ions. These orbits are associated with definite energies and are also called energy shells or
5983:
John L, Heilbron (1985). "Bohr's First Theories of the Atom". In French, A. P.; Kennedy, P. J. (eds.).
3718:
The total kinetic energy is half what it would be for a single electron moving around a heavy nucleus.
1141:
497:
in 1885 that described wavelengths of some spectral lines of hydrogen. This was further generalized by
5984:
5291:). All these techniques essentially make use of Bohr's Newtonian energy-potential picture of the atom.
1574:
7618:
7426:
7385:
7352:
7319:
7286:
7173:
7051:
6939:
6775:
6694:
6655:
6460:
6077:
5794:
5768:
5703:
5636:
5508:
4739:
1558:
812:
83:
6448:
5592:
3181:{\displaystyle R_{\mathrm {E} }={\frac {(k_{\mathrm {e} }e^{2})^{2}m_{\mathrm {e} }}{2\hbar ^{2}}}.}
1084:), the radiation frequency has no unambiguous classical interpretation. This marks the birth of the
628:
512:
put forth three postulates to provide an electron model consistent with Rutherford's nuclear model:
90:). The orbits in which the electron may travel are shown as grey circles; their radius increases as
7843:
6928:
M.A.B. Whitaker (1999). "The Bohr–Moseley synthesis and a simple model for atomic x-ray energies".
5669:
5576:
5516:
5473:
5306:
4809:
346:
When Bohr began his work on a new atomic theory in the summer of 1912 the atomic model proposed by
17:
7794:
4408:{\displaystyle {\frac {1}{\lambda }}=R\left({\frac {1}{n_{f}^{2}}}-{\frac {1}{n_{i}^{2}}}\right).}
1486:
7769:
7732:
7634:
7442:
7401:
7163:
7042:
7018:
6955:
6828:
6710:
6518:
6484:
6275:
6267:
6164:
6039:
5962:
5852:
5357:
2149:
1563:
574:
474:
351:
341:
287:
216:
158:
4460:
To apply to atoms with more than one electron, the Rydberg formula can be modified by replacing
7112:
The Life of Stars: The Controversial Inception and Emergence of the Theory of Stellar Structure
6585:
4849:
this X-ray line came from a transition between energy levels with quantum numbers 1 and 2, and
4795:. One property was the size of atoms, which could be determined approximately by measuring the
3050:
less energy than a motionless electron infinitely far from the nucleus. The next energy level (
82:
and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of
7595:
7570:
7551:
7521:
7483:
7224:
7201:
7115:
6820:
6605:
6561:
6538:
6476:
6375:
6349:
6303:
6200:
6156:
6031:
5990:
5918:
5880:
5721:
5647:
5581:
5572:
5365:
5232:
4839:
3910:
Despite many attempts, no theory of the atom could reproduce these relatively simple formula.
2239:
1752:
1632:
1550:
1542:
434:
420:
386:
278:
270:
236:
232:
150:
910:
7802:
7738:
7626:
7434:
7393:
7360:
7327:
7294:
7191:
7181:
7092:
7059:
7010:
6947:
6869:
6783:
6741:
6702:
6663:
6597:
6530:
6468:
6339:
6259:
6192:
6148:
6116:
6085:
6023:
5954:
5844:
5776:
5602:
5496:
5461:
4770:
4761:, after that the orbit is full, the next level would have to be used. This gives the atom a
4498:
is constant representing a screening effect due to the inner-shell and other electrons (see
3831:
3802:
3238:
1952:
1932:
1763:
1546:
1110:
498:
258:
224:
185:
165:
only to be replaced by the quantum atomic model in the 1920s. It consists of a small, dense
154:
4776:
For example, the lithium atom has two electrons in the lowest 1s orbit, and these orbit at
3921:
1729:{\displaystyle {\frac {m_{\mathrm {e} }v^{2}}{r}}={\frac {Zk_{\mathrm {e} }e^{2}}{r^{2}}},}
1379:
883:
7725:
7583:
6853:
5698:
5615:
5536:
5500:
5288:
4879:
4749:
4189:
3792:
2545:{\displaystyle m_{\text{e}}{\sqrt {\dfrac {k_{\text{e}}Ze^{2}}{m_{\text{e}}r}}}r=n\hbar ,}
2038:
classical radiation law, giving rise to distinct spectral lines in the emitted radiation.
856:
836:
722:
704:
524:
502:
459:
430:
391:
385:
occasionally scatter at large angles, a result inconsistent with Thomson's model. In 1911
243:
208:
4765:
designed by Kossel, Langmuir, and Bury, in which each shell corresponds to a Bohr orbit.
3075:
The combination of natural constants in the energy formula is called the Rydberg energy (
1454:
1353:
7622:
7430:
7389:
7356:
7323:
7290:
7177:
7055:
6943:
6779:
6698:
6659:
6464:
6186:
6081:
5772:
5479:
The Bohr–Sommerfeld model was fundamentally inconsistent and led to many paradoxes. The
4858:
when used in the formula for atoms heavier than hydrogen, should be diminished by 1, to
3308:{\displaystyle {\frac {k_{\mathrm {e} }e^{2}}{\hbar c}}=\alpha \approx {\frac {1}{137}}}
2653:{\displaystyle r_{n}={\frac {n^{2}\hbar ^{2}}{Zk_{\mathrm {e} }e^{2}m_{\mathrm {e} }}}.}
7798:
7788:
7763:
7514:
7196:
7151:
5528:
5492:
5317:
5302:
5267:
4874:
4745:
4735:
4511:
4499:
4445:
3813:
3512:
3505:
3504:
The actual energy levels cannot be solved analytically for more than one electron (see
3069:
1936:
1603:
1599:
1545:, in which Bohr's model of electrons traveling in quantized orbits was extended into a
1520:
1067:
1047:
1023:
1003:
983:
963:
943:
786:
665:
395:
382:
378:
329:
310:
285:(1900) and the advent of a mature quantum mechanics (1925) is often referred to as the
166:
130:
79:
1179:
According to de Broglie's hypothesis, matter particles such as the electron behave as
7817:
7638:
6959:
6951:
6432:
6279:
6250:
McCormmach, Russell (1 January 1966). "The atomic theory of John William Nicholson".
5684:
5488:
5313:
4825:
4432:
3798:
3516:
3192:
1771:
1607:
1583:
1132:
1041:
494:
325:
254:
196:
107:
53:
38:
7405:
6832:
6488:
5900:
1602:
of any atom where one electron is far away from everything else. It can be used for
7718:
7446:
7244:
7150:
Svidzinsky, Anatoly A.; Scully, Marlan O.; Herschbach, Dudley R. (23 August 2005).
7022:
6714:
6502:
Bohr, N. (1985). "Rydberg's discovery of the spectral laws". In Kalckar, J. (ed.).
6168:
5798:
5643:
5520:
5342:
5295:
4419:
4319:
these results can be expressed in terms of the wavelength of the photon given off:
3806:
3553:
3047:
2047:
695:
470:
423:
in 1911, on the subject of Radiation and Quanta. Much of the discussions concerned
274:
204:
181:
75:
5759:
Lakhtakia, Akhlesh; Salpeter, Edwin E. (1996). "Models and Modelers of Hydrogen".
7630:
6601:
3394:{\displaystyle R_{\mathrm {E} }={\frac {1}{2}}(m_{\mathrm {e} }c^{2})\alpha ^{2}}
7704:
6196:
5539:
2675:
2034:
transform of the pattern will only have frequencies which are multiples of 1/T.
1636:
1595:
1480:
1180:
683:
374:
347:
220:
200:
162:
42:
7345:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
7312:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
7279:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
7156:
Proceedings of the National Academy of Sciences of the United States of America
6648:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
6523:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
6188:
Old Quantum Theory and Early Quantum Mechanics. Challenges in Physics Education
6109:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
6070:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
3556:
of electron and proton in all situations, instead of the mass of the electron,
3191:
This expression is clarified by interpreting it in combinations that form more
7487:
7476:
de Broglie, Maurice; Langevin, Paul; Solvay, Ernest; Einstein, Albert (1912).
7364:
7331:
7298:
7096:
7014:
6887:
6667:
6534:
6447:
Müller, U.; de Reus, T.; Reinhardt, J.; Müller, B.; Greiner, W. (1988-03-01).
6225:
6120:
6089:
4753:
1845:{\displaystyle v={\sqrt {\frac {Zk_{\mathrm {e} }e^{2}}{m_{\mathrm {e} }r}}}.}
1578:
Models depicting electron energy levels in hydrogen, helium, lithium, and neon
1128:
1095:(BKS theory) is a failed attempt to extend the Bohr model, which violates the
1092:
509:
424:
355:
282:
146:
118:
48:
7221:
Compendium of quantum physics: concepts, experiments, history, and philosophy
7063:
6824:
6787:
6609:
6542:
6472:
6379:
6353:
6344:
6327:
6035:
5877:
Niels Bohr and the Quantum Atom: The Bohr Model of Atomic Structure 1913–1925
5217:
line of Moseley's time is now known to be a pair of close lines, written as (
1557:
of the same theory, wave mechanics, was discovered by the Austrian physicist
192:, and with the electron energies quantized (assuming only discrete values).
7186:
4801:
4796:
877:
490:
122:
7205:
6978:"Die Radioelemente, das periodische System und die Konstitution der. Atome"
6160:
5945:
Heilbron, John L.; Kuhn, Thomas S. (1969). "The Genesis of the Bohr Atom".
4734:
In 1921, following the work of chemists and others involved in work on the
6730:"Langmuir's Theory of the Arrangement of Electrons in Atoms and Molecules"
6480:
5917:(1. paperback ed., reprinted ed.). Cambridge: Cambridge Univ. Press.
1631:
The electron is held in a circular orbit by electrostatic attraction. The
1610:
below). In high energy physics, it can be used to calculate the masses of
819:
amount of energy is radiated. However, unlike Einstein, Bohr stuck to the
6395:
Quantum Reality, Relativistic Causality, and Closing the Epistemic Circle
5584:– the electrons in the ring are at the maximum distance from each other.
4517:
electrons in a shell is eight, writing: "We see, further, that a ring of
3903:{\displaystyle \nu =R\left({\frac {1}{m^{2}}}-{\frac {1}{n^{2}}}\right).}
399:
173:
71:
7168:
6873:
6745:
6271:
6043:
6011:
5966:
5856:
6263:
6027:
5732:
Theoretical and experimental justification for the Schrödinger equation
5658:
5651:
5356:
Several enhancements to the Bohr model were proposed, most notably the
5283:
5214:
4829:
2818:-th level for any atom is determined by the radius and quantum number:
1591:
189:
177:
6428:"Revealing the hidden connection between pi and Bohr's hydrogen model"
1107:
Bohr's condition, that the angular momentum be an integer multiple of
7468:, A. Engel translator, (1997) Princeton University Press, Princeton.
7438:
7397:
6706:
5958:
5848:
5346:
Elliptical orbits with the same energy and quantized angular momentum
4758:
1587:
1103:
in quantum jumps, with the conservation laws only holding on average.
827:
212:
7414:
7373:
7340:
7307:
7274:
7078:
7037:
6857:
6766:[On molecular formation as a question of atomic structure].
6763:
6729:
6682:
6643:
6506:. Vol. 10. Amsterdam: North-Holland Publ. Cy. pp. 373–379.
6152:
6104:
6065:
5835:
Kragh, Helge (1 January 1979). "Niels Bohr's Second Atomic Theory".
5780:
5664:
code point U+269B (⚛) for an atom looks like a planetary atom model.
2129:{\displaystyle \Delta E\propto {\frac {1}{r^{3/2}}}\propto E^{3/2}.}
1566:, by being trapped by the potential of the positive nuclear charge.
682:
is 1; this gives the smallest possible orbital radius, known as the
6185:
Giliberti, Marco; Lovisetti, Luisa (2024). "Bohr's Hydrogen Atom".
5614:, which was in part responsible for its later usage in relation to
5274:
The Bohr model also has difficulty with, or else fails to explain:
4521:
electrons cannot rotate in a single ring round a nucleus of charge
4015:{\displaystyle W_{\tau }={\frac {2\pi ^{2}me^{4}}{h^{2}\tau ^{2}}}}
2451:
Substituting the expression for the velocity gives an equation for
721:
determined by the energy difference of the levels according to the
7454:
A. Einstein (1917). "Zum Quantensatz von Sommerfeld und Epstein".
5626:
5591:
5341:
4815:
2222:{\displaystyle E\propto {\frac {1}{r}}\propto {\frac {1}{L^{2}}}.}
1614:
1611:
1606:
X-ray transition calculations if other assumptions are added (see
1573:
304:
170:
5915:
The tiger and the shark: empirical roots of wave-particle dualism
7658:
6105:"VII. On electrical vibrations and the constitution of the atom"
5815:
5639:' logo shows baseballs as electrons orbiting a large letter "A".
142:
7662:
7548:
Atomic and Molecular Structure: the development of our concepts
6139:
Heilbron, John L. (June 2013). "The path to the quantum atom".
5449:
is the radial momentum canonically conjugate to the coordinate
6586:"Early atomic models – from mechanical to quantum (1904–1913)"
3494:{\displaystyle E_{n}=-{\frac {Z^{2}R_{\mathrm {E} }}{n^{2}}}.}
1856:
It also determines the electron's total energy at any radius:
6449:"Positron production in crossed beams of bare uranium nuclei"
2170:. The energy in terms of the angular momentum then obeys to:
6370:
Bohr, Niels; Rosenfeld, Léon Jacques Henri Constant (1963).
5754:
5752:
4816:
Moseley's law and calculation (K-alpha X-ray emission lines)
6012:"The Scattering of α and β Particles and Rutherford's Atom"
5499:. The current picture of the hydrogen atom is based on the
5266:
In modern quantum mechanics, the electron in hydrogen is a
3913:
In Bohr's theory describing the energies of transitions or
3424:
protons, the energy levels are (to a rough approximation):
2383:, so the angular momentum should be an integer multiple of
1923:
The total energy is negative and inversely proportional to
5940:
5938:
5936:
5934:
5472:, is the only one possible, since the quantum numbers are
1376:
is the angular momentum of the orbiting electron. Writing
853:
of classical radiation is equal to the rotation frequency
1451:
Bohr described angular momentum of the electron orbit as
1396:
for this angular momentum, the previous equation becomes
6806:"Lars Vegard, atomic structure, and the periodic system"
6300:
Inward bound: of matter and forces in the physical world
4263:{\displaystyle cR_{H}={\frac {2\pi ^{2}me^{4}}{h^{3}}}.}
3775:{\displaystyle E_{n}={\frac {R_{\mathrm {E} }}{2n^{2}}}}
505:. After this, Bohr declared, "everything became clear".
106:
transition depicted here produces the first line of the
7456:
Verhandlungen der Deutschen Physikalischen Gesellschaft
5483:
measured the tilt of the orbital plane relative to the
4022:
The energy difference between two such levels is then:
1912:{\displaystyle E=-{\frac {1}{2}}m_{\mathrm {e} }v^{2}.}
6560:(Impression: 3 ed.). Oxford: Oxford Univ. Press.
5870:
5868:
5866:
815:, Bohr's formula assumes that during a quantum jump a
253:
The Bohr model is a relatively primitive model of the
7506:(3rd ed.). San Francisco: W.H. Freeman & Co.
6858:"The Arrangement of Electrons in Atoms and Molecules"
6180:
6178:
5377:
5051:
4903:
4328:
4281:
4198:
4028:
3944:
3924:
3840:
3816:
3727:
3565:
3511:) because the electrons are not only affected by the
3433:
3327:
3249:
3204:
3091:
3064:
3) is −1.51 eV, and so on. For larger values of
2827:
2687:
2568:
2481:
2468:
2396:
2268:
2259:, the spacing between neighboring energies obeys to;
2242:
2179:
2152:
2067:
1982:
1864:
1782:
1647:
1523:
1489:
1457:
1405:
1382:
1356:
1301:
1243:
1196:
1144:
1113:
1070:
1050:
1026:
1006:
986:
966:
946:
913:
886:
859:
839:
789:
731:
707:
668:
631:
577:
533:
7341:"LXXIII. On the constitution of atoms and molecules"
7308:"XXXVII. On the constitution of atoms and molecules"
6644:"LXXIII. On the constitution of atoms and molecules"
4780: = 2. Each one sees the nuclear charge of
3039:
An electron in the lowest energy level of hydrogen (
1620:
Calculation of the orbits requires two assumptions.
7755:
7696:
5901:
Before Bohr: Theories of atomic structure 1850-1913
5724:
is adequately explained by means of the Bohr model.
5625:is a "crest-and-spinning-atom emblem", enclosed in
4744:Bohr's partner in research during 1914 to 1916 was
1549:of electron motion. The new theory was proposed by
7513:
7079:"The quantum theory of radiation and line spectra"
6408:Gilder, Louisa (2009). "The Arguments 1909—1935".
6293:
6291:
6289:
5431:
5176:
5031:
4407:
4308:
4262:
4179:
4014:
3930:
3902:
3822:
3774:
3702:
3493:
3393:
3307:
3229:
3180:
3028:
2803:
2652:
2544:
2437:
2368:
2251:
2221:
2162:
2128:
2015:
1911:
1844:
1728:
1529:
1509:
1471:
1437:
1388:
1368:
1339:
1281:
1223:
1168:
1119:
1076:
1056:
1032:
1012:
992:
972:
952:
932:
899:
865:
845:
795:
775:
713:
674:
654:
613:
563:
6332:Monthly Notices of the Royal Astronomical Society
6245:
6243:
6241:
6239:
30:"Bohr's law" redirects here. For other uses, see
7590:Steven and Susan Zumdahl (2010). "Chapter 7.4".
6365:
6363:
6321:
6319:
5460:is one full orbital period. The integral is the
2058:, so the energy level spacing formula obeys to:
691:
7275:"I. On the constitution of atoms and molecules"
6519:"I. On the constitution of atoms and molecules"
6134:
6132:
6130:
6066:"I. On the constitution of atoms and molecules"
3537:), the motion becomes highly relativistic, and
1541:In 1925, a new kind of mechanics was proposed,
501:in 1888, resulting in what is now known as the
242:The model's key success lies in explaining the
5978:
5976:
5673:uses a planetary-like image in its print logo.
5527:particular, the symplectic form should be the
5432:{\displaystyle \int _{0}^{T}p_{r}\,dq_{r}=nh,}
2438:{\displaystyle L={\frac {nh}{2\pi }}=n\hbar .}
2232:Assuming, with Bohr, that quantized values of
833:According to the Maxwell theory the frequency
419:Rutherford, Bohr's mentor, attended the first
7674:
6757:
6755:
6393:Stachel, John (2009). "Bohr and the Photon".
6302:(Reprint ed.). Oxford: Clarendon Press
5989:. Cambridge, Mass: Harvard University Press.
980:. These jumps reproduce the frequency of the
27:Atomic model introduced by Niels Bohr in 1913
8:
6907:"The high-frequency spectra of the elements"
6799:
6797:
6764:"Über Molekülbildung als Frage des Atombaus"
6579:
6577:
5683:On maps, it is generally used to indicate a
5567:Niels Bohr proposed a model of the atom and
5316:– changes in spectral lines due to external
3834:and a pair of integers indexing the lines:
5947:Historical Studies in the Physical Sciences
5837:Historical Studies in the Physical Sciences
5830:
5828:
5826:
5824:
5596:Shield of the U.S. Atomic Energy Commission
4788:doesn't usually come out to be an integer.
2016:{\displaystyle m_{\mathrm {e} }vr=n\hbar .}
687:
261:model. As a theory, it can be derived as a
7681:
7667:
7659:
7502:Linus Carl Pauling (1970). "Chapter 5-1".
7137:Избранные научные труды (статьи 1909–1925)
6558:The quantum story: a history in 40 moments
6328:"The Constitution of the Solar Corona. IL"
6220:
6218:
6216:
5717:provided early support for the Bohr model.
3515:but also interact with each other via the
1438:{\displaystyle \ell ={\frac {nh}{2\pi }},}
776:{\displaystyle \Delta E=E_{2}-E_{1}=h\nu }
564:{\displaystyle m_{\mathrm {e} }vr=n\hbar }
7195:
7185:
7167:
6683:"The Constitution of Atoms and Molecules"
6343:
5650:, and has come to be used as a symbol of
5411:
5403:
5397:
5387:
5382:
5376:
5165:
5141:
5132:
5110:
5080:
5069:
5068:
5050:
5013:
5004:
4993:
4984:
4973:
4950:
4949:
4936:
4923:
4902:
4389:
4384:
4375:
4364:
4359:
4350:
4329:
4327:
4309:{\displaystyle E={\frac {hc}{\lambda }},}
4288:
4280:
4249:
4238:
4225:
4215:
4206:
4197:
4164:
4159:
4150:
4139:
4134:
4125:
4112:
4101:
4088:
4078:
4067:
4062:
4047:
4042:
4027:
4003:
3993:
3981:
3968:
3958:
3949:
3943:
3923:
3884:
3875:
3864:
3855:
3839:
3815:
3763:
3748:
3747:
3741:
3732:
3726:
3687:
3686:
3677:
3670:
3669:
3653:
3646:
3645:
3628:
3627:
3613:
3612:
3599:
3598:
3587:
3586:
3579:
3570:
3564:
3480:
3468:
3467:
3457:
3450:
3438:
3432:
3385:
3372:
3361:
3360:
3343:
3333:
3332:
3326:
3295:
3269:
3258:
3257:
3250:
3248:
3221:
3210:
3209:
3203:
3166:
3150:
3149:
3139:
3129:
3118:
3117:
3107:
3097:
3096:
3090:
3015:
3004:
2993:
2980:
2968:
2958:
2942:
2941:
2931:
2921:
2910:
2909:
2896:
2889:
2874:
2859:
2848:
2847:
2837:
2826:
2790:
2776:
2764:
2741:
2740:
2730:
2719:
2718:
2707:
2701:
2692:
2686:
2637:
2636:
2626:
2615:
2614:
2599:
2589:
2582:
2573:
2567:
2513:
2501:
2488:
2479:
2473:
2467:
2403:
2395:
2355:
2338:
2324:
2315:
2303:
2278:
2267:
2241:
2208:
2199:
2186:
2178:
2153:
2151:
2113:
2109:
2090:
2086:
2077:
2066:
1988:
1987:
1981:
1900:
1889:
1888:
1874:
1863:
1825:
1824:
1812:
1801:
1800:
1789:
1781:
1715:
1704:
1693:
1692:
1682:
1667:
1656:
1655:
1648:
1646:
1522:
1499:
1488:
1461:
1456:
1412:
1404:
1381:
1355:
1302:
1300:
1244:
1242:
1224:{\displaystyle \lambda ={\frac {h}{mv}},}
1203:
1195:
1143:
1112:
1069:
1049:
1025:
1005:
985:
965:
945:
918:
912:
891:
885:
858:
838:
788:
758:
745:
730:
706:
667:
641:
630:
576:
539:
538:
532:
7565:Paul Tipler and Ralph Llewellyn (2002).
7546:Walter J. Lehmann (1972). "Chapter 18".
7139:. Vol. 1. М.: «Наука». p. 133.
6862:Journal of the American Chemical Society
6734:Journal of the American Chemical Society
6059:
6057:
6055:
6053:
5243:The Bohr model gives an incorrect value
4538:
2555:so that the allowed orbit radius at any
1340:{\displaystyle {\frac {nh}{2\pi }}=mvr,}
1282:{\displaystyle {\frac {nh}{mv}}=2\pi r,}
47:
7466:The Collected Papers of Albert Einstein
7152:"Bohr's 1913 molecular model revisited"
7038:"Zur Quantentheorie der Spektrallinien"
6374:. Copenhagen; W.A. Benjamin: New York.
6338:(8). Oxford University Press: 677–693.
5879:. Oxford University Press. p. 18.
5748:
3277:
3163:
2955:
2704:
2596:
2536:
2448:This is how Bohr arrived at his model.
2429:
2007:
1114:
632:
558:
5680:uses planetary-like image as its logo.
5612:United States Atomic Energy Commission
5260:fully quantum treatment in phase space
141:was the first successful model of the
78:encircles a small, positively charged
7653:Standing waves in Bohr's atomic model
6813:Bulletin for the History of Chemistry
6252:Archive for History of Exact Sciences
6191:. Cham: Springer Nature Switzerland.
6016:Archive for History of Exact Sciences
3230:{\displaystyle m_{\mathrm {e} }c^{2}}
1127:, was later reinterpreted in 1924 by
7:
7374:"The Spectra of Helium and Hydrogen"
5588:Symbolism of planetary atomic models
5456:, which is the radial position, and
5358:Sommerfeld or Bohr–Sommerfeld models
4540:Bohr's 1913 proposed configurations
5646:, was chosen as the symbol for the
5468:. This condition, suggested by the
3552:The Bohr formula properly uses the
1020:. For sufficiently large values of
876:of the electron in its orbit, with
18:Niels Bohr's model of the atom
7537:George Gamow (1985). "Chapter 2".
6397:. Dordrecht: Springer. p. 79.
5623:International Atomic Energy Agency
5070:
4951:
4800:of the table is chemically inert (
3749:
3688:
3671:
3647:
3629:
3614:
3600:
3588:
3469:
3362:
3334:
3259:
3211:
3151:
3119:
3098:
3019:
3016:
2943:
2911:
2849:
2794:
2791:
2777:
2742:
2720:
2638:
2616:
2344:
2293:
2269:
2243:
2068:
1989:
1890:
1826:
1802:
1694:
1657:
1448:which is Bohr's second postulate.
732:
540:
458:orbital angular momentum with the
415:Influence of the Solvay Conference
248:hydrogen's spectral emission lines
184:, but with attraction provided by
145:. Developed from 1911 to 1918 by
25:
7223:. Heidelberg New York: Springer.
6326:Nicholson, J. W. (14 June 1912).
5728:Introduction to quantum mechanics
1169:{\displaystyle n\lambda =2\pi r.}
369:Rutherford scattering experiments
283:Planck's discovery of the quantum
7793:
7784:
7783:
7520:. New York: Dover Publications.
6842:from the original on 2022-10-09.
4272:Since the energy of a photon is
3046:) therefore has about 13.6
2146:of the circular orbit scales as
70:), where the negatively charged
7611:The European Physical Journal H
7569:(4th ed.). W. H. Freeman.
7539:Thirty Years That Shook Physics
7482:(in French). Gauthier-Villars.
6590:The European Physical Journal H
6103:Rayleigh, Lord (January 1906).
5571:. According to his model for a
5563:Bohr model of the chemical bond
4832:line), and their atomic number
3241:of the electron (511 keV),
2663:The smallest possible value of
690:of the hydrogen atom and other
6728:Bury, Charles R. (July 1921).
5986:Niels Bohr: a centenary volume
5268:spherical cloud of probability
5162:
5149:
5146:
5119:
5107:
5094:
4970:
4957:
4793:periodic table of the elements
3378:
3353:
3136:
3110:
3057:) is −3.4 eV. The third (
2928:
2902:
2300:
2284:
811:Like Einstein's theory of the
688:energies of the allowed orbits
655:{\displaystyle \hbar =h/2\pi }
398:attracting negatively charged
199:'s Solar System model (1897),
1:
7594:(8th ed.). Brooks/Cole.
6681:Nicholson, J. W. (May 1914).
5799:"Les Hypothèses moléculaires"
223:'s quantum model (1910), the
7834:Foundational quantum physics
7772:(relativistic quantum model)
7339:Bohr, N. (1 November 1913).
6642:Bohr, N. (1 November 1913).
5569:a model of the chemical bond
5495:in 1925, using Heisenberg's
5323:The model also violates the
5257:Nevertheless, in the modern
1510:{\displaystyle \lambda =h/p}
117:) it results in a photon of
7306:Bohr, N. (September 1913).
6931:European Journal of Physics
6197:10.1007/978-3-031-57934-9_6
5761:American Journal of Physics
5491:, which was first given by
4506:Shell model (heavier atoms)
2163:{\displaystyle {\sqrt {r}}}
614:{\displaystyle n=1,2,3,...}
32:Bohr's law (disambiguation)
7865:
7631:10.1140/epjh/e2011-20031-x
6952:10.1088/0143-0807/20/3/312
6602:10.1140/epjh/e2012-30009-7
6230:"Niels Bohr – Session III"
6010:Heilbron, John L. (1968).
5913:Wheaton, Bruce R. (1992).
5560:
5557:Model of the chemical bond
5349:
4509:
3790:
1970:is an integer multiple of
1093:Bohr–Kramers–Slater theory
518:classical electromagnetism
366:
339:
335:
298:
267:obsolete scientific theory
63:) or a hydrogen-like ion (
36:
29:
7779:
7712:vortex theory of the atom
7372:Bohr, N. (October 1913).
7365:10.1080/14786441308635031
7332:10.1080/14786441308634993
7299:10.1080/14786441308634955
7114:. Springer. p. 203.
7097:10.1080/14786440608635362
7015:10.1080/00268978200100752
6983:Physikalische Zeitschrift
6668:10.1080/14786441308635031
6535:10.1080/14786441308634955
6121:10.1080/14786440609463428
6090:10.1080/14786441308634955
5899:Helge Kragh (Oct. 2010).
5618:technology in particular.
4870:theoretical explanation.
4852:, that the atomic number
3797:Beginning in late 1860s,
2054:. The energy scales as 1/
359:lines in atomic spectra.
263:first-order approximation
7064:10.1002/andp.19163561702
6905:Moseley, H.G.J. (1913).
6892:"Niels Bohr – Session I"
6788:10.1002/andp.19163540302
6584:Baily, C. (2013-01-01).
6473:10.1103/PhysRevA.37.1449
5470:correspondence principle
5466:action-angle coordinates
2252:{\displaystyle \Delta L}
1747:is the electron's mass,
1086:correspondence principle
623:principal quantum number
363:Rutherford nuclear model
301:History of atomic theory
180:to the structure of the
100:principal quantum number
37:Not to be confused with
7550:. John Wiley and Sons.
7413:Bohr, N. (March 1921).
7245:"Logo Usage Guidelines"
7187:10.1073/pnas.0505778102
6974:van den Broek, Antonius
6556:Baggott, J. E. (2013).
6410:The Age of Entanglement
5715:Franck–Hertz experiment
5676:The JavaScript library
5481:magnetic quantum number
5368:quantization condition
3317:fine-structure constant
1481:de Broglie's wavelength
933:{\displaystyle E_{n-k}}
803:is the Planck constant.
493:equation discovered by
52:The Bohr model of the
7512:Linus Pauling (1988).
7273:Bohr, N. (July 1913).
7110:Shaviv, Glora (2010).
7084:Philosophical Magazine
7036:A. Sommerfeld (1916).
6911:Philosophical Magazine
6625:Philosophical Magazine
6517:Bohr, N. (July 1913).
6345:10.1093/mnras/72.8.677
6298:Pais, Abraham (2002).
6064:Bohr, N. (July 1913).
5816:de Broglie et al. 1912
5642:A similar symbol, the
5597:
5433:
5347:
5178:
5033:
4844:Antonius Van den Broek
4729:John William Nicholson
4409:
4310:
4264:
4181:
4016:
3932:
3904:
3824:
3776:
3704:
3495:
3395:
3309:
3231:
3182:
3030:
2805:
2667:in the hydrogen atom (
2654:
2546:
2439:
2370:
2253:
2236:are equally spaced by
2223:
2164:
2130:
2017:
1913:
1846:
1730:
1579:
1570:Electron energy levels
1531:
1511:
1473:
1439:
1390:
1370:
1341:
1283:
1225:
1170:
1121:
1120:{\displaystyle \hbar }
1097:conservation of energy
1078:
1058:
1034:
1014:
1000:-th harmonic of orbit
994:
974:
954:
934:
901:
867:
847:
797:
777:
715:
676:
662:. The lowest value of
656:
615:
565:
455:John William Nicholson
313:
229:John William Nicholson
126:
84:electromagnetic energy
7707:(billiard ball model)
7541:. Dover Publications.
6804:Kragh, Helge (2012).
5875:Kragh, Helge (2012).
5803:La Revue scientifique
5709:Bohr–Sommerfeld model
5662:Miscellaneous Symbols
5634:minor league baseball
5595:
5434:
5352:Bohr–Sommerfeld model
5345:
5325:uncertainty principle
5179:
5034:
4410:
4311:
4265:
4182:
4017:
3933:
3931:{\displaystyle \tau }
3905:
3825:
3782: (positronium).
3777:
3705:
3496:
3396:
3310:
3232:
3183:
3031:
2806:
2655:
2547:
2440:
2371:
2254:
2224:
2165:
2142:The angular momentum
2131:
2018:
1914:
1847:
1731:
1590:, and doubly ionized
1577:
1532:
1512:
1474:
1440:
1391:
1389:{\displaystyle \ell }
1371:
1342:
1284:
1226:
1185:de Broglie wavelength
1171:
1122:
1079:
1059:
1035:
1015:
995:
975:
960:is much smaller than
955:
935:
902:
900:{\displaystyle E_{n}}
868:
848:
798:
778:
716:
677:
657:
616:
566:
449:Nicholson atom theory
308:
275:energy level diagrams
203:'s model (1901), the
139:Rutherford–Bohr model
51:
7764:electron cloud model
7721:(cubical atom model)
6436:. November 17, 2015.
5667:The television show
5637:Albuquerque Isotopes
5542:, which is called a
5474:adiabatic invariants
5375:
5049:
4901:
4740:Charles Rugeley Bury
4560:Electrons per shell
4326:
4279:
4196:
4026:
3942:
3922:
3838:
3814:
3725:
3563:
3431:
3325:
3247:
3202:
3089:
2825:
2685:
2566:
2466:
2394:
2266:
2240:
2177:
2150:
2065:
1980:
1862:
1780:
1645:
1521:
1487:
1455:
1403:
1380:
1354:
1299:
1241:
1194:
1142:
1111:
1068:
1048:
1024:
1004:
984:
964:
944:
911:
884:
866:{\displaystyle \nu }
857:
846:{\displaystyle \nu }
837:
813:photoelectric effect
787:
729:
714:{\displaystyle \nu }
705:
666:
629:
575:
531:
336:Thomson's atom model
157:, it supplanted the
110:, and for hydrogen (
7748:(old quantum model)
7623:2011EPJH...36..327K
7431:1921Natur.107..104B
7390:1913Natur..92..231B
7357:1913PMag...26..857B
7324:1913PMag...26..476B
7291:1913PMag...26....1B
7178:2005PNAS..10211985S
7162:(34): 11985–11988.
7056:1916AnP...356....1S
6944:1999EJPh...20..213W
6890:(31 October 1962).
6874:10.1021/ja02227a002
6780:1916AnP...354..229K
6762:Kossel, W. (1916).
6746:10.1021/ja01440a023
6699:1914Natur..93..268N
6660:1913PMag...26..857B
6465:1988PhRvA..37.1449M
6228:(7 November 1962).
6082:1913PMag...26....1B
5818:, pp. 122–123.
5773:1997AmJPh..65..933L
5670:The Big Bang Theory
5577:dynamic equilibrium
5511:developed in 1926.
5392:
5307:hyperfine structure
4810:transition elements
4554:Electrons per shell
4548:Electrons per shell
4541:
4394:
4369:
4169:
4144:
1625:Classical mechanics
1547:more accurate model
1472:{\displaystyle 2/h}
1369:{\displaystyle mvr}
1234:which implies that
350:, now known as the
186:electrostatic force
7849:Old quantum theory
7770:Dirac–Gordon model
7733:plum pudding model
7415:"Atomic Structure"
7077:W. Wilson (1915).
7043:Annalen der Physik
6768:Annalen der Physik
6264:10.1007/BF00357268
6028:10.1007/BF00411591
5598:
5429:
5378:
5348:
5174:
5029:
4539:
4405:
4380:
4355:
4306:
4260:
4177:
4155:
4130:
4012:
3928:
3900:
3820:
3772:
3700:
3491:
3391:
3305:
3227:
3178:
3026:
2814:The energy of the
2801:
2650:
2542:
2524:
2435:
2366:
2249:
2219:
2160:
2126:
2048:Kepler's third law
2013:
1909:
1842:
1726:
1594:, but it includes
1580:
1564:hydrogen-like atom
1527:
1507:
1469:
1435:
1386:
1366:
1337:
1279:
1221:
1187:of an electron is
1166:
1117:
1074:
1054:
1030:
1010:
990:
970:
950:
930:
897:
863:
843:
807:Other points are:
793:
773:
711:
672:
652:
611:
561:
475:plum pudding model
381:demonstrated that
352:Plum pudding model
342:Plum pudding model
314:
288:old quantum theory
257:, compared to the
233:quantum mechanical
217:plum pudding model
215:model (1904), the
159:plum pudding model
127:
7811:
7810:
7741:(planetary model)
7728:(Saturnian model)
7601:978-0-495-82992-8
7516:General Chemistry
7504:General Chemistry
7425:(2682): 104–107.
7384:(2295): 231–232.
7230:978-3-540-70626-7
7003:Molecular Physics
6693:(2324): 268–269.
6567:978-0-19-965597-7
6453:Physical Review A
6309:978-0-19-851997-3
6206:978-3-031-57933-2
5996:978-0-674-62415-3
5924:978-0-521-35892-7
5886:978-0-19-163046-0
5722:inert-pair effect
5704:Balmer's Constant
5648:American Atheists
5582:Coulomb repulsion
5573:diatomic molecule
5509:Erwin Schrödinger
5301:The existence of
5233:Siegbahn notation
5144:
5140:
5088:
5019:
4999:
4840:Ernest Rutherford
4724:
4723:
4395:
4370:
4337:
4301:
4255:
4170:
4145:
4118:
4010:
3890:
3870:
3823:{\displaystyle R}
3770:
3695:
3636:
3573:
3486:
3351:
3303:
3284:
3173:
3014:
3010:
2975:
2881:
2789:
2775:
2749:
2678:and is equal to:
2645:
2525:
2523:
2516:
2491:
2476:
2421:
2361:
2330:
2310:
2214:
2194:
2158:
2100:
1882:
1837:
1836:
1753:elementary charge
1721:
1677:
1633:centripetal force
1586:, singly ionized
1559:Erwin Schrödinger
1551:Werner Heisenberg
1543:quantum mechanics
1530:{\displaystyle h}
1430:
1320:
1262:
1216:
1077:{\displaystyle k}
1057:{\displaystyle n}
1033:{\displaystyle n}
1013:{\displaystyle n}
993:{\displaystyle k}
973:{\displaystyle n}
953:{\displaystyle k}
796:{\displaystyle h}
675:{\displaystyle n}
435:Arthur Erich Haas
421:Solvay Conference
392:model of the atom
387:Ernest Rutherford
279:Arthur Erich Haas
271:quantum mechanics
237:classical physics
151:Ernest Rutherford
16:(Redirected from
7856:
7839:Hydrogen physics
7797:
7787:
7786:
7739:Rutherford model
7683:
7676:
7669:
7660:
7642:
7605:
7580:
7561:
7542:
7531:
7519:
7507:
7491:
7463:
7450:
7439:10.1038/107104a0
7409:
7398:10.1038/092231d0
7368:
7351:(155): 857–875.
7335:
7318:(153): 476–502.
7302:
7260:
7259:
7257:
7256:
7241:
7235:
7234:
7216:
7210:
7209:
7199:
7189:
7171:
7147:
7141:
7140:
7132:
7126:
7125:
7107:
7101:
7100:
7091:(174): 795–802.
7074:
7068:
7067:
7033:
7027:
7026:
7009:(5): 1001–1019.
6998:
6992:
6991:
6976:(January 1913).
6970:
6964:
6963:
6925:
6919:
6918:
6902:
6896:
6895:
6884:
6878:
6877:
6854:Langmuir, Irving
6850:
6844:
6843:
6841:
6810:
6801:
6792:
6791:
6759:
6750:
6749:
6740:(7): 1602–1609.
6725:
6719:
6718:
6707:10.1038/093268a0
6678:
6672:
6671:
6654:(155): 857–875.
6639:
6633:
6632:
6620:
6614:
6613:
6581:
6572:
6571:
6553:
6547:
6546:
6514:
6508:
6507:
6499:
6493:
6492:
6459:(5): 1449–1455.
6444:
6438:
6437:
6424:
6418:
6417:
6405:
6399:
6398:
6390:
6384:
6383:
6367:
6358:
6357:
6347:
6323:
6314:
6313:
6295:
6284:
6283:
6247:
6234:
6233:
6222:
6211:
6210:
6182:
6173:
6172:
6136:
6125:
6124:
6100:
6094:
6093:
6061:
6048:
6047:
6007:
6001:
6000:
5980:
5971:
5970:
5959:10.2307/27757291
5942:
5929:
5928:
5910:
5904:
5897:
5891:
5890:
5872:
5861:
5860:
5849:10.2307/27757389
5832:
5819:
5813:
5807:
5806:
5791:
5785:
5784:
5756:
5621:The flag of the
5610:The logo of the
5603:planetary system
5497:matrix mechanics
5438:
5436:
5435:
5430:
5416:
5415:
5402:
5401:
5391:
5386:
5252:
5183:
5181:
5180:
5175:
5170:
5169:
5145:
5142:
5138:
5137:
5136:
5115:
5114:
5093:
5089:
5081:
5075:
5074:
5073:
5038:
5036:
5035:
5030:
5025:
5021:
5020:
5018:
5017:
5005:
5000:
4998:
4997:
4985:
4978:
4977:
4956:
4955:
4954:
4941:
4940:
4928:
4927:
4865:
4857:
4837:
4542:
4497:
4491:
4481:
4475:
4465:
4456:
4443:
4430:
4414:
4412:
4411:
4406:
4401:
4397:
4396:
4393:
4388:
4376:
4371:
4368:
4363:
4351:
4338:
4330:
4315:
4313:
4312:
4307:
4302:
4297:
4289:
4269:
4267:
4266:
4261:
4256:
4254:
4253:
4244:
4243:
4242:
4230:
4229:
4216:
4211:
4210:
4186:
4184:
4183:
4178:
4176:
4172:
4171:
4168:
4163:
4151:
4146:
4143:
4138:
4126:
4119:
4117:
4116:
4107:
4106:
4105:
4093:
4092:
4079:
4074:
4073:
4072:
4071:
4054:
4053:
4052:
4051:
4021:
4019:
4018:
4013:
4011:
4009:
4008:
4007:
3998:
3997:
3987:
3986:
3985:
3973:
3972:
3959:
3954:
3953:
3937:
3935:
3934:
3929:
3909:
3907:
3906:
3901:
3896:
3892:
3891:
3889:
3888:
3876:
3871:
3869:
3868:
3856:
3832:Rydberg constant
3830:, now known the
3829:
3827:
3826:
3821:
3803:Johannes Rydberg
3781:
3779:
3778:
3773:
3771:
3769:
3768:
3767:
3754:
3753:
3752:
3742:
3737:
3736:
3709:
3707:
3706:
3701:
3696:
3694:
3693:
3692:
3691:
3681:
3676:
3675:
3674:
3654:
3652:
3651:
3650:
3637:
3635:
3634:
3633:
3632:
3619:
3618:
3617:
3606:
3605:
3604:
3603:
3593:
3592:
3591:
3580:
3575:
3574:
3571:
3536:
3500:
3498:
3497:
3492:
3487:
3485:
3484:
3475:
3474:
3473:
3472:
3462:
3461:
3451:
3443:
3442:
3414:
3400:
3398:
3397:
3392:
3390:
3389:
3377:
3376:
3367:
3366:
3365:
3352:
3344:
3339:
3338:
3337:
3314:
3312:
3311:
3306:
3304:
3296:
3285:
3283:
3275:
3274:
3273:
3264:
3263:
3262:
3251:
3239:rest mass energy
3236:
3234:
3233:
3228:
3226:
3225:
3216:
3215:
3214:
3187:
3185:
3184:
3179:
3174:
3172:
3171:
3170:
3157:
3156:
3155:
3154:
3144:
3143:
3134:
3133:
3124:
3123:
3122:
3108:
3103:
3102:
3101:
3063:
3056:
3045:
3035:
3033:
3032:
3027:
3022:
3012:
3011:
3009:
3008:
2999:
2998:
2997:
2981:
2976:
2974:
2973:
2972:
2963:
2962:
2949:
2948:
2947:
2946:
2936:
2935:
2926:
2925:
2916:
2915:
2914:
2901:
2900:
2890:
2882:
2880:
2879:
2878:
2865:
2864:
2863:
2854:
2853:
2852:
2838:
2810:
2808:
2807:
2802:
2797:
2787:
2780:
2773:
2772:
2771:
2750:
2748:
2747:
2746:
2745:
2735:
2734:
2725:
2724:
2723:
2712:
2711:
2702:
2697:
2696:
2674:) is called the
2673:
2659:
2657:
2656:
2651:
2646:
2644:
2643:
2642:
2641:
2631:
2630:
2621:
2620:
2619:
2605:
2604:
2603:
2594:
2593:
2583:
2578:
2577:
2551:
2549:
2548:
2543:
2526:
2522:
2518:
2517:
2514:
2507:
2506:
2505:
2493:
2492:
2489:
2482:
2480:
2478:
2477:
2474:
2444:
2442:
2441:
2436:
2422:
2420:
2412:
2404:
2375:
2373:
2372:
2367:
2362:
2360:
2359:
2350:
2339:
2331:
2329:
2328:
2316:
2311:
2309:
2308:
2307:
2279:
2258:
2256:
2255:
2250:
2228:
2226:
2225:
2220:
2215:
2213:
2212:
2200:
2195:
2187:
2169:
2167:
2166:
2161:
2159:
2154:
2135:
2133:
2132:
2127:
2122:
2121:
2117:
2101:
2099:
2098:
2094:
2078:
2022:
2020:
2019:
2014:
1994:
1993:
1992:
1969:
1953:angular momentum
1933:potential energy
1918:
1916:
1915:
1910:
1905:
1904:
1895:
1894:
1893:
1883:
1875:
1851:
1849:
1848:
1843:
1838:
1835:
1831:
1830:
1829:
1818:
1817:
1816:
1807:
1806:
1805:
1791:
1790:
1764:Coulomb constant
1735:
1733:
1732:
1727:
1722:
1720:
1719:
1710:
1709:
1708:
1699:
1698:
1697:
1683:
1678:
1673:
1672:
1671:
1662:
1661:
1660:
1649:
1635:is equal to the
1536:
1534:
1533:
1528:
1516:
1514:
1513:
1508:
1503:
1478:
1476:
1475:
1470:
1465:
1444:
1442:
1441:
1436:
1431:
1429:
1421:
1413:
1395:
1393:
1392:
1387:
1375:
1373:
1372:
1367:
1346:
1344:
1343:
1338:
1321:
1319:
1311:
1303:
1288:
1286:
1285:
1280:
1263:
1261:
1253:
1245:
1230:
1228:
1227:
1222:
1217:
1215:
1204:
1175:
1173:
1172:
1167:
1126:
1124:
1123:
1118:
1083:
1081:
1080:
1075:
1063:
1061:
1060:
1055:
1039:
1037:
1036:
1031:
1019:
1017:
1016:
1011:
999:
997:
996:
991:
979:
977:
976:
971:
959:
957:
956:
951:
939:
937:
936:
931:
929:
928:
906:
904:
903:
898:
896:
895:
872:
870:
869:
864:
852:
850:
849:
844:
802:
800:
799:
794:
782:
780:
779:
774:
763:
762:
750:
749:
720:
718:
717:
712:
681:
679:
678:
673:
661:
659:
658:
653:
645:
620:
618:
617:
612:
570:
568:
567:
562:
545:
544:
543:
499:Johannes Rydberg
320:Planetary models
225:Rutherford model
149:and building on
116:
105:
69:
62:
21:
7864:
7863:
7859:
7858:
7857:
7855:
7854:
7853:
7824:1913 in science
7814:
7813:
7812:
7807:
7775:
7751:
7697:Historic models
7692:
7687:
7649:
7608:
7602:
7589:
7584:Klaus Hentschel
7577:
7564:
7558:
7545:
7536:
7528:
7511:
7501:
7498:
7496:Further reading
7475:
7453:
7412:
7371:
7338:
7305:
7272:
7269:
7267:Primary sources
7264:
7263:
7254:
7252:
7243:
7242:
7238:
7231:
7218:
7217:
7213:
7169:physics/0508161
7149:
7148:
7144:
7135:Бор Н. (1970).
7134:
7133:
7129:
7122:
7109:
7108:
7104:
7076:
7075:
7071:
7035:
7034:
7030:
7000:
6999:
6995:
6972:
6971:
6967:
6927:
6926:
6922:
6904:
6903:
6899:
6886:
6885:
6881:
6852:
6851:
6847:
6839:
6808:
6803:
6802:
6795:
6761:
6760:
6753:
6727:
6726:
6722:
6680:
6679:
6675:
6641:
6640:
6636:
6622:
6621:
6617:
6583:
6582:
6575:
6568:
6555:
6554:
6550:
6516:
6515:
6511:
6504:Collected works
6501:
6500:
6496:
6446:
6445:
6441:
6426:
6425:
6421:
6407:
6406:
6402:
6392:
6391:
6387:
6369:
6368:
6361:
6325:
6324:
6317:
6310:
6297:
6296:
6287:
6249:
6248:
6237:
6224:
6223:
6214:
6207:
6184:
6183:
6176:
6153:10.1038/498027a
6147:(7452): 27–30.
6138:
6137:
6128:
6115:(61): 117–123.
6102:
6101:
6097:
6063:
6062:
6051:
6009:
6008:
6004:
5997:
5982:
5981:
5974:
5944:
5943:
5932:
5925:
5912:
5911:
5907:
5898:
5894:
5887:
5874:
5873:
5864:
5834:
5833:
5822:
5814:
5810:
5793:
5792:
5788:
5781:10.1119/1.18691
5758:
5757:
5750:
5745:
5740:
5735:
5699:1913 in science
5694:
5616:nuclear fission
5590:
5565:
5559:
5544:prequantization
5501:atomic orbitals
5454:
5447:
5407:
5393:
5373:
5372:
5354:
5340:
5318:magnetic fields
5289:Rydberg formula
5244:
5241:
5229:
5222:
5203:
5194:
5161:
5128:
5106:
5076:
5064:
5047:
5046:
5009:
4989:
4983:
4979:
4969:
4945:
4932:
4919:
4899:
4898:
4880:Irving Langmuir
4864: − 1)
4859:
4853:
4833:
4818:
4763:shell structure
4750:Irving Langmuir
4526:
4514:
4508:
4493:
4483:
4477:
4467:
4461:
4454:
4449:
4441:
4436:
4428:
4423:
4349:
4345:
4324:
4323:
4290:
4277:
4276:
4245:
4234:
4221:
4217:
4202:
4194:
4193:
4190:Planck constant
4124:
4120:
4108:
4097:
4084:
4080:
4063:
4058:
4043:
4038:
4024:
4023:
3999:
3989:
3988:
3977:
3964:
3960:
3945:
3940:
3939:
3920:
3919:
3880:
3860:
3854:
3850:
3836:
3835:
3812:
3811:
3795:
3793:Rydberg formula
3789:
3787:Rydberg formula
3759:
3755:
3743:
3728:
3723:
3722:
3682:
3665:
3658:
3641:
3623:
3608:
3607:
3594:
3582:
3581:
3566:
3561:
3560:
3531:
3476:
3463:
3453:
3452:
3434:
3429:
3428:
3406:
3381:
3368:
3356:
3328:
3323:
3322:
3276:
3265:
3253:
3252:
3245:
3244:
3217:
3205:
3200:
3199:
3162:
3158:
3145:
3135:
3125:
3113:
3109:
3092:
3087:
3086:
3081:
3058:
3051:
3040:
3000:
2989:
2982:
2964:
2954:
2950:
2937:
2927:
2917:
2905:
2892:
2891:
2870:
2866:
2855:
2843:
2839:
2823:
2822:
2760:
2736:
2726:
2714:
2713:
2703:
2688:
2683:
2682:
2668:
2632:
2622:
2610:
2606:
2595:
2585:
2584:
2569:
2564:
2563:
2509:
2508:
2497:
2484:
2483:
2469:
2464:
2463:
2413:
2405:
2392:
2391:
2351:
2340:
2320:
2299:
2283:
2264:
2263:
2238:
2237:
2204:
2175:
2174:
2148:
2147:
2105:
2082:
2063:
2062:
2031:
1983:
1978:
1977:
1965:
1955:
1896:
1884:
1860:
1859:
1820:
1819:
1808:
1796:
1792:
1778:
1777:
1761:
1746:
1711:
1700:
1688:
1684:
1663:
1651:
1650:
1643:
1642:
1572:
1519:
1518:
1485:
1484:
1453:
1452:
1422:
1414:
1401:
1400:
1378:
1377:
1352:
1351:
1312:
1304:
1297:
1296:
1254:
1246:
1239:
1238:
1208:
1192:
1191:
1140:
1139:
1109:
1108:
1066:
1065:
1046:
1045:
1022:
1021:
1002:
1001:
982:
981:
962:
961:
942:
941:
914:
909:
908:
887:
882:
881:
875:
855:
854:
835:
834:
785:
784:
754:
741:
727:
726:
723:Planck relation
703:
702:
664:
663:
627:
626:
573:
572:
534:
529:
528:
525:Planck constant
503:Rydberg formula
487:
460:Planck constant
451:
431:Hendrik Lorentz
417:
408:
371:
365:
344:
338:
322:
303:
297:
244:Rydberg formula
209:Hantaro Nagaoka
111:
103:
74:confined to an
64:
57:
46:
35:
28:
23:
22:
15:
12:
11:
5:
7862:
7860:
7852:
7851:
7846:
7841:
7836:
7831:
7829:Atomic physics
7826:
7816:
7815:
7809:
7808:
7806:
7805:
7799:Portal:Physics
7791:
7789:Category:Atoms
7780:
7777:
7776:
7774:
7773:
7766:
7759:
7757:
7756:Current models
7753:
7752:
7750:
7749:
7742:
7735:
7729:
7722:
7715:
7708:
7700:
7698:
7694:
7693:
7688:
7686:
7685:
7678:
7671:
7663:
7657:
7656:
7648:
7647:External links
7645:
7644:
7643:
7617:(3): 327–352.
7606:
7600:
7587:
7581:
7575:
7567:Modern Physics
7562:
7556:
7543:
7534:
7533:
7532:
7526:
7497:
7494:
7493:
7492:
7473:
7451:
7410:
7369:
7336:
7303:
7268:
7265:
7262:
7261:
7236:
7229:
7211:
7142:
7127:
7121:978-3642020872
7120:
7102:
7069:
7028:
6993:
6965:
6938:(3): 213–220.
6920:
6913:. 6th series.
6897:
6879:
6868:(6): 868–934.
6845:
6793:
6774:(3): 229–362.
6751:
6720:
6673:
6634:
6615:
6573:
6566:
6548:
6509:
6494:
6439:
6419:
6412:. p. 55.
6400:
6385:
6359:
6315:
6308:
6285:
6258:(2): 160–184.
6235:
6212:
6205:
6174:
6126:
6095:
6049:
6022:(4): 247–307.
6002:
5995:
5972:
5930:
5923:
5905:
5892:
5885:
5862:
5820:
5808:
5786:
5747:
5746:
5744:
5741:
5739:
5736:
5734:
5733:
5730:
5725:
5718:
5711:
5706:
5701:
5695:
5693:
5690:
5689:
5688:
5681:
5674:
5665:
5655:
5640:
5630:
5619:
5589:
5586:
5561:Main article:
5558:
5555:
5529:curvature form
5505:wave mechanics
5493:Wolfgang Pauli
5452:
5445:
5440:
5439:
5428:
5425:
5422:
5419:
5414:
5410:
5406:
5400:
5396:
5390:
5385:
5381:
5350:Main article:
5339:
5336:
5335:
5334:
5331:
5328:
5321:
5310:
5303:fine structure
5299:
5292:
5240:
5237:
5227:
5220:
5201:
5192:
5185:
5184:
5173:
5168:
5164:
5160:
5157:
5154:
5151:
5148:
5135:
5131:
5127:
5124:
5121:
5118:
5113:
5109:
5105:
5102:
5099:
5096:
5092:
5087:
5084:
5079:
5072:
5067:
5063:
5060:
5057:
5054:
5040:
5039:
5028:
5024:
5016:
5012:
5008:
5003:
4996:
4992:
4988:
4982:
4976:
4972:
4968:
4965:
4962:
4959:
4953:
4948:
4944:
4939:
4935:
4931:
4926:
4922:
4918:
4915:
4912:
4909:
4906:
4875:Walther Kossel
4817:
4814:
4746:Walther Kossel
4736:periodic table
4722:
4721:
4720:8, 8, 4, 2, 2
4718:
4715:
4712:
4709:
4706:
4702:
4701:
4698:
4695:
4692:
4689:
4686:
4682:
4681:
4678:
4675:
4672:
4669:
4666:
4662:
4661:
4658:
4655:
4652:
4649:
4646:
4642:
4641:
4638:
4635:
4632:
4629:
4626:
4622:
4621:
4618:
4615:
4612:
4609:
4606:
4602:
4601:
4598:
4595:
4592:
4589:
4586:
4582:
4581:
4578:
4575:
4572:
4569:
4566:
4562:
4561:
4558:
4555:
4552:
4549:
4546:
4524:
4512:Electron shell
4510:Main article:
4507:
4504:
4500:Electron shell
4452:
4439:
4426:
4416:
4415:
4404:
4400:
4392:
4387:
4383:
4379:
4374:
4367:
4362:
4358:
4354:
4348:
4344:
4341:
4336:
4333:
4317:
4316:
4305:
4300:
4296:
4293:
4287:
4284:
4259:
4252:
4248:
4241:
4237:
4233:
4228:
4224:
4220:
4214:
4209:
4205:
4201:
4175:
4167:
4162:
4158:
4154:
4149:
4142:
4137:
4133:
4129:
4123:
4115:
4111:
4104:
4100:
4096:
4091:
4087:
4083:
4077:
4070:
4066:
4061:
4057:
4050:
4046:
4041:
4037:
4034:
4031:
4006:
4002:
3996:
3992:
3984:
3980:
3976:
3971:
3967:
3963:
3957:
3952:
3948:
3927:
3899:
3895:
3887:
3883:
3879:
3874:
3867:
3863:
3859:
3853:
3849:
3846:
3843:
3819:
3791:Main article:
3788:
3785:
3784:
3783:
3766:
3762:
3758:
3751:
3746:
3740:
3735:
3731:
3711:
3710:
3699:
3690:
3685:
3680:
3673:
3668:
3664:
3661:
3657:
3649:
3644:
3640:
3631:
3626:
3622:
3616:
3611:
3602:
3597:
3590:
3585:
3578:
3569:
3502:
3501:
3490:
3483:
3479:
3471:
3466:
3460:
3456:
3449:
3446:
3441:
3437:
3403:
3402:
3388:
3384:
3380:
3375:
3371:
3364:
3359:
3355:
3350:
3347:
3342:
3336:
3331:
3320:
3302:
3299:
3294:
3291:
3288:
3282:
3279:
3272:
3268:
3261:
3256:
3242:
3224:
3220:
3213:
3208:
3189:
3188:
3177:
3169:
3165:
3161:
3153:
3148:
3142:
3138:
3132:
3128:
3121:
3116:
3112:
3106:
3100:
3095:
3079:
3070:Wallis product
3037:
3036:
3025:
3021:
3018:
3007:
3003:
2996:
2992:
2988:
2985:
2979:
2971:
2967:
2961:
2957:
2953:
2945:
2940:
2934:
2930:
2924:
2920:
2913:
2908:
2904:
2899:
2895:
2888:
2885:
2877:
2873:
2869:
2862:
2858:
2851:
2846:
2842:
2836:
2833:
2830:
2812:
2811:
2800:
2796:
2793:
2786:
2783:
2779:
2770:
2767:
2763:
2759:
2756:
2753:
2744:
2739:
2733:
2729:
2722:
2717:
2710:
2706:
2700:
2695:
2691:
2661:
2660:
2649:
2640:
2635:
2629:
2625:
2618:
2613:
2609:
2602:
2598:
2592:
2588:
2581:
2576:
2572:
2553:
2552:
2541:
2538:
2535:
2532:
2529:
2521:
2512:
2504:
2500:
2496:
2487:
2472:
2446:
2445:
2434:
2431:
2428:
2425:
2419:
2416:
2411:
2408:
2402:
2399:
2377:
2376:
2365:
2358:
2354:
2349:
2346:
2343:
2337:
2334:
2327:
2323:
2319:
2314:
2306:
2302:
2298:
2295:
2292:
2289:
2286:
2282:
2277:
2274:
2271:
2248:
2245:
2230:
2229:
2218:
2211:
2207:
2203:
2198:
2193:
2190:
2185:
2182:
2157:
2137:
2136:
2125:
2120:
2116:
2112:
2108:
2104:
2097:
2093:
2089:
2085:
2081:
2076:
2073:
2070:
2046:determined by
2030:
2027:
2026:
2025:
2024:
2023:
2012:
2009:
2006:
2003:
2000:
1997:
1991:
1986:
1963:
1948:
1947:
1945:A quantum rule
1941:
1940:
1937:virial theorem
1921:
1920:
1919:
1908:
1903:
1899:
1892:
1887:
1881:
1878:
1873:
1870:
1867:
1854:
1853:
1852:
1841:
1834:
1828:
1823:
1815:
1811:
1804:
1799:
1795:
1788:
1785:
1770:is the atom's
1759:
1744:
1738:
1737:
1736:
1725:
1718:
1714:
1707:
1703:
1696:
1691:
1687:
1681:
1676:
1670:
1666:
1659:
1654:
1628:
1627:
1600:Rydberg states
1571:
1568:
1526:
1506:
1502:
1498:
1495:
1492:
1468:
1464:
1460:
1446:
1445:
1434:
1428:
1425:
1420:
1417:
1411:
1408:
1385:
1365:
1362:
1359:
1348:
1347:
1336:
1333:
1330:
1327:
1324:
1318:
1315:
1310:
1307:
1290:
1289:
1278:
1275:
1272:
1269:
1266:
1260:
1257:
1252:
1249:
1232:
1231:
1220:
1214:
1211:
1207:
1202:
1199:
1177:
1176:
1165:
1162:
1159:
1156:
1153:
1150:
1147:
1116:
1105:
1104:
1089:
1073:
1053:
1042:Rydberg states
1029:
1009:
989:
969:
949:
927:
924:
921:
917:
894:
890:
873:
862:
842:
831:
824:Maxwell theory
805:
804:
792:
772:
769:
766:
761:
757:
753:
748:
744:
740:
737:
734:
710:
699:
671:
651:
648:
644:
640:
637:
634:
621:is called the
610:
607:
604:
601:
598:
595:
592:
589:
586:
583:
580:
560:
557:
554:
551:
548:
542:
537:
521:
486:
483:
450:
447:
416:
413:
407:
406:Atomic spectra
404:
383:alpha particle
379:Ernest Marsden
367:Main article:
364:
361:
356:beta particles
340:Main article:
337:
334:
330:Larmor formula
321:
318:
311:X-ray notation
299:Main article:
296:
293:
169:surrounded by
131:atomic physics
80:atomic nucleus
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
7861:
7850:
7847:
7845:
7842:
7840:
7837:
7835:
7832:
7830:
7827:
7825:
7822:
7821:
7819:
7804:
7800:
7796:
7792:
7790:
7782:
7781:
7778:
7771:
7767:
7765:
7761:
7760:
7758:
7754:
7747:
7743:
7740:
7736:
7734:
7730:
7727:
7726:Nagaoka model
7723:
7720:
7716:
7713:
7709:
7706:
7702:
7701:
7699:
7695:
7691:
7690:Atomic models
7684:
7679:
7677:
7672:
7670:
7665:
7664:
7661:
7654:
7651:
7650:
7646:
7640:
7636:
7632:
7628:
7624:
7620:
7616:
7612:
7607:
7603:
7597:
7593:
7588:
7585:
7582:
7578:
7576:0-7167-4345-0
7572:
7568:
7563:
7559:
7557:0-471-52440-9
7553:
7549:
7544:
7540:
7535:
7529:
7527:0-486-65622-5
7523:
7518:
7517:
7509:
7508:
7505:
7500:
7499:
7495:
7489:
7485:
7481:
7480:
7474:
7471:
7467:
7464:Reprinted in
7461:
7457:
7452:
7448:
7444:
7440:
7436:
7432:
7428:
7424:
7420:
7416:
7411:
7407:
7403:
7399:
7395:
7391:
7387:
7383:
7379:
7375:
7370:
7366:
7362:
7358:
7354:
7350:
7346:
7342:
7337:
7333:
7329:
7325:
7321:
7317:
7313:
7309:
7304:
7300:
7296:
7292:
7288:
7285:(151): 1–25.
7284:
7280:
7276:
7271:
7270:
7266:
7250:
7246:
7240:
7237:
7232:
7226:
7222:
7215:
7212:
7207:
7203:
7198:
7193:
7188:
7183:
7179:
7175:
7170:
7165:
7161:
7157:
7153:
7146:
7143:
7138:
7131:
7128:
7123:
7117:
7113:
7106:
7103:
7098:
7094:
7090:
7086:
7085:
7080:
7073:
7070:
7065:
7061:
7057:
7053:
7049:
7046:(in German).
7045:
7044:
7039:
7032:
7029:
7024:
7020:
7016:
7012:
7008:
7004:
6997:
6994:
6989:
6986:(in German).
6985:
6984:
6979:
6975:
6969:
6966:
6961:
6957:
6953:
6949:
6945:
6941:
6937:
6933:
6932:
6924:
6921:
6916:
6912:
6908:
6901:
6898:
6893:
6889:
6883:
6880:
6875:
6871:
6867:
6863:
6859:
6856:(June 1919).
6855:
6849:
6846:
6838:
6834:
6830:
6826:
6822:
6818:
6814:
6807:
6800:
6798:
6794:
6789:
6785:
6781:
6777:
6773:
6770:(in German).
6769:
6765:
6758:
6756:
6752:
6747:
6743:
6739:
6735:
6731:
6724:
6721:
6716:
6712:
6708:
6704:
6700:
6696:
6692:
6688:
6684:
6677:
6674:
6669:
6665:
6661:
6657:
6653:
6649:
6645:
6638:
6635:
6630:
6626:
6619:
6616:
6611:
6607:
6603:
6599:
6595:
6591:
6587:
6580:
6578:
6574:
6569:
6563:
6559:
6552:
6549:
6544:
6540:
6536:
6532:
6529:(151): 1–25.
6528:
6524:
6520:
6513:
6510:
6505:
6498:
6495:
6490:
6486:
6482:
6478:
6474:
6470:
6466:
6462:
6458:
6454:
6450:
6443:
6440:
6435:
6434:
6433:Physics World
6429:
6423:
6420:
6416:
6411:
6404:
6401:
6396:
6389:
6386:
6381:
6377:
6373:
6366:
6364:
6360:
6355:
6351:
6346:
6341:
6337:
6333:
6329:
6322:
6320:
6316:
6311:
6305:
6301:
6294:
6292:
6290:
6286:
6281:
6277:
6273:
6269:
6265:
6261:
6257:
6253:
6246:
6244:
6242:
6240:
6236:
6231:
6227:
6221:
6219:
6217:
6213:
6208:
6202:
6198:
6194:
6190:
6189:
6181:
6179:
6175:
6170:
6166:
6162:
6158:
6154:
6150:
6146:
6142:
6135:
6133:
6131:
6127:
6122:
6118:
6114:
6110:
6106:
6099:
6096:
6091:
6087:
6083:
6079:
6076:(151): 1–25.
6075:
6071:
6067:
6060:
6058:
6056:
6054:
6050:
6045:
6041:
6037:
6033:
6029:
6025:
6021:
6017:
6013:
6006:
6003:
5998:
5992:
5988:
5987:
5979:
5977:
5973:
5968:
5964:
5960:
5956:
5952:
5948:
5941:
5939:
5937:
5935:
5931:
5926:
5920:
5916:
5909:
5906:
5902:
5896:
5893:
5888:
5882:
5878:
5871:
5869:
5867:
5863:
5858:
5854:
5850:
5846:
5842:
5838:
5831:
5829:
5827:
5825:
5821:
5817:
5812:
5809:
5804:
5800:
5796:
5790:
5787:
5782:
5778:
5774:
5770:
5766:
5762:
5755:
5753:
5749:
5742:
5737:
5731:
5729:
5726:
5723:
5719:
5716:
5712:
5710:
5707:
5705:
5702:
5700:
5697:
5696:
5691:
5687:installation.
5686:
5685:nuclear power
5682:
5679:
5675:
5672:
5671:
5666:
5663:
5660:
5656:
5653:
5649:
5645:
5641:
5638:
5635:
5631:
5628:
5624:
5620:
5617:
5613:
5609:
5608:
5607:
5604:
5594:
5587:
5585:
5583:
5578:
5574:
5570:
5564:
5556:
5554:
5552:
5551:closed shells
5547:
5545:
5541:
5538:
5534:
5530:
5524:
5522:
5518:
5517:perturbations
5512:
5510:
5506:
5502:
5498:
5494:
5490:
5489:hydrogen atom
5486:
5482:
5477:
5475:
5471:
5467:
5463:
5459:
5455:
5448:
5426:
5423:
5420:
5417:
5412:
5408:
5404:
5398:
5394:
5388:
5383:
5379:
5371:
5370:
5369:
5367:
5363:
5359:
5353:
5344:
5337:
5332:
5329:
5326:
5322:
5319:
5315:
5314:Zeeman effect
5311:
5308:
5304:
5300:
5297:
5293:
5290:
5285:
5281:
5277:
5276:
5275:
5272:
5269:
5264:
5262:
5261:
5255:
5251:
5247:
5238:
5236:
5234:
5230:
5223:
5216:
5211:
5208:
5207:
5200:
5195:
5191:
5171:
5166:
5158:
5155:
5152:
5133:
5129:
5125:
5122:
5116:
5111:
5103:
5100:
5097:
5090:
5085:
5082:
5077:
5065:
5061:
5058:
5055:
5052:
5045:
5044:
5043:
5026:
5022:
5014:
5010:
5006:
5001:
4994:
4990:
4986:
4980:
4974:
4966:
4963:
4960:
4946:
4942:
4937:
4933:
4929:
4924:
4920:
4916:
4913:
4910:
4907:
4904:
4897:
4896:
4895:
4893:
4889:
4883:
4881:
4876:
4871:
4867:
4863:
4856:
4851:
4848:
4845:
4841:
4836:
4831:
4827:
4826:Henry Moseley
4822:
4813:
4811:
4805:
4803:
4798:
4794:
4789:
4787:
4783:
4779:
4774:
4772:
4766:
4764:
4760:
4755:
4751:
4747:
4742:
4741:
4737:
4732:
4730:
4719:
4716:
4713:
4710:
4707:
4704:
4703:
4699:
4696:
4693:
4690:
4687:
4684:
4683:
4679:
4676:
4673:
4670:
4667:
4664:
4663:
4659:
4656:
4653:
4650:
4647:
4644:
4643:
4639:
4636:
4633:
4630:
4627:
4624:
4623:
4619:
4616:
4613:
4610:
4607:
4604:
4603:
4599:
4596:
4593:
4590:
4587:
4584:
4583:
4579:
4576:
4573:
4570:
4567:
4564:
4563:
4559:
4556:
4553:
4550:
4547:
4544:
4543:
4537:
4535:
4531:
4527:
4520:
4513:
4505:
4503:
4501:
4496:
4490:
4487: −
4486:
4480:
4474:
4471: −
4470:
4464:
4458:
4455:
4447:
4442:
4434:
4429:
4421:
4402:
4398:
4390:
4385:
4381:
4377:
4372:
4365:
4360:
4356:
4352:
4346:
4342:
4339:
4334:
4331:
4322:
4321:
4320:
4303:
4298:
4294:
4291:
4285:
4282:
4275:
4274:
4273:
4270:
4257:
4250:
4246:
4239:
4235:
4231:
4226:
4222:
4218:
4212:
4207:
4203:
4199:
4191:
4173:
4165:
4160:
4156:
4152:
4147:
4140:
4135:
4131:
4127:
4121:
4113:
4109:
4102:
4098:
4094:
4089:
4085:
4081:
4075:
4068:
4064:
4059:
4055:
4048:
4044:
4039:
4035:
4032:
4029:
4004:
4000:
3994:
3990:
3982:
3978:
3974:
3969:
3965:
3961:
3955:
3950:
3946:
3925:
3916:
3915:quantum jumps
3911:
3897:
3893:
3885:
3881:
3877:
3872:
3865:
3861:
3857:
3851:
3847:
3844:
3841:
3833:
3817:
3808:
3804:
3800:
3799:Johann Balmer
3794:
3786:
3764:
3760:
3756:
3744:
3738:
3733:
3729:
3721:
3720:
3719:
3715:
3697:
3683:
3678:
3666:
3662:
3659:
3655:
3642:
3638:
3624:
3620:
3609:
3595:
3583:
3576:
3567:
3559:
3558:
3557:
3555:
3550:
3548:
3544:
3540:
3534:
3529:
3525:
3520:
3518:
3517:Coulomb force
3514:
3510:
3509:-body problem
3508:
3488:
3481:
3477:
3464:
3458:
3454:
3447:
3444:
3439:
3435:
3427:
3426:
3425:
3423:
3418:
3413:
3409:
3386:
3382:
3373:
3369:
3357:
3348:
3345:
3340:
3329:
3321:
3318:
3300:
3297:
3292:
3289:
3286:
3280:
3270:
3266:
3254:
3243:
3240:
3222:
3218:
3206:
3198:
3197:
3196:
3194:
3193:natural units
3175:
3167:
3159:
3146:
3140:
3130:
3126:
3114:
3104:
3093:
3085:
3084:
3083:
3078:
3073:
3071:
3067:
3061:
3054:
3049:
3043:
3023:
3005:
3001:
2994:
2990:
2986:
2983:
2977:
2969:
2965:
2959:
2951:
2938:
2932:
2922:
2918:
2906:
2897:
2893:
2886:
2883:
2875:
2871:
2867:
2860:
2856:
2844:
2840:
2834:
2831:
2828:
2821:
2820:
2819:
2817:
2798:
2784:
2781:
2768:
2765:
2761:
2757:
2754:
2751:
2737:
2731:
2727:
2715:
2708:
2698:
2693:
2689:
2681:
2680:
2679:
2677:
2671:
2666:
2647:
2633:
2627:
2623:
2611:
2607:
2600:
2590:
2586:
2579:
2574:
2570:
2562:
2561:
2560:
2558:
2539:
2533:
2530:
2527:
2519:
2510:
2502:
2498:
2494:
2485:
2470:
2462:
2461:
2460:
2458:
2454:
2449:
2432:
2426:
2423:
2417:
2414:
2409:
2406:
2400:
2397:
2390:
2389:
2388:
2386:
2382:
2363:
2356:
2352:
2347:
2341:
2335:
2332:
2325:
2321:
2317:
2312:
2304:
2296:
2290:
2287:
2280:
2275:
2272:
2262:
2261:
2260:
2246:
2235:
2216:
2209:
2205:
2201:
2196:
2191:
2188:
2183:
2180:
2173:
2172:
2171:
2155:
2145:
2140:
2123:
2118:
2114:
2110:
2106:
2102:
2095:
2091:
2087:
2083:
2079:
2074:
2071:
2061:
2060:
2059:
2057:
2053:
2049:
2045:
2039:
2035:
2028:
2010:
2004:
2001:
1998:
1995:
1984:
1976:
1975:
1973:
1968:
1962:
1958:
1954:
1950:
1949:
1946:
1943:
1942:
1938:
1934:
1930:
1926:
1922:
1906:
1901:
1897:
1885:
1879:
1876:
1871:
1868:
1865:
1858:
1857:
1855:
1839:
1832:
1821:
1813:
1809:
1797:
1793:
1786:
1783:
1776:
1775:
1773:
1772:atomic number
1769:
1765:
1758:
1754:
1750:
1743:
1739:
1723:
1716:
1712:
1705:
1701:
1689:
1685:
1679:
1674:
1668:
1664:
1652:
1641:
1640:
1638:
1637:Coulomb force
1634:
1630:
1629:
1626:
1623:
1622:
1621:
1618:
1616:
1613:
1609:
1608:Moseley's law
1605:
1601:
1597:
1593:
1589:
1585:
1584:hydrogen atom
1576:
1569:
1567:
1565:
1560:
1556:
1552:
1548:
1544:
1539:
1524:
1504:
1500:
1496:
1493:
1490:
1482:
1466:
1462:
1458:
1449:
1432:
1426:
1423:
1418:
1415:
1409:
1406:
1399:
1398:
1397:
1383:
1363:
1360:
1357:
1334:
1331:
1328:
1325:
1322:
1316:
1313:
1308:
1305:
1295:
1294:
1293:
1276:
1273:
1270:
1267:
1264:
1258:
1255:
1250:
1247:
1237:
1236:
1235:
1218:
1212:
1209:
1205:
1200:
1197:
1190:
1189:
1188:
1186:
1182:
1163:
1160:
1157:
1154:
1151:
1148:
1145:
1138:
1137:
1136:
1134:
1133:standing wave
1130:
1102:
1098:
1094:
1090:
1087:
1071:
1051:
1043:
1027:
1007:
987:
967:
947:
925:
922:
919:
915:
892:
888:
879:
860:
840:
832:
829:
825:
822:
818:
814:
810:
809:
808:
790:
770:
767:
764:
759:
755:
751:
746:
742:
738:
735:
724:
708:
700:
697:
696:energy levels
693:
692:hydrogen-like
689:
685:
669:
649:
646:
642:
638:
635:
624:
608:
605:
602:
599:
596:
593:
590:
587:
584:
581:
578:
555:
552:
549:
546:
535:
526:
522:
519:
515:
514:
513:
511:
506:
504:
500:
496:
495:Johann Balmer
492:
484:
482:
478:
476:
472:
467:
463:
461:
456:
448:
446:
442:
438:
436:
432:
428:
426:
422:
414:
412:
405:
403:
401:
397:
393:
388:
384:
380:
376:
370:
362:
360:
357:
353:
349:
343:
333:
331:
327:
326:Joseph Larmor
319:
317:
312:
307:
302:
294:
292:
290:
289:
284:
280:
276:
272:
268:
264:
260:
259:valence shell
256:
255:hydrogen atom
251:
249:
245:
240:
238:
234:
230:
226:
222:
218:
214:
210:
206:
205:cubical model
202:
198:
197:Joseph Larmor
193:
191:
187:
183:
179:
175:
172:
168:
164:
160:
156:
152:
148:
144:
140:
136:
132:
124:
120:
114:
109:
108:Balmer series
101:
97:
93:
89:
85:
81:
77:
73:
67:
60:
55:
54:hydrogen atom
50:
44:
40:
39:Bohr equation
33:
19:
7745:
7714:(knot model)
7705:Dalton model
7614:
7610:
7591:
7566:
7547:
7538:
7515:
7503:
7478:
7469:
7465:
7459:
7455:
7422:
7418:
7381:
7377:
7348:
7344:
7315:
7311:
7282:
7278:
7253:. Retrieved
7251:. 2021-11-23
7249:www.iaea.org
7248:
7239:
7220:
7214:
7159:
7155:
7145:
7136:
7130:
7111:
7105:
7088:
7082:
7072:
7050:(17): 1–94.
7047:
7041:
7031:
7006:
7002:
6996:
6987:
6981:
6968:
6935:
6929:
6923:
6917:: 1024–1034.
6914:
6910:
6900:
6882:
6865:
6861:
6848:
6819:(1): 42–49.
6816:
6812:
6771:
6767:
6737:
6733:
6723:
6690:
6686:
6676:
6651:
6647:
6637:
6628:
6624:
6618:
6593:
6589:
6557:
6551:
6526:
6522:
6512:
6503:
6497:
6456:
6452:
6442:
6431:
6422:
6413:
6409:
6403:
6394:
6388:
6371:
6335:
6331:
6299:
6255:
6251:
6187:
6144:
6140:
6112:
6108:
6098:
6073:
6069:
6019:
6015:
6005:
5985:
5950:
5946:
5914:
5908:
5895:
5876:
5840:
5836:
5811:
5802:
5795:Perrin, Jean
5789:
5764:
5760:
5668:
5644:atomic whirl
5599:
5566:
5548:
5525:
5521:Stark effect
5513:
5484:
5478:
5457:
5450:
5443:
5441:
5355:
5296:Stark effect
5279:
5273:
5265:
5259:
5256:
5249:
5245:
5242:
5239:Shortcomings
5225:
5218:
5212:
5205:
5198:
5197:
5189:
5188:
5186:
5041:
4891:
4887:
4884:
4872:
4868:
4861:
4854:
4850:
4847:
4834:
4823:
4819:
4806:
4790:
4785:
4781:
4777:
4775:
4767:
4743:
4733:
4725:
4533:
4529:
4522:
4518:
4515:
4494:
4488:
4484:
4478:
4472:
4468:
4462:
4459:
4450:
4437:
4424:
4417:
4318:
4271:
3912:
3807:Walther Ritz
3796:
3716:
3712:
3554:reduced mass
3551:
3546:
3542:
3541:cancels the
3538:
3532:
3527:
3523:
3521:
3506:
3503:
3421:
3416:
3411:
3407:
3404:
3190:
3076:
3074:
3065:
3059:
3052:
3041:
3038:
2815:
2813:
2669:
2664:
2662:
2556:
2554:
2456:
2455:in terms of
2452:
2450:
2447:
2384:
2380:
2378:
2233:
2231:
2143:
2141:
2138:
2055:
2051:
2050:to scale as
2043:
2040:
2036:
2032:
1971:
1966:
1960:
1956:
1944:
1928:
1924:
1767:
1756:
1748:
1741:
1624:
1619:
1581:
1555:Another form
1540:
1450:
1447:
1349:
1291:
1233:
1178:
1106:
820:
816:
806:
507:
488:
479:
471:J.J. Thomson
468:
464:
452:
443:
439:
429:
418:
409:
372:
345:
323:
315:
286:
252:
241:
227:(1911), and
194:
188:rather than
182:Solar System
138:
134:
128:
125:(red light).
112:
95:
91:
87:
76:atomic shell
65:
58:
7719:Lewis model
6888:Bohr, Niels
6596:(1): 1–38.
6226:Bohr, Niels
5843:: 123–186.
5654:in general.
5540:line bundle
5338:Refinements
4700:8, 8, 4, 3
4680:8, 8, 2, 4
4660:8, 8, 2, 3
4640:8, 8, 2, 2
4620:8, 8, 2, 1
4580:8, 4, 4, 1
2676:Bohr radius
1612:heavy quark
1596:positronium
1040:(so-called
684:Bohr radius
485:Development
375:Hans Geiger
348:J J Thomson
221:Arthur Haas
201:Jean Perrin
163:J J Thomson
153:'s nuclear
104:3 → 2
43:Bohr effect
7844:Niels Bohr
7818:Categories
7746:Bohr model
7488:1048217622
7255:2024-09-04
6631:: 476–502.
5953:: vi–290.
5767:(9): 933.
5738:References
5533:connection
5366:Sommerfeld
4754:octet rule
4714:8, 4, 2, 2
3801:and later
2029:Derivation
1517:described
1129:de Broglie
1064:(or large
510:Niels Bohr
425:Max Planck
295:Background
147:Niels Bohr
135:Bohr model
119:wavelength
7803:Chemistry
7639:120859582
7592:Chemistry
7510:Reprint:
6960:250901403
6825:797965772
6610:2102-6467
6543:1941-5982
6380:557599205
6354:0035-8711
6280:120797894
6036:0003-9519
5743:Footnotes
5629:branches.
5537:Hermitian
5380:∫
5156:−
5126:×
5101:−
5059:ν
5002:−
4964:−
4930:−
4914:ν
4824:In 1913,
4802:noble gas
4797:viscosity
4771:screening
4444:=2), and
4373:−
4335:λ
4299:λ
4223:π
4157:τ
4148:−
4132:τ
4086:π
4065:τ
4056:−
4045:τ
4033:ν
4001:τ
3966:π
3951:τ
3926:τ
3873:−
3842:ν
3448:−
3383:α
3293:≈
3290:α
3278:ℏ
3164:ℏ
2984:−
2978:≈
2956:ℏ
2887:−
2835:−
2766:−
2758:×
2752:≈
2705:ℏ
2597:ℏ
2537:ℏ
2430:ℏ
2418:π
2345:Δ
2336:−
2333:≈
2313:−
2294:Δ
2276:∝
2270:Δ
2244:Δ
2197:∝
2184:∝
2103:∝
2075:∝
2069:Δ
2008:ℏ
1872:−
1491:λ
1427:π
1407:ℓ
1384:ℓ
1317:π
1271:π
1198:λ
1158:π
1149:λ
1115:ℏ
923:−
878:harmonics
861:ν
841:ν
821:classical
771:ν
752:−
733:Δ
709:ν
650:π
633:ℏ
559:ℏ
491:empirical
400:electrons
373:In 1908,
213:Saturnian
178:analogous
174:electrons
121:656
7462:: 82–92.
7406:11988018
7206:16103360
6990:: 32–41.
6837:Archived
6833:53520045
6489:35364965
6272:41133258
6161:23739408
6044:41133273
5967:27757291
5857:27757389
5797:(1901).
5692:See also
5507:, which
4600:8, 8, 2
3415:, where
1101:momentum
817:discrete
783:, where
571:, where
508:In 1913
453:In 1911
219:(1904),
207:(1902),
176:. It is
171:orbiting
94:, where
72:electron
7619:Bibcode
7447:4035652
7427:Bibcode
7386:Bibcode
7353:Bibcode
7320:Bibcode
7287:Bibcode
7197:1186029
7174:Bibcode
7052:Bibcode
7023:9628509
6940:Bibcode
6776:Bibcode
6715:3977652
6695:Bibcode
6656:Bibcode
6481:9899816
6461:Bibcode
6169:4355108
6078:Bibcode
5769:Bibcode
5659:Unicode
5652:atheism
5632:The US
5284:lithium
5215:K-alpha
4873:It was
4830:K-alpha
4708:4, 2, 2
4694:8, 4, 3
4674:8, 2, 4
4654:8, 2, 3
4634:8, 2, 2
4614:8, 2, 1
4574:4, 4, 1
4557:Element
4551:Element
4545:Element
4528:unless
4446:Paschen
3513:nucleus
3315:is the
3237:is the
1762:is the
1751:is the
1592:lithium
828:photons
396:nucleus
190:gravity
167:nucleus
98:is the
88:hν
7637:
7598:
7573:
7554:
7524:
7486:
7445:
7419:Nature
7404:
7378:Nature
7227:
7204:
7194:
7118:
7021:
6958:
6831:
6823:
6713:
6687:Nature
6608:
6564:
6541:
6487:
6479:
6415:waves.
6378:
6352:
6306:
6278:
6270:
6203:
6167:
6159:
6141:Nature
6042:
6034:
5993:
5965:
5921:
5883:
5855:
5805:: 463.
5462:action
5442:where
5362:Wilson
5187:Here,
5139:
4759:Kossel
4492:where
4433:Balmer
3013:
2788:
2774:
1740:where
1615:mesons
1604:K-line
1588:helium
1479:while
1350:where
1183:. The
625:, and
133:, the
102:. The
68:> 1
7768:1928
7762:1926
7744:1913
7737:1911
7731:1904
7724:1904
7717:1902
7710:1867
7703:1804
7635:S2CID
7443:S2CID
7402:S2CID
7164:arXiv
7019:S2CID
6956:S2CID
6840:(PDF)
6829:S2CID
6809:(PDF)
6711:S2CID
6485:S2CID
6276:S2CID
6268:JSTOR
6165:S2CID
6040:JSTOR
5963:JSTOR
5853:JSTOR
5678:React
5627:olive
5535:of a
5531:of a
5231:) in
4482:with
4466:with
4431:=1),
4420:Lyman
3535:≈ 137
3522:When
1181:waves
1131:as a
940:when
155:model
7596:ISBN
7571:ISBN
7552:ISBN
7522:ISBN
7484:OCLC
7225:ISBN
7202:PMID
7116:ISBN
6821:OCLC
6606:ISSN
6562:ISBN
6539:ISSN
6477:PMID
6376:OCLC
6350:ISSN
6304:ISBN
6201:ISBN
6157:PMID
6032:ISSN
5991:ISBN
5919:ISBN
5881:ISBN
5720:The
5713:The
5657:The
5312:The
5305:and
5224:and
5213:The
5123:2.46
4842:and
4688:4, 3
4668:2, 4
4648:2, 3
4628:2, 2
4608:2, 1
4594:8, 2
3805:and
3526:= 1/
2987:13.6
2785:52.9
2755:5.29
1951:The
1766:and
1598:and
1099:and
1091:The
907:and
377:and
246:for
143:atom
7627:doi
7435:doi
7423:107
7394:doi
7361:doi
7328:doi
7295:doi
7192:PMC
7182:doi
7160:102
7093:doi
7060:doi
7011:doi
6948:doi
6870:doi
6784:doi
6772:354
6742:doi
6703:doi
6664:doi
6598:doi
6531:doi
6469:doi
6340:doi
6260:doi
6193:doi
6149:doi
6145:498
6117:doi
6086:doi
6024:doi
5955:doi
5845:doi
5777:doi
5553:".
5503:of
5464:of
5280:two
5042:or
4804:).
4476:or
3572:red
3545:in
3301:137
3082:):
3055:= 2
3044:= 1
2672:= 1
2559:is
1483:of
1292:or
874:rot
473:'s
402:.
273:or
211:'s
161:of
137:or
129:In
115:= 1
61:= 1
41:or
7820::
7801:/
7633:.
7625:.
7615:36
7613:.
7460:19
7458:.
7441:.
7433:.
7421:.
7417:.
7400:.
7392:.
7382:92
7380:.
7376:.
7359:.
7349:26
7347:.
7343:.
7326:.
7316:26
7314:.
7310:.
7293:.
7283:26
7281:.
7277:.
7247:.
7200:.
7190:.
7180:.
7172:.
7158:.
7154:.
7089:29
7087:.
7081:.
7058:.
7048:51
7040:.
7017:.
7007:47
7005:.
6988:14
6980:.
6954:.
6946:.
6936:20
6934:.
6915:26
6909:.
6866:41
6864:.
6860:.
6835:.
6827:.
6817:37
6815:.
6811:.
6796:^
6782:.
6754:^
6738:43
6736:.
6732:.
6709:.
6701:.
6691:93
6689:.
6685:.
6662:.
6652:26
6650:.
6646:.
6629:26
6627:.
6604:.
6594:38
6592:.
6588:.
6576:^
6537:.
6527:26
6525:.
6521:.
6483:.
6475:.
6467:.
6457:37
6455:.
6451:.
6430:.
6362:^
6348:.
6336:72
6334:.
6330:.
6318:^
6288:^
6274:.
6266:.
6254:.
6238:^
6215:^
6199:.
6177:^
6163:.
6155:.
6143:.
6129:^
6113:11
6111:.
6107:.
6084:.
6074:26
6072:.
6068:.
6052:^
6038:.
6030:.
6018:.
6014:.
5975:^
5961:.
5949:.
5933:^
5865:^
5851:.
5841:10
5839:.
5823:^
5801:.
5775:.
5765:65
5763:.
5751:^
5546:.
5485:xy
5476:.
5298:).
5235:.
5226:Kα
5219:Kα
5196:=
5143:Hz
5134:15
5130:10
4866:.
4717:24
4711:16
4697:23
4691:15
4677:22
4671:14
4657:21
4651:13
4637:20
4631:12
4617:19
4611:11
4597:18
4591:10
4577:17
4192::
3938::
3519:.
3412:Ze
3410:=
3195::
3072:.
3062:=
3048:eV
2769:11
2762:10
2459::
2387:,
1974::
1967:vr
1959:=
1755:,
1639:.
1617:.
1553:.
725::
527::
291:.
239:.
123:nm
7682:e
7675:t
7668:v
7641:.
7629::
7621::
7604:.
7579:.
7560:.
7530:.
7490:.
7470:6
7449:.
7437::
7429::
7408:.
7396::
7388::
7367:.
7363::
7355::
7334:.
7330::
7322::
7301:.
7297::
7289::
7258:.
7233:.
7208:.
7184::
7176::
7166::
7124:.
7099:.
7095::
7066:.
7062::
7054::
7025:.
7013::
6962:.
6950::
6942::
6876:.
6872::
6790:.
6786::
6778::
6748:.
6744::
6717:.
6705::
6697::
6670:.
6666::
6658::
6612:.
6600::
6570:.
6545:.
6533::
6491:.
6471::
6463::
6382:.
6356:.
6342::
6312:.
6282:.
6262::
6256:3
6209:.
6195::
6171:.
6151::
6123:.
6119::
6092:.
6088::
6080::
6046:.
6026::
6020:4
5999:.
5969:.
5957::
5951:1
5927:.
5889:.
5859:.
5847::
5783:.
5779::
5771::
5458:T
5453:r
5451:q
5446:r
5444:p
5427:,
5424:h
5421:n
5418:=
5413:r
5409:q
5405:d
5399:r
5395:p
5389:T
5384:0
5364:–
5250:ħ
5248:=
5246:L
5228:2
5221:1
5206:h
5204:/
5202:E
5199:R
5193:v
5190:R
5172:.
5167:2
5163:)
5159:1
5153:Z
5150:(
5147:)
5120:(
5117:=
5112:2
5108:)
5104:1
5098:Z
5095:(
5091:)
5086:4
5083:3
5078:(
5071:v
5066:R
5062:=
5056:=
5053:f
5027:,
5023:)
5015:2
5011:2
5007:1
4995:2
4991:1
4987:1
4981:(
4975:2
4971:)
4967:1
4961:Z
4958:(
4952:E
4947:R
4943:=
4938:f
4934:E
4925:i
4921:E
4917:=
4911:h
4908:=
4905:E
4892:Z
4888:Z
4862:Z
4860:(
4855:Z
4835:Z
4786:Z
4782:Z
4778:Z
4705:8
4685:7
4665:6
4645:5
4625:4
4605:3
4588:2
4585:2
4571:9
4568:1
4565:1
4534:N
4530:n
4525:e
4523:n
4519:n
4495:b
4489:b
4485:n
4479:n
4473:b
4469:Z
4463:Z
4453:f
4451:n
4448:(
4440:f
4438:n
4435:(
4427:f
4425:n
4422:(
4403:.
4399:)
4391:2
4386:i
4382:n
4378:1
4366:2
4361:f
4357:n
4353:1
4347:(
4343:R
4340:=
4332:1
4304:,
4295:c
4292:h
4286:=
4283:E
4258:.
4251:3
4247:h
4240:4
4236:e
4232:m
4227:2
4219:2
4213:=
4208:H
4204:R
4200:c
4174:)
4166:2
4161:1
4153:1
4141:2
4136:2
4128:1
4122:(
4114:2
4110:h
4103:4
4099:e
4095:m
4090:2
4082:2
4076:=
4069:1
4060:W
4049:2
4040:W
4036:=
4030:h
4005:2
3995:2
3991:h
3983:4
3979:e
3975:m
3970:2
3962:2
3956:=
3947:W
3898:.
3894:)
3886:2
3882:n
3878:1
3866:2
3862:m
3858:1
3852:(
3848:R
3845:=
3818:R
3765:2
3761:n
3757:2
3750:E
3745:R
3739:=
3734:n
3730:E
3698:.
3689:p
3684:m
3679:/
3672:e
3667:m
3663:+
3660:1
3656:1
3648:e
3643:m
3639:=
3630:p
3625:m
3621:+
3615:e
3610:m
3601:p
3596:m
3589:e
3584:m
3577:=
3568:m
3547:R
3543:α
3539:Z
3533:Z
3530:(
3528:α
3524:Z
3507:n
3489:.
3482:2
3478:n
3470:E
3465:R
3459:2
3455:Z
3445:=
3440:n
3436:E
3422:Z
3417:Z
3408:q
3401:.
3387:2
3379:)
3374:2
3370:c
3363:e
3358:m
3354:(
3349:2
3346:1
3341:=
3335:E
3330:R
3319:,
3298:1
3287:=
3281:c
3271:2
3267:e
3260:e
3255:k
3223:2
3219:c
3212:e
3207:m
3176:.
3168:2
3160:2
3152:e
3147:m
3141:2
3137:)
3131:2
3127:e
3120:e
3115:k
3111:(
3105:=
3099:E
3094:R
3080:E
3077:R
3066:n
3060:n
3053:n
3042:n
3024:.
3020:V
3017:e
3006:2
3002:n
2995:2
2991:Z
2970:2
2966:n
2960:2
2952:2
2944:e
2939:m
2933:2
2929:)
2923:2
2919:e
2912:e
2907:k
2903:(
2898:2
2894:Z
2884:=
2876:n
2872:r
2868:2
2861:2
2857:e
2850:e
2845:k
2841:Z
2832:=
2829:E
2816:n
2799:.
2795:m
2792:p
2782:=
2778:m
2743:e
2738:m
2732:2
2728:e
2721:e
2716:k
2709:2
2699:=
2694:1
2690:r
2670:Z
2665:r
2648:.
2639:e
2634:m
2628:2
2624:e
2617:e
2612:k
2608:Z
2601:2
2591:2
2587:n
2580:=
2575:n
2571:r
2557:n
2540:,
2534:n
2531:=
2528:r
2520:r
2515:e
2511:m
2503:2
2499:e
2495:Z
2490:e
2486:k
2475:e
2471:m
2457:n
2453:r
2433:.
2427:n
2424:=
2415:2
2410:h
2407:n
2401:=
2398:L
2385:ħ
2381:ħ
2364:.
2357:3
2353:L
2348:L
2342:2
2326:2
2322:L
2318:1
2305:2
2301:)
2297:L
2291:+
2288:L
2285:(
2281:1
2273:E
2247:L
2234:L
2217:.
2210:2
2206:L
2202:1
2192:r
2189:1
2181:E
2156:r
2144:L
2124:.
2119:2
2115:/
2111:3
2107:E
2096:2
2092:/
2088:3
2084:r
2080:1
2072:E
2056:r
2052:r
2044:T
2011:.
2005:n
2002:=
1999:r
1996:v
1990:e
1985:m
1972:ħ
1964:e
1961:m
1957:L
1939:.
1929:r
1925:r
1907:.
1902:2
1898:v
1891:e
1886:m
1880:2
1877:1
1869:=
1866:E
1840:.
1833:r
1827:e
1822:m
1814:2
1810:e
1803:e
1798:k
1794:Z
1787:=
1784:v
1768:Z
1760:e
1757:k
1749:e
1745:e
1742:m
1724:,
1717:2
1713:r
1706:2
1702:e
1695:e
1690:k
1686:Z
1680:=
1675:r
1669:2
1665:v
1658:e
1653:m
1525:h
1505:p
1501:/
1497:h
1494:=
1467:h
1463:/
1459:2
1433:,
1424:2
1419:h
1416:n
1410:=
1364:r
1361:v
1358:m
1335:,
1332:r
1329:v
1326:m
1323:=
1314:2
1309:h
1306:n
1277:,
1274:r
1268:2
1265:=
1259:v
1256:m
1251:h
1248:n
1219:,
1213:v
1210:m
1206:h
1201:=
1164:.
1161:r
1155:2
1152:=
1146:n
1072:k
1052:n
1028:n
1008:n
988:k
968:n
948:k
926:k
920:n
916:E
893:n
889:E
830:.
791:h
768:h
765:=
760:1
756:E
747:2
743:E
739:=
736:E
670:n
647:2
643:/
639:h
636:=
609:.
606:.
603:.
600:,
597:3
594:,
591:2
588:,
585:1
582:=
579:n
556:n
553:=
550:r
547:v
541:e
536:m
113:Z
96:n
92:n
86:(
66:Z
59:Z
56:(
45:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.