241:
226:
312:
286:
153:
192:
271:
331:
211:
1233:
256:
442:
168:
2974:
2825:
2893:
2730:
785:, also known as thionitrites, are compounds containing a nitroso group attached to the sulfur atom of a thiol, e.g. R−S−N=O. They have received considerable attention in biochemistry because they serve as donors of the nitrosonium ion, NO, and nitric oxide, NO, which may serve as signaling molecules in living systems, especially related to vasodilation.
1125:
is a positively charged ion featuring three organic substituents attached to sulfur, with the formula . Together with their negatively charged counterpart, the anion, the compounds are called sulfonium salts. An oxosulfonium ion is a positively charged ion featuring three organic substituents and an
1453:
of low-valent organosulfur compounds such as thiols, sulfides, and disulfides. Malodorous volatile thiols are protein-degradation products found in putrid food, so sensitive identification of these compounds is crucial to avoiding intoxication. Low-valent volatile sulfur compounds are also found in
1126:
oxygen attached to sulfur, with the formula . Together with their negatively charged counterpart, the anion, the compounds are called oxosulfonium salts. Related species include alkoxysulfonium and chlorosulfonium ions, and , respectively.
749:
S(O)=NR′. When two different R groups are attached to sulfur, sulfoximides are chiral. Much of the interest in this class of compounds is derived from the discovery that methionine sulfoximide (methionine sulfoximine) is an inhibitor of
1457:
Copper is required for the highly sensitive detection of certain volatile thiols and related organosulfur compounds by olfactory receptors in mice. Whether humans, too, require copper for sensitive detection of thiols is not yet known.
760:(also called sulfodiimines, sulfodiimides or sulfonediimides) are tetracoordinate sulfur–nitrogen compounds, isoelectronic with sulfones, in which both oxygen atoms of the sulfone are replaced by a substituted nitrogen atom, e.g., R
382:
for thiomethane is 89 kcal/mol (370 kJ/mol) compared to methane's 100 kcal/mol (420 kJ/mol) and when hydrogen is replaced by a methyl group the energy decreases to 73 kcal/mol (305 kJ/mol). The single
740:
S=NR′, the nitrogen analog of sulfoxides. They are of interest in part due to their pharmacological properties. When two different R groups are attached to sulfur, sulfimides are chiral. Sulfimides form stable α-carbanions.
744:
Sulfoximides (also called sulfoximines) are tetracoordinate sulfur–nitrogen compounds, isoelectronic with sulfones, in which one oxygen atom of the sulfone is replaced by a substituted nitrogen atom, e.g.,
2332:
Duan, X.; Block, E.; Li, Z.; Connelly, T.; Zhang, J.; Huang, Z.; Su, X.; Pan, Y.; Wu, L.; Chi, Q.; Thomas, S.; Zhang, S.; Ma, M.; Matsunami, H.; Chen, G.-Q.; Zhang, H. (2012).
240:
2061:
Buschmann, J.; Damerius, R.; Gerhardt, R.; Lentz, D.; Luger, P.; Marschall, R.; Preugschat, D.; Seppelt, K.; Simon, A. (1992). "(Trifluoroethylidyne)sulfur trifluoride, F
1887:
311:
1046:(RC(O)SH) and dithiocarboxylic acids (RC(S)SH) are well known. They are structurally similar to carboxylic acids but more acidic. Thioamides are analogous to amides.
2408:
285:
1174:, the ylidic carbon–sulfur bond is highly polarized and is better described as being ionic. Sulfonium ylides are key intermediates in the synthetically useful
1917:
McCaw, Patrick G.; Buckley, Naomi M.; Collins, Stuart G.; Maguire, Anita R. (March 2016). "Generation, Reactivity and Uses of
Sulfines in Organic Synthesis".
270:
255:
550:(see drawing), tricyclic heterocycles consisting of two benzene rings fused to a central thiophene ring, occurs widely in heavier fractions of petroleum.
225:
1159:
330:
1109:. Chiral sulfinamides are used in asymmetric synthesis, while sulfenamides are used extensively in the vulcanization process to assist cross-linking.
1077:
have functionality R−S−OH. In the series sulfonic—sulfinic—sulfenic acids, both the acid strength and stability diminish in that order. Sulfonamides,
152:
191:
2316:
2219:
2194:
1872:
1819:
1755:
1722:
1689:
992:
considers this term obsolete, the name persists in the literature. These compounds are well known with extensive chemistry. Examples include
362:
Relative to C−C bonds, C−S bonds are both longer, because sulfur atoms are larger than carbon atoms, and about 10% weaker. Representative
2263:
Sato, S.; Matsunaga, K.; Horn, E.; Furukawa, N.; Nabeshima, T. (2006). "Isolation and
Molecular Structure of the Organo-persulfuranes ".
210:
2401:
120:, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds.
1543:
1656:
1564:
1555:
1483:
3116:
167:
3106:
2394:
2210:
Drabowicz, J.; Kiełbasiński, P.; Łyżwa, P.; Zając, A.; Mikołajczyk, M. (2008). "Alkanesulfenic Acids". In Kambe, N. (ed.).
1284:
1062:
45:. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g.,
2235:
Schultz, A. G.; DeTar, M. B. (1976). "Thiocarbonyl ylides. Photogeneration, rearrangement, and cycloaddition reactions".
1442:, and tropical fruit flavors. Many of these natural products also have important medicinal properties such as preventing
897:, with formula R−N=C=S, are found naturally. Vegetable foods with characteristic flavors due to isothiocyanates include
246:
1283:
ranging between 189 and 193 pm (longer than the standard bond length) with the central sulfur atom in a distorted
2139:
2111:
2035:
1232:
2172:
Organic chemistry IUPAC Blue Book. C-6 Sulfur
Halides, Sulfoxides, Sulfones, and Sulfur Acids and Their Derivatives
1359:
Common organosulfur compounds present in petroleum fractions at the level of 200–500 ppm. Common compounds are
459:" of carbonyl groups. Thioacetals and thioketals can also be used to protect a carbonyl group in organic syntheses.
2963:
2958:
2953:
2948:
2943:
2938:
2933:
2928:
2923:
2918:
2913:
2908:
2898:
2841:
2735:
2656:
2651:
2508:
2137:
Schreiner, P.; Reisenauer, H.; Romanski, J.; Mloston, G. (2009). "A formal carbon–sulfur triple bond: H−C≡S−O−H".
1944:
Opitz, G. (February 1967). "Sulfines and
Sulfenes– theS-Oxides andS,S-Dioxides of Thioaldehydes and Thioketones".
1583:
Chauhan, Pankaj; Mahajan, Suruchi; Enders, Dieter (2014). "Organocatalytic Carbon–Sulfur Bond-Forming
Reactions".
2999:
2701:
2671:
2661:
2641:
2629:
2597:
2562:
2530:
2498:
2493:
2453:
2468:
2432:
2386:
1863:
Drabowicz, J.; Lewkowski, J.; Kudelska, W.; Girek, T. (2008). "Dialkylsulfur
Tetrahalides". In Kambe, N. (ed.).
522:, among others. The latter three compounds represent a special class of sulfur-containing heterocycles that are
3044:
3039:
3034:
3029:
3024:
3019:
3014:
3009:
3004:
2989:
2979:
2830:
2805:
2800:
2785:
2770:
2750:
2745:
2696:
2624:
2607:
2557:
2552:
2547:
2542:
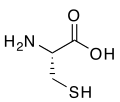
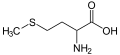
2518:
2478:
993:
388:
379:
768:. They are of interest because of their biological activity and as building blocks for heterocycle synthesis.
3111:
2994:
2984:
2795:
2780:
2765:
2755:
2740:
2681:
2666:
2646:
2636:
2617:
2612:
2602:
2592:
2535:
2503:
542:
for oxygen drawing away electrons to itself at the expense of the aromatic ring current. Yet as an aromatic
534:
is 29 kcal/mol (121 kJ/mol) compared to 20 kcal/mol (84 kJ/mol) for the oxygen analogue
527:
295:
2473:
2463:
384:
2903:
2818:
2760:
2723:
2718:
2706:
2686:
2676:
2582:
2577:
2572:
2488:
2185:
Braverman, S.; Cherkinsky, M.; Levinger, S. (2008). "Alkanesulfinic Acids and Salts". In Kambe, N. (ed.).
441:
414:
321:
2711:
2523:
2458:
2448:
1175:
128:
890:
455:
feature C−S−C−S−C bond sequence. They represent a subclass of sulfides. The thioacetals are useful in "
336:
2790:
2775:
2587:
2567:
2345:
1368:
1106:
751:
1542:
Organic chemistry IUPAC Blue Book. Recommendation R-5.7.1.3.4 Thiocarboxylic and thiocarbonic acids.
1371:(HDS) in refineries, these compounds are removed as illustrated by the hydrogenolysis of thiophene:
2691:
1502:
Martin, J. C.; Arhart, R. J.; Franz, J. A.; Perozzi, E. F.; Kaplan, L. J. "Bisdiphenyl sulfurane".
1043:
906:
825:. Less well known are dialkylsulfur tetrahalides, mainly represented by the tetrafluorides, e.g., R
562:
1810:
Braverman, S.; Cherkinsky, M.; Levinger, S. (2008). "Alkylsulfur
Trihalides". In Kambe, N. (ed.).
565:
group, but these functionalities are very different in their chemical properties. Thiols are more
1423:
1016:
714:
433:
216:
406:
of thiols. Alkylating agents include not only alkyl halides, but also epoxides, aziridines, and
1061:−OH. They are strong acids that are typically soluble in organic solvents. Sulfonic acids like
713:-dioxide of a disulfide. All of these compounds are well known with extensive chemistry, e.g.,
2373:
2312:
2281:
2265:
2237:
2215:
2190:
2155:
2071:
1868:
1837:
1815:
1792:
1751:
1718:
1685:
1652:
1620:
1600:
1560:
1504:
1479:
1276:
1248:
1066:
624:
583:
539:
421:
353:
2363:
2353:
2304:
2273:
2245:
2147:
2119:
2079:
2043:
2007:
1980:
1953:
1926:
1901:
1845:
1784:
1629:
1592:
1513:
1364:
1244:
870:
862:
850:
846:
718:
587:
547:
407:
392:
231:
140:
38:
645:
have general structure R−C(O)−S−R. They are related to regular esters (R−C(O)−O−R) but are
634:
which contains an unusual pentathiepin ring (5-sulfur chain cyclised onto a benzene ring).
2173:
1531:
1264:
1155:
914:
806:
802:
798:
90:
49:. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common
2349:
1788:
89:, which are derived from ancient organisms, necessarily contain organosulfur compounds,
2368:
2333:
1431:
1348:
1295:
A variety of organosulfur compounds occur in nature. Most abundant are the amino acids
894:
845:
between carbon and sulfur are relatively uncommon, but include the important compounds
779:
757:
612:
599:
396:
317:
98:
70:
797:
atom ("X" in the chemical formulas that follow) bonded to a single sulfur atom, e.g.:
3100:
3063:
1998:
Moltzen, E. K.; Klabunde, K. J.; Senning, A. (1988). "Carbon monosulfide: a review".
1332:
1260:
1122:
1074:
1070:
1054:
698:
690:
606:
359:
298:
291:
124:
94:
17:
1897:
1015:
Triple bonds between sulfur and carbon in sulfaalkynes are rare and can be found in
1672:
García Ruano, J. L.; Cid, M. B.; Martín Castro, A. M.; Alemán, J. (2008). "Acyclic
1530:
Organic chemistry IUPAC Blue Book. Rules C-5: Compounds
Containing Bivalent Sulfur
1426:. Volatile organosulfur compounds also contribute subtle flavor characteristics to
1252:
866:
854:
646:
620:
463:
425:
371:
74:
2097:
Gerhardt, R.; Gerlbig, T.; Buschamann, J.; Luger, P.; Seppelt, K. (1988). "The SF
1449:
Humans and other animals have an exquisitely sensitive sense of smell toward the
1892:
1419:
1324:
1320:
1316:
1280:
1194:
1110:
1082:
1078:
902:
842:
733:
566:
543:
515:
467:
363:
261:
86:
66:
50:
1984:
1336:
1328:
1296:
1272:
1206:
1102:
922:
918:
858:
654:
650:
616:
561:
groups contain the functionality R−SH. Thiols are structurally similar to the
487:
462:
The above classes of sulfur compounds also exist in saturated and unsaturated
448:
403:
302:
276:
201:
197:
182:
62:
58:
1896:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
1633:
1517:
1454:
areas where oxygen levels in the air are low, posing a risk of suffocation.
1113:, R−S−CN, are related to sulfenyl halides and esters in terms of reactivity.
2358:
1905:
1435:
1360:
1344:
1340:
1050:
Sulfonic, sulfinic and sulfenic acids, esters, amides, and related compounds
889:
are more common. They are typically prepared by the reaction of amides with
874:
666:
642:
591:
531:
519:
452:
375:
367:
143:, which are listed (approximately) in decreasing order of their occurrence.
139:
Organosulfur compounds can be classified according to the sulfur-containing
117:
105:
82:
46:
2377:
2285:
2159:
2151:
2123:
2047:
1957:
1930:
1796:
1604:
793:
A wide range of organosulfur compounds are known which contain one or more
1182:, photocyclization of aryl vinyl sulfides, as well as by other processes.
69:
both contain sulfur. While sulfur-containing antibiotics save many lives,
2308:
2025:
Pötter, B.; Seppelt, K. (1984). "Trifluoroethylidynesulfur
Trifluoride, F
1443:
1312:
1300:
1221:
1179:
1035:. The compound HCSOH is also represented as having a formal triple bond.
736:(also called a sulfilimines) are sulfur–nitrogen compounds of structure R
523:
511:
507:
503:
499:
491:
479:
475:
471:
456:
178:
113:
54:
2303:. ACS Symposium Series 1068. Vol. 1068. American Chemical Society.
2249:
2083:
2011:
1849:
1407:
1304:
1163:
1004:
985:
969:
794:
722:
674:
631:
569:, more acidic, and more readily oxidized. This acidity can differ by 5
158:
2277:
1596:
2418:
1439:
1415:
1411:
1308:
1224:
1217:
910:
898:
495:
483:
109:
42:
2334:"Crucial role of copper in detection of metal-coordinating odorants"
861:(RC(=S)R′) are uncommon with alkyl substituents, but one example is
630:
Longer sulfur chains are also known, such as in the natural product
399:
are respectively 73 and 77 kcal/mol (305 and 322 kJ/mol).
358:
Sulfides, formerly known as thioethers, are characterized by C−S−C
1135:
989:
926:
558:
535:
1178:. Thiocarbonyl ylides (RR′C=S−C−RR′) can form by ring-opening of
586:
between sulfur (2.58) and hydrogen (2.20) is small and therefore
546:
the thio group is less electron-releasing than the alkoxy group.
1450:
1427:
615:
R−S−S−R with a covalent sulfur to sulfur bond are important for
595:
78:
2390:
1738:
Drabowicz, J.; Lewkowski, J.; Kudelska, W.; Girek, T. (2008). "
1705:
Drabowicz, J.; Lewkowski, J.; Kudelska, W.; Girek, T. (2008). "
1347:
produced by several species of fungi under investigation as an
264:, an essential cofactor of four mitochondrial enzyme complexes.
2101:-Unit as Steric Protecting Group; Synthesis and Structure of F
869:
are rarer still, reflecting their lack of steric protection ("
570:
952:-oxides of thiocarbonyl compounds are known as thiocarbonyl
2069:: two solid-state structures and reactivity as a carbene".
1134:
Deprotonation of sulfonium and oxosulfonium salts affords
1618:
Suter, C. M.; Maxwell, Charles E. (1938). "Phenoxthin ".
1193:
are relatively specialized functional group that feature
1101:, respectively, each have a rich chemistry. For example,
1973:
Phosphorus, Sulfur, and
Silicon and the Related Elements
2299:
Qian, M. C.; Fan, X.; Mahattanatawee, K., eds. (2011).
2174:
http://www.acdlabs.com/iupac/nomenclature/79/r79_26.htm
1532:
http://www.acdlabs.com/iupac/nomenclature/79/r79_25.htm
623:
for the folding and stability of some proteins and in
1835:
Sheppard, W. A. (1962). "Arylsulfur Pentafluorides".
1166:, are sometimes drawn with a C=S double bond, e.g., R
3055:
1971:Zwanenburg, Binne (May 1989). "Sulfine Chemistry".
1775:-Nitrosothiols: cellular formation and transport".
1946:Angewandte Chemie International Edition in English
605:Certain aromatic thiols can be accessed through a
590:in thiols is not prominent. Aliphatic thiols form
61:) are organosulfur compounds, and the antibiotics
1019:(CS) and have been suggested for the compounds F
837:Thioketones, thioaldehydes, and related compounds
1239:It is prepared from the corresponding sulfurane
1216:One of the few all-carbon persulfuranes has two
279:core structure, where "R" is the variable group.
33:is the study of the properties and synthesis of
1130:Sulfonium, oxosulfonium and thiocarbonyl ylides
649:and related reactions. Thioesters formed from
538:. The reason for this difference is the higher
1746:-Dialkylsulfonediimines". In Kambe, N. (ed.).
821:; and alkyl and arylsulfur pentafluorides, RSF
161:, the active flavor compound in crushed garlic
2402:
653:are prominent in biochemistry, especially in
127:for the detection of sulfur compounds is the
8:
466:structures, often in combination with other
1713:-Dialkylsulfoximides". In Kambe, N. (ed.).
2409:
2395:
2387:
2214:. Vol. 39. Thieme. pp. 550–557.
2189:. Vol. 39. Thieme. pp. 196–211.
1867:. Vol. 39. Thieme. pp. 123–124.
1814:. Vol. 39. Thieme. pp. 187–188.
1750:. Vol. 39. Thieme. pp. 173–180.
1717:. Vol. 39. Thieme. pp. 154–173.
1684:. Vol. 39. Thieme. pp. 352–375.
813:X; alkyl and arylsulfur trichlorides, RSCl
387:is shorter than that of the C−C bond. The
2421:with other elements in the periodic table
2367:
2357:
1680:-Dialkylsulfimides". In Kambe, N. (ed.).
1649:An Introduction to Organosulfur Chemistry
1117:Sulfonium, oxosulfonium and related salts
729:Sulfimides, sulfoximides, sulfonediimines
673:-oxide of a sulfide ("sulfide oxide"), a
339:with a see-saw structure, like that of SF
1578:
1576:
1394:
1390:
1386:
1382:
1378:
1374:
1559:(81st ed.). CRC Press. June 2000.
1466:
661:Sulfoxides, sulfones and thiosulfinates
145:
2438:
1011:Triple bonds between carbon and sulfur
1919:European Journal of Organic Chemistry
1497:
1495:
27:Organic compounds that contain sulfur
7:
3080:Academic research, no widespread use
73:is a deadly chemical warfare agent.
1789:10.1016/j.freeradbiomed.2004.12.016
1651:. Chichester: John Wiley and Sons.
1476:Reactions of Organosulfur Compounds
1073:have functionality R−S(O)−OH while
1039:Thiocarboxylic acids and thioamides
944:-dioxides of thiocarbonyl compounds
402:Sulfides are typically prepared by
147:Illustrative organosulfur compounds
1893:Compendium of Chemical Terminology
432:by action of elemental sulfur and
413:They can also be prepared via the
25:
2301:Volatile Sulfur Compounds in Food
1556:Handbook of Chemistry and Physics
1160:Johnson–Corey–Chaykovsky reaction
366:in sulfur compounds are 183
2972:
2891:
2823:
2728:
2428:
1446:aggregation or fighting cancer.
1414:are responsible for the odor of
1291:Organosulfur compounds in nature
1231:
1065:is a frequently used reagent in
984:-oxides have also been known as
627:for the crosslinking of rubber.
554:Thiols, disulfides, polysulfides
440:
329:
310:
284:
269:
254:
239:
224:
209:
190:
166:
151:
1105:are sulfonamides derived from
873:" exists as a cyclic trimer).
647:more susceptible to hydrolysis
320:, a type of sulfide used as a
1:
1771:Zhang, Y.; Hogg, N. (2005). "
1422:contributes to the flavor of
1323:is the primary intracellular
1319:contain sulfur heterocycles.
1285:octahedral molecular geometry
1063:trifluoromethanesulfonic acid
697:-oxide of a disulfide, and a
1186:Sulfuranes and persulfuranes
249:, a controversial surfactant
247:Perfluorooctanesulfonic acid
219:, a representative disulfide
2140:Angew. Chem. Int. Ed. Engl.
2112:Angew. Chem. Int. Ed. Engl.
2036:Angew. Chem. Int. Ed. Engl.
1267:to (a stable) persulfurane
1255:to the sulfuranyl dication
1197:sulfur, with the formula SR
370:for the S−C single bond in
234:, a component of crude oil
3133:
2338:Proc. Natl. Acad. Sci. USA
1259:followed by reaction with
1057:have functionality R−S(=O)
389:bond dissociation energies
351:
2969:
2888:
2440:
2436:
2426:
1985:10.1080/10426508908040276
689:-dioxide of a sulfide, a
1634:10.15227/orgsyn.018.0064
1518:10.15227/orgsyn.057.0022
960:C=S=O, and thiocarbonyl
380:bond dissociation energy
185:containing a thiol group
3117:Foul-smelling chemicals
2359:10.1073/pnas.1111297109
1906:10.1351/goldbook.S06108
1647:Cremlyn, R. J. (1996).
1343:is a sulfur-containing
598:, which are topical in
528:resonance stabilization
3107:Organosulfur compounds
3075:Many uses in chemistry
3070:Core organic chemistry
2152:10.1002/anie.200903969
2124:10.1002/anie.198815341
2048:10.1002/anie.198401501
1958:10.1002/anie.196701071
1931:10.1002/ejoc.201501538
1339:, derived from fungi.
1158:, for instance in the
415:Pummerer rearrangement
322:chemical warfare agent
35:organosulfur compounds
31:Organosulfur chemistry
1777:Free Radic. Biol. Med
1176:Stevens rearrangement
817:and trifluorides, RSF
693:, R−S(O)−S−R, is the
385:carbon to oxygen bond
129:Carius halogen method
18:Organosulfur compound
2309:10.1021/bk-2011-1068
2212:Science of Synthesis
2187:Science of Synthesis
1865:Science of Synthesis
1812:Science of Synthesis
1748:Science of Synthesis
1715:Science of Synthesis
1682:Science of Synthesis
1369:hydrodesulfurization
1367:. By the process of
1107:aromatic sulfonation
1085:, with formulas R−SO
1044:Thiocarboxylic acids
877:, with the formula R
752:glutamine synthetase
470:, as illustrated by
204:containing a sulfide
2350:2012PNAS..109.3492D
2250:10.1021/ja00428a029
2084:10.1021/ja00050a027
2012:10.1021/cr00084a003
1850:10.1021/ja00875a006
1162:used to synthesize
669:, R−S(O)−R, is the
374:and 173 pm in
1478:. Academic Press.
1474:Block, E. (1978).
1424:shiitake mushrooms
1017:carbon monosulfide
891:Lawesson's reagent
715:dimethyl sulfoxide
582:The difference in
434:aluminium chloride
337:Martin's sulfurane
217:Diphenyl disulfide
135:Structural classes
104:Sulfur shares the
3094:
3093:
3050:
3049:
2318:978-0-8412-2616-6
2278:10.1021/ja060497y
2272:(21): 6778–6779.
2266:J. Am. Chem. Soc.
2244:(12): 3564–3572.
2238:J. Am. Chem. Soc.
2221:978-1-58890-530-7
2196:978-1-58890-530-7
2146:(43): 8133–8136.
2072:J. Am. Chem. Soc.
1874:978-1-58890-530-7
1844:(16): 3064–3072.
1838:J. Am. Chem. Soc.
1821:978-1-58890-530-7
1757:978-1-58890-530-7
1724:978-1-58890-530-7
1691:978-1-58890-530-7
1621:Organic Syntheses
1597:10.1021/cr500235v
1591:(18): 8807–8864.
1505:Organic Syntheses
1365:dibenzothiophenes
1277:X-ray diffraction
1249:boron trifluoride
1067:organic chemistry
625:polymer chemistry
584:electronegativity
548:Dibenzothiophenes
540:electronegativity
422:Ferrario reaction
408:Michael acceptors
354:Sulfide (organic)
141:functional groups
39:organic compounds
16:(Redirected from
3124:
3086:
3081:
3076:
3071:
2976:
2975:
2895:
2894:
2827:
2826:
2732:
2731:
2429:
2411:
2404:
2397:
2388:
2382:
2381:
2371:
2361:
2344:(9): 3492–3497.
2329:
2323:
2322:
2296:
2290:
2289:
2260:
2254:
2253:
2232:
2226:
2225:
2207:
2201:
2200:
2182:
2176:
2170:
2164:
2163:
2134:
2128:
2127:
2094:
2088:
2087:
2058:
2052:
2051:
2022:
2016:
2015:
1995:
1989:
1988:
1968:
1962:
1961:
1941:
1935:
1934:
1925:(9): 1630–1650.
1914:
1908:
1885:
1879:
1878:
1860:
1854:
1853:
1832:
1826:
1825:
1807:
1801:
1800:
1768:
1762:
1761:
1735:
1729:
1728:
1702:
1696:
1695:
1669:
1663:
1662:
1644:
1638:
1636:
1615:
1609:
1608:
1585:Chemical Reviews
1580:
1571:
1570:
1551:
1545:
1540:
1534:
1528:
1522:
1520:
1499:
1490:
1489:
1471:
1398:
1335:are life-saving
1245:xenon difluoride
1235:
1156:sulfonium ylides
1138:, of structure R
915:Brussels sprouts
871:thioformaldehyde
863:thiobenzophenone
851:carbonyl sulfide
847:carbon disulfide
807:sulfonyl halides
803:sulfinyl halides
799:sulfenyl halides
719:dimethyl sulfone
588:hydrogen bonding
444:
428:is converted to
393:dimethyl sulfide
333:
314:
288:
273:
258:
243:
232:Dibenzothiophene
228:
213:
194:
170:
155:
21:
3132:
3131:
3127:
3126:
3125:
3123:
3122:
3121:
3097:
3096:
3095:
3090:
3089:
3084:
3079:
3074:
3069:
3051:
2973:
2892:
2824:
2729:
2422:
2415:
2385:
2331:
2330:
2326:
2319:
2298:
2297:
2293:
2262:
2261:
2257:
2234:
2233:
2229:
2222:
2209:
2208:
2204:
2197:
2184:
2183:
2179:
2171:
2167:
2136:
2135:
2131:
2108:
2104:
2100:
2096:
2095:
2091:
2068:
2064:
2060:
2059:
2055:
2032:
2028:
2024:
2023:
2019:
1997:
1996:
1992:
1970:
1969:
1965:
1943:
1942:
1938:
1916:
1915:
1911:
1886:
1882:
1875:
1862:
1861:
1857:
1834:
1833:
1829:
1822:
1809:
1808:
1804:
1770:
1769:
1765:
1758:
1737:
1736:
1732:
1725:
1704:
1703:
1699:
1692:
1671:
1670:
1666:
1659:
1646:
1645:
1641:
1617:
1616:
1612:
1582:
1581:
1574:
1567:
1553:
1552:
1548:
1541:
1537:
1529:
1525:
1501:
1500:
1493:
1486:
1473:
1472:
1468:
1464:
1406:Compounds like
1404:
1402:Flavor and odor
1396:
1392:
1388:
1384:
1380:
1376:
1372:
1357:
1355:In fossil fuels
1307:. The vitamins
1293:
1265:tetrahydrofuran
1212:
1200:
1188:
1173:
1169:
1153:
1149:
1145:
1141:
1132:
1119:
1100:
1096:
1092:
1088:
1060:
1052:
1041:
1034:
1030:
1026:
1022:
1013:
979:
975:
959:
946:
895:Isothiocyanates
888:
884:
880:
841:Compounds with
839:
832:
828:
824:
820:
816:
812:
791:
777:
767:
763:
758:Sulfonediimines
748:
739:
731:
725:(see drawing).
704:
680:
663:
640:
577:
556:
356:
350:
343:
342:
334:
325:
315:
306:
289:
280:
274:
265:
259:
250:
244:
235:
229:
220:
214:
205:
195:
186:
171:
162:
156:
137:
28:
23:
22:
15:
12:
11:
5:
3130:
3128:
3120:
3119:
3114:
3112:Soil chemistry
3109:
3099:
3098:
3092:
3091:
3088:
3087:
3082:
3077:
3072:
3067:
3064:Chemical bonds
3060:
3059:
3057:
3053:
3052:
3048:
3047:
3042:
3037:
3032:
3027:
3022:
3017:
3012:
3007:
3002:
2997:
2992:
2987:
2982:
2977:
2970:
2967:
2966:
2961:
2956:
2951:
2946:
2941:
2936:
2931:
2926:
2921:
2916:
2911:
2906:
2901:
2896:
2889:
2886:
2885:
2881:
2880:
2877:
2874:
2871:
2868:
2865:
2862:
2859:
2856:
2853:
2850:
2847:
2844:
2839:
2836:
2833:
2828:
2821:
2816:
2812:
2811:
2808:
2803:
2798:
2793:
2788:
2783:
2778:
2773:
2768:
2763:
2758:
2753:
2748:
2743:
2738:
2733:
2726:
2721:
2715:
2714:
2709:
2704:
2699:
2694:
2689:
2684:
2679:
2674:
2669:
2664:
2659:
2654:
2649:
2644:
2639:
2634:
2632:
2627:
2621:
2620:
2615:
2610:
2605:
2600:
2595:
2590:
2585:
2580:
2575:
2570:
2565:
2560:
2555:
2550:
2545:
2540:
2538:
2533:
2527:
2526:
2521:
2516:
2511:
2506:
2501:
2496:
2491:
2485:
2484:
2481:
2476:
2471:
2466:
2461:
2456:
2451:
2445:
2444:
2441:
2439:
2437:
2435:
2427:
2424:
2423:
2416:
2414:
2413:
2406:
2399:
2391:
2384:
2383:
2324:
2317:
2291:
2255:
2227:
2220:
2202:
2195:
2177:
2165:
2129:
2106:
2102:
2098:
2089:
2066:
2062:
2053:
2030:
2026:
2017:
1990:
1963:
1952:(2): 107–123.
1936:
1909:
1880:
1873:
1855:
1827:
1820:
1802:
1783:(7): 831–838.
1763:
1756:
1730:
1723:
1697:
1690:
1664:
1657:
1639:
1610:
1572:
1565:
1546:
1535:
1523:
1491:
1484:
1465:
1463:
1460:
1432:cheddar cheese
1403:
1400:
1356:
1353:
1292:
1289:
1237:
1236:
1210:
1198:
1187:
1184:
1171:
1167:
1151:
1147:
1143:
1139:
1131:
1128:
1118:
1115:
1098:
1094:
1090:
1086:
1075:sulfenic acids
1071:Sulfinic acids
1058:
1055:Sulfonic acids
1051:
1048:
1040:
1037:
1032:
1028:
1024:
1020:
1012:
1009:
997:-propanethial-
980:). The thione
977:
973:
957:
945:
931:
886:
882:
878:
838:
835:
830:
826:
822:
818:
814:
810:
790:
789:Sulfur halides
787:
783:-Nitrosothiols
776:
775:-Nitrosothiols
770:
765:
761:
746:
737:
730:
727:
702:
678:
662:
659:
639:
636:
600:nanotechnology
575:
555:
552:
446:
445:
397:dimethyl ether
352:Main article:
349:
346:
345:
344:
340:
335:
328:
326:
318:Sulfur mustard
316:
309:
307:
290:
283:
281:
275:
268:
266:
260:
253:
251:
245:
238:
236:
230:
223:
221:
215:
208:
206:
196:
189:
187:
172:
165:
163:
157:
150:
148:
136:
133:
99:oil refineries
93:of which is a
71:sulfur mustard
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3129:
3118:
3115:
3113:
3110:
3108:
3105:
3104:
3102:
3083:
3078:
3073:
3068:
3065:
3062:
3061:
3058:
3054:
3046:
3043:
3041:
3038:
3036:
3033:
3031:
3028:
3026:
3023:
3021:
3018:
3016:
3013:
3011:
3008:
3006:
3003:
3001:
2998:
2996:
2993:
2991:
2988:
2986:
2983:
2981:
2978:
2971:
2968:
2965:
2962:
2960:
2957:
2955:
2952:
2950:
2947:
2945:
2942:
2940:
2937:
2935:
2932:
2930:
2927:
2925:
2922:
2920:
2917:
2915:
2912:
2910:
2907:
2905:
2902:
2900:
2897:
2890:
2887:
2883:
2882:
2878:
2875:
2872:
2869:
2866:
2863:
2860:
2857:
2854:
2851:
2848:
2845:
2843:
2840:
2837:
2834:
2832:
2829:
2822:
2820:
2817:
2814:
2813:
2809:
2807:
2804:
2802:
2799:
2797:
2794:
2792:
2789:
2787:
2784:
2782:
2779:
2777:
2774:
2772:
2769:
2767:
2764:
2762:
2759:
2757:
2754:
2752:
2749:
2747:
2744:
2742:
2739:
2737:
2734:
2727:
2725:
2722:
2720:
2717:
2716:
2713:
2710:
2708:
2705:
2703:
2700:
2698:
2695:
2693:
2690:
2688:
2685:
2683:
2680:
2678:
2675:
2673:
2670:
2668:
2665:
2663:
2660:
2658:
2655:
2653:
2650:
2648:
2645:
2643:
2640:
2638:
2635:
2633:
2631:
2628:
2626:
2623:
2622:
2619:
2616:
2614:
2611:
2609:
2606:
2604:
2601:
2599:
2596:
2594:
2591:
2589:
2586:
2584:
2581:
2579:
2576:
2574:
2571:
2569:
2566:
2564:
2561:
2559:
2556:
2554:
2551:
2549:
2546:
2544:
2541:
2539:
2537:
2534:
2532:
2529:
2528:
2525:
2522:
2520:
2517:
2515:
2512:
2510:
2507:
2505:
2502:
2500:
2497:
2495:
2492:
2490:
2487:
2486:
2482:
2480:
2477:
2475:
2472:
2470:
2467:
2465:
2462:
2460:
2457:
2455:
2452:
2450:
2447:
2446:
2442:
2434:
2431:
2430:
2425:
2420:
2417:Compounds of
2412:
2407:
2405:
2400:
2398:
2393:
2392:
2389:
2379:
2375:
2370:
2365:
2360:
2355:
2351:
2347:
2343:
2339:
2335:
2328:
2325:
2320:
2314:
2310:
2306:
2302:
2295:
2292:
2287:
2283:
2279:
2275:
2271:
2268:
2267:
2259:
2256:
2251:
2247:
2243:
2240:
2239:
2231:
2228:
2223:
2217:
2213:
2206:
2203:
2198:
2192:
2188:
2181:
2178:
2175:
2169:
2166:
2161:
2157:
2153:
2149:
2145:
2142:
2141:
2133:
2130:
2125:
2121:
2117:
2114:
2113:
2093:
2090:
2085:
2081:
2077:
2074:
2073:
2057:
2054:
2049:
2045:
2041:
2038:
2037:
2021:
2018:
2013:
2009:
2005:
2001:
1994:
1991:
1986:
1982:
1979:(1–2): 1–24.
1978:
1974:
1967:
1964:
1959:
1955:
1951:
1947:
1940:
1937:
1932:
1928:
1924:
1920:
1913:
1910:
1907:
1903:
1899:
1895:
1894:
1889:
1884:
1881:
1876:
1870:
1866:
1859:
1856:
1851:
1847:
1843:
1840:
1839:
1831:
1828:
1823:
1817:
1813:
1806:
1803:
1798:
1794:
1790:
1786:
1782:
1778:
1774:
1767:
1764:
1759:
1753:
1749:
1745:
1741:
1734:
1731:
1726:
1720:
1716:
1712:
1708:
1701:
1698:
1693:
1687:
1683:
1679:
1675:
1668:
1665:
1660:
1658:0-471-95512-4
1654:
1650:
1643:
1640:
1635:
1631:
1627:
1623:
1622:
1614:
1611:
1606:
1602:
1598:
1594:
1590:
1586:
1579:
1577:
1573:
1568:
1566:0-8493-0481-4
1562:
1558:
1557:
1550:
1547:
1544:
1539:
1536:
1533:
1527:
1524:
1519:
1515:
1511:
1507:
1506:
1498:
1496:
1492:
1487:
1485:0-12-107050-6
1481:
1477:
1470:
1467:
1461:
1459:
1455:
1452:
1447:
1445:
1441:
1437:
1433:
1429:
1425:
1421:
1417:
1413:
1409:
1401:
1399:
1370:
1366:
1363:, especially
1362:
1354:
1352:
1350:
1346:
1342:
1338:
1334:
1333:cephalosporin
1330:
1326:
1322:
1318:
1315:, as well as
1314:
1310:
1306:
1302:
1298:
1290:
1288:
1286:
1282:
1278:
1274:
1270:
1266:
1262:
1261:methyllithium
1258:
1254:
1250:
1246:
1242:
1234:
1230:
1229:
1228:
1226:
1223:
1219:
1214:
1208:
1204:
1203:persulfuranes
1196:
1192:
1185:
1183:
1181:
1177:
1165:
1161:
1157:
1137:
1129:
1127:
1124:
1123:sulfonium ion
1116:
1114:
1112:
1108:
1104:
1084:
1080:
1076:
1072:
1068:
1064:
1056:
1049:
1047:
1045:
1038:
1036:
1018:
1010:
1008:
1006:
1002:
1000:
996:
991:
987:
983:
971:
968:-dioxides or
967:
963:
955:
951:
943:
939:
935:
932:
930:
928:
924:
920:
916:
912:
908:
904:
900:
896:
892:
876:
872:
868:
867:Thioaldehydes
864:
860:
856:
852:
848:
844:
836:
834:
808:
804:
800:
796:
788:
786:
784:
782:
774:
771:
769:
759:
755:
753:
742:
735:
728:
726:
724:
720:
716:
712:
708:
705:−S−R, is the
700:
699:thiosulfonate
696:
692:
691:thiosulfinate
688:
684:
676:
672:
668:
660:
658:
656:
652:
648:
644:
637:
635:
633:
628:
626:
622:
618:
614:
610:
608:
607:Herz reaction
603:
601:
597:
593:
589:
585:
580:
578:
574:
568:
564:
560:
553:
551:
549:
545:
541:
537:
533:
529:
525:
521:
517:
513:
509:
505:
501:
497:
493:
489:
485:
481:
477:
473:
469:
465:
460:
458:
454:
450:
443:
439:
438:
437:
435:
431:
427:
423:
418:
416:
411:
409:
405:
400:
398:
394:
390:
386:
381:
377:
373:
369:
365:
361:
355:
347:
338:
332:
327:
323:
319:
313:
308:
304:
300:
299:antibacterial
297:
293:
292:Sulfanilamide
287:
282:
278:
272:
267:
263:
257:
252:
248:
242:
237:
233:
227:
222:
218:
212:
207:
203:
199:
193:
188:
184:
180:
176:
169:
164:
160:
154:
149:
146:
144:
142:
134:
132:
130:
126:
125:chemical test
121:
119:
115:
111:
107:
102:
100:
96:
92:
88:
84:
80:
76:
72:
68:
64:
60:
56:
52:
48:
44:
41:that contain
40:
36:
32:
19:
3085:Bond unknown
2513:
2341:
2337:
2327:
2300:
2294:
2269:
2264:
2258:
2241:
2236:
2230:
2211:
2205:
2186:
2180:
2168:
2143:
2138:
2132:
2118:(11): 1534.
2115:
2110:
2092:
2078:(24): 9465.
2075:
2070:
2056:
2039:
2034:
2020:
2003:
1999:
1993:
1976:
1972:
1966:
1949:
1945:
1939:
1922:
1918:
1912:
1891:
1883:
1864:
1858:
1841:
1836:
1830:
1811:
1805:
1780:
1776:
1772:
1766:
1747:
1743:
1739:
1733:
1714:
1710:
1706:
1700:
1681:
1677:
1673:
1667:
1648:
1642:
1625:
1619:
1613:
1588:
1584:
1554:
1549:
1538:
1526:
1509:
1503:
1475:
1469:
1456:
1448:
1405:
1358:
1294:
1281:bond lengths
1268:
1256:
1253:acetonitrile
1240:
1238:
1215:
1202:
1190:
1189:
1133:
1120:
1111:Thiocyanates
1097:, and R−SNR′
1083:sulfenamides
1079:sulfinamides
1053:
1042:
1014:
998:
994:
988:, and while
981:
965:
961:
953:
949:
947:
941:
937:
936:-Oxides and
933:
855:thiophosgene
843:double bonds
840:
792:
780:
778:
772:
756:
743:
732:
710:
706:
694:
686:
682:
670:
664:
641:
629:
621:biochemistry
617:crosslinking
611:
604:
581:
572:
567:nucleophilic
557:
516:isothiazoles
464:heterocyclic
461:
447:
430:phenoxathiin
429:
426:phenyl ether
419:
412:
401:
372:methanethiol
364:bond lengths
357:
174:
138:
123:A classical
122:
103:
75:Fossil fuels
37:, which are
34:
30:
29:
1420:Lenthionine
1337:antibiotics
1325:antioxidant
1321:Glutathione
1317:lipoic acid
1222:biphenylene
1195:tetravalent
1103:sulfa drugs
1093:, R−S(O)NR′
956:-oxides: (R
923:nasturtiums
903:horseradish
859:Thioketones
681:−R, is the
657:synthesis.
544:substituent
488:dithietanes
468:heteroatoms
449:Thioacetals
301:, called a
296:sulfonamide
262:Lipoic acid
108:group with
95:major focus
91:the removal
87:natural gas
67:sulfa drugs
51:amino acids
3101:Categories
2042:(2): 150.
2006:(2): 391.
1462:References
1361:thiophenes
1329:Penicillin
1297:methionine
1279:shows C−S
1273:cis isomer
1207:hexavalent
1201:Likewise,
1191:Sulfuranes
919:watercress
875:Thioamides
805:, RS(O)X;
734:Sulfimides
655:fatty acid
651:coenzyme A
643:Thioesters
638:Thioesters
613:Disulfides
592:monolayers
520:thiophenes
453:thioketals
404:alkylation
378:. The C−S
303:sulfa drug
277:Penicillin
202:amino acid
198:Methionine
183:amino acid
63:penicillin
59:methionine
3066:to carbon
2000:Chem. Rev
1436:chocolate
1381:S + 8 H
1349:antiviral
1345:mycotoxin
1341:Gliotoxin
1180:thiiranes
1150:S(O)−C−R′
667:sulfoxide
532:thiophene
512:thiazoles
508:thiepines
504:thiepanes
500:dithianes
492:thiolanes
480:thietanes
476:thiirenes
472:thiiranes
376:thiophene
118:tellurium
106:chalcogen
83:petroleum
47:saccharin
2378:22328155
2286:16719444
2160:19768827
1898:sulfines
1797:15749378
1605:25144663
1444:platelet
1430:, nuts,
1351:agent.
1313:thiamine
1301:cysteine
1220:and two
1205:feature
1164:epoxides
1154:. While
986:sulfines
970:sulfenes
881:C(=S)N(R
701:, R−S(O)
677:, R−S(O)
524:aromatic
457:umpolung
348:Sulfides
179:Cysteine
114:selenium
55:cysteine
2884:
2369:3295281
2346:Bibcode
1408:allicin
1305:cystine
1271:as the
1225:ligands
1005:sulfene
907:mustard
801:, RSX;
795:halogen
764:S(=NR′)
723:allicin
675:sulfone
632:varacin
579:units.
563:alcohol
496:thianes
484:thietes
420:In the
159:Allicin
53:, two (
3056:Legend
2419:carbon
2376:
2366:
2315:
2284:
2218:
2193:
2158:
2105:S−C≡SF
2029:C−C≡SF
1871:
1818:
1795:
1754:
1721:
1688:
1655:
1628:: 64.
1603:
1563:
1512:: 22.
1482:
1440:coffee
1416:garlic
1412:ajoene
1309:biotin
1303:, and
1218:methyl
1142:S−C−R′
1136:ylides
1001:-oxide
927:capers
925:, and
911:radish
899:wasabi
853:, and
721:, and
526:. The
518:, and
116:, and
110:oxygen
85:, and
43:sulfur
2065:CC≡SF
1888:IUPAC
1385:→ C
1243:with
1170:S=CR′
1146:and R
1027:and F
990:IUPAC
809:, RSO
619:: in
559:Thiol
536:furan
360:bonds
200:, an
181:, an
2374:PMID
2313:ISBN
2282:PMID
2216:ISBN
2191:ISBN
2156:PMID
1923:2016
1869:ISBN
1816:ISBN
1793:PMID
1752:ISBN
1719:ISBN
1686:ISBN
1653:ISBN
1601:PMID
1561:ISBN
1480:ISBN
1451:odor
1428:wine
1410:and
1393:+ H
1331:and
1311:and
1081:and
1031:SCSF
1023:CCSF
1003:and
976:C=SO
948:The
596:gold
451:and
395:and
391:for
294:, a
79:coal
65:and
57:and
3030:CEs
3025:CCf
3020:CBk
3015:CCm
3010:CAm
3005:CPu
3000:CNp
2990:CPa
2985:CTh
2964:CYb
2959:CTm
2954:CEr
2949:CHo
2944:CDy
2939:CTb
2934:CGd
2929:CEu
2924:CSm
2919:CPm
2914:CNd
2909:CPr
2904:CCe
2899:CLa
2879:Og
2876:Ts
2873:Lv
2870:Mc
2867:Fl
2864:Nh
2861:Cn
2858:Rg
2855:Ds
2852:Mt
2849:Hs
2846:Bh
2842:CSg
2838:Db
2835:Rf
2819:CRa
2815:Fr
2810:Rn
2806:CAt
2801:CPo
2796:CBi
2791:CPb
2786:CTl
2781:CHg
2776:CAu
2771:CPt
2766:CIr
2761:COs
2756:CRe
2746:CTa
2741:CHf
2736:CLu
2724:CBa
2719:CCs
2712:CXe
2702:CTe
2697:CSb
2692:CSn
2687:CIn
2682:CCd
2677:CAg
2672:CPd
2667:CRh
2662:CRu
2657:CTc
2652:CMo
2647:CNb
2642:CZr
2630:CSr
2625:CRb
2618:CKr
2613:CBr
2608:CSe
2603:CAs
2598:CGe
2593:CGa
2588:CZn
2583:CCu
2578:CNi
2573:CCo
2568:CFe
2563:CMn
2558:CCr
2548:CTi
2543:CSc
2536:CCa
2524:CAr
2519:CCl
2504:CSi
2499:CAl
2494:CMg
2489:CNa
2483:Ne
2454:CBe
2449:CLi
2443:He
2364:PMC
2354:doi
2342:109
2305:doi
2274:doi
2270:128
2246:doi
2148:doi
2120:doi
2109:".
2080:doi
2076:114
2044:doi
2033:".
2008:doi
1981:doi
1954:doi
1927:doi
1902:doi
1900:".
1846:doi
1785:doi
1630:doi
1593:doi
1589:114
1514:doi
1263:in
1251:in
1089:NR′
995:syn
972:, R
594:on
530:of
417:.
97:of
3103::
3045:No
3040:Md
3035:Fm
2995:CU
2980:Ac
2831:Lr
2751:CW
2707:CI
2637:CY
2553:CV
2531:CK
2514:CS
2509:CP
2479:CF
2474:CO
2469:CN
2464:CC
2459:CB
2433:CH
2372:.
2362:.
2352:.
2340:.
2336:.
2311:.
2280:.
2242:98
2154:.
2144:48
2116:27
2040:23
2004:88
2002:.
1977:43
1975:.
1948:.
1921:.
1890:,
1842:84
1791:.
1781:38
1779:.
1626:18
1624:.
1599:.
1587:.
1575:^
1510:57
1508:.
1494:^
1438:,
1434:,
1418:.
1391:10
1327:.
1299:,
1287:.
1275:.
1247:/
1227::
1213:.
1209:SR
1121:A
1069:.
1007:.
929:.
921:,
917:,
913:,
909:,
905:,
901:,
893:.
885:)R
865:.
857:.
849:,
833:.
829:SF
754:.
717:,
665:A
609:.
602:.
514:,
510:,
506:,
502:,
498:,
494:,
490:,
486:,
482:,
478:,
474:,
436:.
424:,
410:.
368:pm
177:)-
131:.
112:,
101:.
81:,
77:,
2410:e
2403:t
2396:v
2380:.
2356::
2348::
2321:.
2307::
2288:.
2276::
2252:.
2248::
2224:.
2199:.
2162:.
2150::
2126:.
2122::
2107:3
2103:5
2099:5
2086:.
2082::
2067:3
2063:3
2050:.
2046::
2031:3
2027:3
2014:.
2010::
1987:.
1983::
1960:.
1956::
1950:6
1933:.
1929::
1904::
1877:.
1852:.
1848::
1824:.
1799:.
1787::
1773:S
1760:.
1744:S
1742:,
1740:S
1727:.
1711:S
1709:,
1707:S
1694:.
1678:S
1676:,
1674:S
1661:.
1637:.
1632::
1607:.
1595::
1569:.
1521:.
1516::
1488:.
1397:S
1395:2
1389:H
1387:4
1383:2
1379:4
1377:H
1375:4
1373:C
1269:3
1257:2
1241:1
1211:6
1199:4
1172:2
1168:2
1152:2
1148:2
1144:2
1140:2
1099:2
1095:2
1091:2
1087:2
1059:2
1033:3
1029:5
1025:3
1021:3
999:S
982:S
978:2
974:2
966:S
964:,
962:S
958:2
954:S
950:S
942:S
940:,
938:S
934:S
887:3
883:2
879:1
831:4
827:2
823:5
819:3
815:3
811:2
781:S
773:S
766:2
762:2
747:2
745:R
738:2
711:S
709:,
707:S
703:2
695:S
687:S
685:,
683:S
679:2
671:S
576:a
573:K
571:p
341:4
324:.
305:.
175:R
173:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.