262:
187:
44:
538:- is a colorless, crystalline solid that melts at 257 °C and decomposes at 254 °C. Nitroguanidine is an extremely insensitive but powerful high explosive. Wetting it with > 20 wt.-% water effects desensitization from HD 1.1 down to HD 4.1 (flammable solid). Nitroguanidine is used as an energetic material, i.e., propellant or high explosive, precursor for insecticides, and for other purposes.
35:
499:
666:
in which it reduces flame temperature, muzzle flash, and erosion of the gun barrel but preserves chamber pressure due to high nitrogen content. Its extreme insensitivity combined with low cost has made it a popular ingredient in insensitive high explosive formulations (e.g AFX-453, AFX-760,
512:
311:
970:
Murmann, R. K.; Glaser, Rainer; Barnes, Charles L. (2005). "Structures of nitroso- and nitroguanidine x - ray crystallography and computational analysis".
760:
933:"Structure of nitroguanidine: nitroamine or nitroimine? New NMR evidence from nitrogen-15 labeled sample and nitrogen-15 spin coupling constants"
835:
United
Nations, Transport of Nitroguanidine, wetted, (UN 1336) in flexible IBCs, ST/SC/AC.10/C.3/2006/52, Geneva, 13 April 2006. Accessed at
604:. However, owing to problems of reliability and safety, this process has never been commercialized despite its attractive economic features.
851:
Koch, Ernst‐Christian (2019). "Insensitive High
Explosives: III. Nitroguanidine – Synthesis – Structure – Spectroscopy – Sensitiveness".
276:
448:
124:
596:
The guanidinium nitrate intermediate may also be produced via the
Boatright–Mackay–Roberts (BMR) process, in which molten
432:
519:
1033:
219:
836:
240:
751:
The nitrosoylated derivative, nitrosoguanidine, is often used to mutagenize bacterial cells for biochemical studies.
795:
Gao, Han; Wang, Qinghua; Ke, Xiang; Liu, Jie; Hao, Gazi; Xiao, Lei; Chen, Teng; Jiang, Wei; Liu, Qiao'e (2017).
1043:
182:
62:
920:
1038:
422:
797:"Preparation and characterization of an ultrafine HMX/NQ co-crystal by vacuum freeze drying method"
768:
555:
257:
90:
987:
952:
772:
895:
868:
818:
764:
481:
412:
17:
979:
944:
860:
808:
663:
551:
396:
334:
908:
E.-C. Koch, Insensitive High
Explosives: IV. Nitroguanidine - Initiation & detonation,
228:
164:
1016:
558:, which is then nitrated by treatment with concentrated sulfuric acid at low temperature.
43:
100:
261:
186:
144:
490:
1027:
887:
547:
385:
175:
991:
956:
837:
https://www.unece.org/fileadmin/DAM/trans/doc/2006/ac10c3/ST-SG-AC10-C3-2006-52e.pdf
546:
Nitroguanidine is produced worldwide on a large scale starting with the reaction of
740:
736:
728:
208:
732:
720:
983:
674:
Nitroguanidine's explosive decomposition is given by the following equation: H
601:
357:
155:
872:
822:
477:
34:
948:
864:
775:
724:
1004:
S. Choi, Refinement of 2-Nitroguanidine by
Neutron Powder Diffraction,
813:
796:
668:
375:
195:
932:
489:
Except where otherwise noted, data are given for materials in their
659:
Nitroguanidine has been in use since the 1930s as an ingredient in
135:
123:
113:
597:
892:
Process
Engineering Design for Manufacture of Guanidine Nitrate
759:
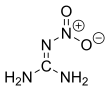
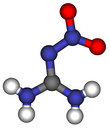
Following several decades of debate, it could be confirmed by
886:
Steele, N. W.; Doyle, J. A.; Whippen, M. G.; Gorton, J. A.;
245:
42:
33:
600:
is reacted with molten ammonium nitrate in the presence of
507:
931:Bulusu, S.; Dudley, R. L.; Autera, J. R. (1987).
207:
99:
771:that nitroguanidine exclusively exists as the
8:
285:InChI=1S/CH4N4O2/c2-1(3)4-5(6)7/h(H4,2,3,4)
846:
844:
295:InChI=1/CH4N4O2/c2-1(3)4-5(6)7/h(H4,2,3,4)
260:
185:
163:
22:
812:
227:
642:
638:
634:
630:
626:
622:
618:
614:
610:
588:
584:
580:
576:
572:
568:
564:
787:
719:Nitroguanidine derivatives are used as
316:
281:
256:
390:257 °C (495 °F; 530 K)
176:
853:Propellants, Explosives, Pyrotechnics
288:Key: IDCPFAYURAQKDZ-UHFFFAOYSA-N
143:
7:
972:Journal of Chemical Crystallography
298:Key: IDCPFAYURAQKDZ-UHFFFAOYAN
198:
778:both in solid state and solution.
14:
723:, having a comparable effect to
497:
937:Magnetic Resonance in Chemistry
671:, AL-IMX-101, IMX-103, etc.).
493:(at 25 °C , 100 kPa).
449:Occupational safety and health
402:3.45 g/kg (in water at 25 °C)
1:
370:Colorless crystalline solid
29:
1060:
362:104.07 g/mol
15:
984:10.1007/s10870-005-3252-y
487:
466:
446:
441:
406:
327:
307:
272:
83:
71:
61:
56:
28:
534:- sometimes abbreviated
16:Not to be confused with
727:. Derivatives include
949:10.1002/mrc.1260250311
865:10.1002/prep.201800253
47:
38:
46:
37:
894:(Technical report).
423:Friction sensitivity
1034:Explosive chemicals
1006:Acta Crystallogr. B
807:(73): 46229–46235.
769:neutron diffraction
556:guanidinium nitrate
554:to afford the salt
397:Solubility in water
25:
814:10.1039/C7RA06646E
520:Infobox references
467:Related compounds
48:
39:
23:
896:Picatinny Arsenal
890:(December 1973).
528:Chemical compound
526:
525:
482:Guanidine nitrate
473:Related compounds
413:Shock sensitivity
241:CompTox Dashboard
125:Interactive image
52:
51:
18:Guanidine nitrate
1051:
1018:
1002:
996:
995:
967:
961:
960:
928:
922:
906:
900:
899:
883:
877:
876:
848:
839:
833:
827:
826:
816:
792:
761:NMR spectroscopy
645:
592:
552:ammonium nitrate
510:
504:
501:
500:
335:Chemical formula
265:
264:
249:
247:
231:
211:
200:
189:
178:
167:
147:
127:
103:
66:1-Nitroguanidine
30:
26:
1059:
1058:
1054:
1053:
1052:
1050:
1049:
1048:
1044:Nitroguanidines
1024:
1023:
1022:
1021:
1003:
999:
969:
968:
964:
930:
929:
925:
907:
903:
885:
884:
880:
850:
849:
842:
834:
830:
794:
793:
789:
784:
757:
749:
717:
711:
705:
698:
692:
688:
681:
677:
664:gun propellants
657:
652:
644:
640:
636:
632:
628:
624:
620:
616:
612:
608:
590:
586:
582:
578:
574:
570:
566:
562:
544:
529:
522:
517:
516:
515: ?)
506:
502:
498:
494:
480:
474:
459:
407:Explosive data
399:
351:
347:
343:
337:
323:
320:
315:
314:
303:
300:
299:
296:
290:
289:
286:
280:
279:
268:
250:
243:
234:
214:
201:
170:
150:
130:
117:
106:
93:
79:
77:
75:
67:
24:Nitroguanidine
21:
12:
11:
5:
1057:
1055:
1047:
1046:
1041:
1036:
1026:
1025:
1020:
1019:
997:
978:(4): 317–325.
962:
943:(3): 234–238.
923:
901:
878:
859:(3): 267–292.
840:
828:
786:
785:
783:
780:
756:
753:
748:
745:
716:
713:
707:
700:
694:
690:
683:
679:
675:
656:
653:
651:
648:
647:
646:
594:
593:
543:
540:
532:Nitroguanidine
527:
524:
523:
518:
496:
495:
491:standard state
488:
485:
484:
475:
472:
469:
468:
464:
463:
460:
457:
454:
453:
444:
443:
439:
438:
435:
429:
428:
425:
419:
418:
415:
409:
408:
404:
403:
400:
395:
392:
391:
388:
382:
381:
378:
372:
371:
368:
364:
363:
360:
354:
353:
349:
345:
341:
338:
333:
330:
329:
325:
324:
322:
321:
318:
310:
309:
308:
305:
304:
302:
301:
297:
294:
293:
291:
287:
284:
283:
275:
274:
273:
270:
269:
267:
266:
253:
251:
239:
236:
235:
233:
232:
224:
222:
216:
215:
213:
212:
204:
202:
194:
191:
190:
180:
172:
171:
169:
168:
160:
158:
152:
151:
149:
148:
140:
138:
132:
131:
129:
128:
120:
118:
111:
108:
107:
105:
104:
96:
94:
89:
86:
85:
81:
80:
73:
69:
68:
65:
59:
58:
54:
53:
50:
49:
40:
13:
10:
9:
6:
4:
3:
2:
1056:
1045:
1042:
1040:
1037:
1035:
1032:
1031:
1029:
1017:
1014:
1010:
1007:
1001:
998:
993:
989:
985:
981:
977:
973:
966:
963:
958:
954:
950:
946:
942:
938:
934:
927:
924:
921:
918:
914:
911:
905:
902:
897:
893:
889:
888:Hercules Inc.
882:
879:
874:
870:
866:
862:
858:
854:
847:
845:
841:
838:
832:
829:
824:
820:
815:
810:
806:
802:
798:
791:
788:
781:
779:
777:
774:
770:
766:
762:
754:
752:
746:
744:
742:
738:
734:
730:
726:
722:
714:
712:
710:
704:
697:
687:
672:
670:
665:
662:
654:
649:
607:
606:
605:
603:
599:
561:
560:
559:
557:
553:
549:
548:dicyandiamide
541:
539:
537:
533:
521:
514:
509:
492:
486:
483:
479:
476:
471:
470:
465:
461:
456:
455:
451:
450:
445:
440:
436:
434:
431:
430:
426:
424:
421:
420:
416:
414:
411:
410:
405:
401:
398:
394:
393:
389:
387:
386:Melting point
384:
383:
379:
377:
374:
373:
369:
366:
365:
361:
359:
356:
355:
339:
336:
332:
331:
326:
317:
313:
306:
292:
282:
278:
271:
263:
259:
258:DTXSID4024222
255:
254:
252:
242:
238:
237:
230:
226:
225:
223:
221:
218:
217:
210:
206:
205:
203:
197:
193:
192:
188:
184:
181:
179:
177:ECHA InfoCard
174:
173:
166:
162:
161:
159:
157:
154:
153:
146:
142:
141:
139:
137:
134:
133:
126:
122:
121:
119:
115:
110:
109:
102:
98:
97:
95:
92:
88:
87:
82:
70:
64:
60:
55:
45:
41:
36:
32:
31:
27:
19:
1015:, 1955-1957.
1012:
1008:
1005:
1000:
975:
971:
965:
940:
936:
926:
916:
912:
909:
904:
898:. AD-772074.
891:
881:
856:
852:
831:
804:
800:
790:
758:
750:
747:Biochemistry
741:thiamethoxam
737:imidacloprid
729:clothianidin
721:insecticides
718:
708:
702:
695:
685:
673:
660:
658:
625:→ [C(NH
595:
545:
535:
531:
530:
458:Main hazards
447:
84:Identifiers
72:Other names
1039:Propellants
763:, and both
733:dinotefuran
661:triple-base
550:(DCD) with
542:Manufacture
452:(OHS/OSH):
427:> 350 N
367:Appearance
328:Properties
319:NC(N)=N()=O
183:100.008.313
145:CHEBI:39180
1028:Categories
919:, 467-487.
910:Def. Tech.
782:References
773:nitroimine
715:Pesticides
655:Explosives
602:silica gel
462:Explosive
417:> 50 J
380:1.77 g/cm
358:Molar mass
229:NAY6KWL67F
156:ChemSpider
112:3D model (
91:CAS Number
63:IUPAC name
873:0721-3115
823:2046-2069
755:Structure
563:[C(NH
478:Guanidine
433:RE factor
992:96090647
957:97416890
776:tautomer
725:nicotine
442:Hazards
101:556-88-7
801:RSC Adv
669:IMX-101
513:what is
511: (
376:Density
352:
196:PubChem
74:Picrite
990:
955:
871:
821:
739:, and
637:+ 2 NH
575:→ (NH
508:verify
505:
312:SMILES
57:Names
988:S2CID
953:S2CID
765:x-ray
699:+ 2 N
689:→ 2 H
437:1.00
277:InChI
209:11174
165:10701
136:ChEBI
114:JSmol
1009:1981
913:2019
869:ISSN
819:ISSN
767:and
650:Uses
641:+ CO
617:+ NH
613:CONH
609:2 NH
598:urea
583:CNNO
220:UNII
980:doi
945:doi
861:doi
809:doi
709:(s)
706:+ C
703:(g)
696:(g)
686:(s)
633:]NO
587:+ H
571:]NO
536:NGu
246:EPA
199:CID
76:NGu
1030::
1013:37
1011:,
986:.
976:35
974:.
951:.
941:25
939:.
935:.
917:15
915:,
867:.
857:44
855:.
843:^
817:.
803:.
799:.
743:.
735:,
731:,
701:2
684:2
682:CO
621:NO
340:CH
78:NQ
994:.
982::
959:.
947::
875:.
863::
825:.
811::
805:7
693:O
691:2
680:4
678:N
676:4
643:2
639:3
635:3
631:3
629:)
627:2
623:3
619:4
615:2
611:2
591:O
589:2
585:2
581:2
579:)
577:2
573:3
569:3
567:)
565:2
503:Y
350:2
348:O
346:4
344:N
342:4
248:)
244:(
116:)
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.