31:
977:). Solutions produced in a laboratory may contain a virtually limitless number of species that contribute to alkalinity. Alkalinity is frequently given as molar equivalents per liter of solution or per kilogram of solvent. In commercial (e.g. the swimming pool industry) and regulatory contexts, alkalinity might also be given in parts per million of equivalent calcium carbonate (ppm CaCO
2312:
2231:
1516:
The lower the pH, the higher the concentration of bicarbonate will be. This shows how a lower pH can lead to higher alkalinity if the amount of bicarbonate produced is greater than the amount of H remaining after the reaction. This is the case since the amount of acid in the rainwater is low. If this
1541:
shows that pH will be related to calcium ion concentration, with lower pH going with higher calcium ion concentration. In this case, the higher the pH, the more bicarbonate and carbonate ion there will be, in contrast to the paradoxical situation described above, where one does not have equilibrium
1449:
will increase alkalinity by 2 units. Increased dissolution of carbonate rock by acidification from acid rain and mining has contributed to increased alkalinity concentrations in some major rivers throughout the eastern U.S. The following reaction shows how acid rain, containing sulfuric acid, can
1730:
techniques add alkalinity to the ocean and therefore immediately buffer pH changes which might help the organisms in the region that the extra alkalinity is added to. The two technologies that fall into this category are ocean alkalinity enhancement and electrochemical methods. Eventually, due to
1091:
Alkalinity can be measured by titrating a sample with a strong acid until all the buffering capacity of the aforementioned ions above the pH of bicarbonate or carbonate is consumed. This point is functionally set to pH 4.5. At this point, all the bases of interest have been protonated to the
868:
1420:
to a solution in contact with a solid can (over time) affect the alkalinity, especially for carbonate minerals in contact with groundwater or seawater. The dissolution (or precipitation) of carbonate rock has a strong influence on the alkalinity. This is because carbonate rock is composed of
1677:
and sedimentation of carbonate minerals (for example, as a function of ocean acidification) are the primary long-term drivers of alkalinity in the ocean. Over human timescales, mean ocean alkalinity is relatively stable. Seasonal and annual variability of mean ocean alkalinity is very low.
379:
are affected by changes in pH, temperature, and pressure. By isolating the conservative ions on one side of this charge balance equation, the nonconservative ions which accept or donate protons and thus define alkalinity are clustered on the other side of the equation.
284:
is 2 molar equivalents because twice as many H ions would be necessary to balance the charge. The total charge of a solution always equals zero. This leads to a parallel definition of alkalinity that is based upon the charge balance of ions in a solution.
1611:(carbonate). The carbonate ion has the potential to absorb two hydrogen ions. Therefore, it causes a net increase in ocean alkalinity. Calcium carbonate dissolution occurs in regions of the ocean which are undersaturated with respect to calcium carbonate.
1120:, although it adds acid and dissolved inorganic carbon, does not change the alkalinity. In natural conditions, the dissolution of basic rocks and addition of ammonia or organic amines leads to the addition of base to natural waters at the CO
386:
1128:
to bicarbonate ion and carbonate ion. At equilibrium, the water contains a certain amount of alkalinity contributed by the concentration of weak acid anions. Conversely, the addition of acid converts weak acid anions to
225:, that is now known as the Principle of Constant Proportions. However, there was one exception. Dittmar found that the concentration of calcium was slightly greater in the deep ocean, and named this increase alkalinity.
189:. It is one of the best measures of the sensitivity of the stream to acid inputs. There can be long-term changes in the alkalinity of streams and rivers in response to human disturbances such as acid rain generated by SO
1735:
is used. Both of these technologies have the potential to operate on a large scale and to be efficient at removing carbon dioxide. However, they are expensive, have many risks and side effects and currently have a low
1092:
zero level species, hence they no longer cause alkalinity. In the carbonate system the bicarbonate ions and the carbonate ions have become converted to carbonic acid at this pH. This pH is also called the CO
334:
1133:
and continuous addition of strong acids can cause the alkalinity to become less than zero. For example, the following reactions take place during the addition of acid to a typical seawater solution:
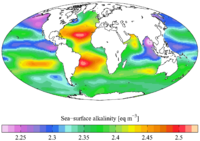
1699:(SST). Therefore, it generally increases with high latitudes and depths. As a result, upwelling areas (where water from the deep ocean is pushed to the surface) also have higher alkalinity values.
391:
877:. Total alkalinity is not (much) affected by temperature, pressure, or pH, and is thus itself a conservative measurement, which increases its usefulness in aquatic systems. All anions except
1084:(Subscript T indicates the total concentration of the species in the solution as measured. This is opposed to the free concentration, which takes into account the significant amount of
1321:
to a solution does not change its alkalinity, since the net reaction produces the same number of equivalents of positively contributing species (H) as negative contributing species (
1702:
There are many programs to measure, record, and study oceanic alkalinity, together with many of the other characteristics of seawater, like temperature and salinity. These include:
1684:
River dominated mixing also occurs close to the shore; it is strongest close to the mouth of a large river. Here, the rivers can act as either a source or a sink of alkalinity. A
2170:
2541:
Thomas, H.; Schiettecatte, L.-S.; et al. Enhanced Ocean Carbon
Storage from Anaerobic Alkalinity Generation in Coastal Sediments. Biogeosciences Discussions. 2008, 5, 3575–3591
1246:
It can be seen from the above protonation reactions that most bases consume one proton (H) to become a neutral species, thus increasing alkalinity by one per equivalent.
2721:
Climate Change 2022: Mitigation of
Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
1521:, precipitate carbonate, and thereby become less alkaline again. When carbonate minerals, water, and the atmosphere are all in equilibrium, the reversible reaction
2259:
Climate Change 2021: The
Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
2712:
260:
Alkalinity roughly refers to the molar amount of bases in a solution that can be converted to uncharged species by a strong acid. For example, 1 mole of
1289:. (The conjunction "as" is appropriate in this case because the alkalinity results from a mixture of ions but is reported "as if" all of this is due to CaCO
2250:
863:{\displaystyle {\begin{aligned}&\sum ({\text{conservative cations}})-\sum ({\text{conservative anions}})=\\&\quad +2++++2+++-----\end{aligned}}}
1108:
in an aqueous solution. There are no strong acids or bases at this point. Therefore, the alkalinity is modeled and quantified with respect to the CO
119:
until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (
1615:
2677:
2550:
Millero, F. J.; Lee, K.; Roche, M. Distribution of alkalinity in the surface waters of the major oceans. Marine
Chemistry. 1998, 60, 111-130.
2460:
2390:
2344:
1938:
1893:
1673:
The ocean's alkalinity varies over time, most significantly over geologic timescales (millennia). Changes in the balance between terrestrial
1984:
Kaushal, S. S.; Likens, G. E.; Utz, R. M.; Pace, M. L.; Grese, M.; Yepsen, M. (2013). "Increased river alkalinization in the
Eastern U.S.".
1681:
Alkalinity varies by location depending on evaporation/precipitation, advection of water, biological processes, and geochemical processes.
1706:(Geochemical Ocean Sections Study), TTO/NAS (Transient Tracers in the Ocean/North Atlantic Study), JGOFS (Joint Global Ocean Flux Study),
2094:"An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data"
1563:
1626:
from the atmosphere into the oceans. This does not affect the ocean's alkalinity but it does result in a reduction in pH value (called
1770:
1707:
35:
1649:
occur in oxygen-limited environments. Both of these processes consume hydrogen ions (thus increasing alkalinity) and release gases (N
2843:
2425:
2149:
2037:
291:
2838:
1703:
150:
terrains, alkalinities are low and involve a lot of ions. In the ocean, on the other hand, alkalinity is completely dominated by
2814:
2833:
1646:
2808:
925:
of carbonate or bicarbonate, defined as pH 4.5 for many oceanographic/limnological studies. The alkalinity is equal to the
1662:
1631:
2766:
2744:
1817:
2709:
181:. Moreover, measuring alkalinity is important in determining a stream's ability to neutralize acidic pollution from
2748:
Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water. Version 2
1737:
945:
processes that produce carbonate anions. Other common natural components that can contribute to alkalinity include
1882:
Chester, R.; Jickells, Tim (2012). "Chapter 9: Nutrients oxygen organic carbon and the carbon cycle in seawater".
1637:
Biological processes have a much greater impact on oceanic alkalinity on short (minutes to centuries) timescales.
2723:
2261:
1883:
214:
2247:
2136:, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, 2017-08-27,
2053:
Wolf-Gladrow, Dieter A.; Zeebe, Richard E.; Klaas, Christine; Körtzinger, Arne; Dickson, Andrew G. (July 2007).
2792:
1619:
245:
84:
2609:
1765:
1696:
222:
1274:. and decrease alkalinity, as they act as sources of protons. They are often represented collectively as
58:
2485:
1918:
1634:
has been proposed as one option to add alkalinity to the ocean and therefore buffer against pH changes.
2055:"Total alkalinity: The explicit conservative expression and its application to biogeochemical processes"
218:
1124:
equivalence point. The dissolved base in water increases the pH and titrates an equivalent amount of CO
2497:
2105:
2066:
1851:
1638:
1293:.) This can be converted into milliequivalents per Liter (meq/L) by dividing by 50 (the approximate
934:
112:
1691:
Oceanic alkalinity also follows general trends based on latitude and depth. It has been shown that A
1720:
1627:
1559:
70:
2584:
2691:
2358:
2301:
2220:
2054:
178:
2093:
1731:
diffusion, that alkalinity addition will be quite small to distant waters. This is why the term
2788:
2759:
The following packages calculate the state of the carbonate system in seawater (including pH):
221:. He found that in seawater the major ions were in a fixed ratio, confirming the hypothesis of
2796:
2683:
2673:
2521:
2513:
2466:
2456:
2431:
2421:
2396:
2386:
2350:
2340:
2164:
2145:
2033:
2001:
1934:
1899:
1889:
1021:
1005:
937:
tends to make up most of the total alkalinity due to the common occurrence and dissolution of
922:
132:
2665:
2505:
2332:
2291:
2210:
2137:
2131:
2113:
2074:
1993:
1926:
1859:
1842:
Dickson, Andrew G. (1992). "The development of the alkalinity concept in marine chemistry".
1755:
1450:
have the effect of increasing river alkalinity by increasing the amount of bicarbonate ion:
982:
930:
249:
229:
120:
88:
24:
367:" such that they are unaffected by changes in temperature, pressure or pH. Others such as
30:
2778:
2770:
2727:
2716:
2316:
2296:
2279:
2265:
2254:
2235:
2215:
2198:
1760:
1642:
1013:
210:
96:
49:
2509:
989:
lowers the pH of a solution, thus reducing basicity while alkalinity remains unchanged (
2501:
2109:
2070:
1855:
2484:
Doney, Scott C.; Fabry, Victoria J.; Feely, Richard A.; Kleypas, Joan A. (2009-01-01).
2280:"The Impacts of Ocean Acidification on Marine Ecosystems and Reliant Human Communities"
2278:
Doney, Scott C.; Busch, D. Shallin; Cooley, Sarah R.; Kroeker, Kristy J. (2020-10-17).
2199:"The Impacts of Ocean Acidification on Marine Ecosystems and Reliant Human Communities"
2197:
Doney, Scott C.; Busch, D. Shallin; Cooley, Sarah R.; Kroeker, Kristy J. (2020-10-17).
2026:
1930:
1795:
1727:
966:
926:
104:
1959:
2827:
2695:
2362:
2305:
2224:
2117:
1863:
1710:(World Ocean Circulation Experiment), CARINA (Carbon dioxide in the Atlantic Ocean).
1665:
both decrease alkalinity by releasing protons as a byproduct of oxidation reactions.
1658:
1599:. Perhaps the most well known is the dissolution of calcium carbonate to form Ca and
1009:
970:
901:
have low concentrations in Earth's surface water (streams, rivers, and lakes). Thus
2560:
1750:
1001:
174:
170:
2078:
217:, analysed 77 pristine seawater samples from around the world brought back by the
1517:
alkaline groundwater later comes into contact with the atmosphere, it can lose CO
1573:
1033:
155:
143:
38:
1688:
follows the outflow of the river and has a linear relationship with salinity.
16:
Capacity of water to resist changes in pH that would make the water more acidic
2774:
1674:
1294:
1017:
942:
186:
2634:
2517:
2470:
2400:
2268:. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
1903:
2763:
2730:, Cambridge University Press, Cambridge, United Kingdom and New York, NY, US
2662:
A Research
Strategy for Ocean-based Carbon Dioxide Removal and Sequestration
2435:
2329:
A Research
Strategy for Ocean-based Carbon Dioxide Removal and Sequestration
1569:
1112:
equivalence point. Because the alkalinity is measured with respect to the CO
997:
954:
950:
938:
241:
237:
182:
166:
151:
147:
108:
100:
2687:
2525:
2354:
2005:
1790:
135:). Each of these measurements corresponds to an amount of acid added as a
2450:
2380:
2141:
1258:
however, will consume two protons before becoming a zero-level species (CO
2800:
2415:
1085:
1037:
958:
76:
2417:
Aquatic chemistry : chemical equilibria and rates in natural waters
2311:
2230:
974:
962:
136:
2720:
2258:
1997:
1721:
Ocean acidification § Carbon removal technologies which add alkalinity
909:
is also approximately equal to the total alkalinity in surface water.
2818:
2782:
1577:
946:
159:
20:
1096:
equivalence point where the major component in water is dissolved CO
2669:
2336:
2804:
2613:
1596:
1551:
124:
80:
29:
981:). Alkalinity is sometimes incorrectly used interchangeably with
1641:
of organic matter can decrease alkalinity by releasing protons.
918:
272:
in solution represents 1 molar equivalent, while 1 mole of
232:
submitted his PhD theses in which he advocated the existence of
1349:
to the solution lowers its pH, but does not affect alkalinity.
1888:(3rd ed.). Chichester, West Sussex, UK: Wiley/Blackwell.
329:{\displaystyle \sum ({\text{cations}})=\sum ({\text{anions}})}
233:
116:
2315:
Text was copied from this source, which is available under a
2234:
Text was copied from this source, which is available under a
1923:
Reference Module in Earth
Systems and Environmental Sciences
917:
Alkalinity measures the ability of a solution to neutralize
1583:
Thus the chemical equation for alkalinity in seawater is:
1555:
1004:
are specified for various alkalinity measurement methods.
92:
2656:
2654:
873:
This combined charge balance and proton balance is called
2588:
2098:
Deep Sea
Research Part A. Oceanographic Research Papers
1595:
There are many methods of alkalinity generation in the
127:), μeq/kg (microequivalents per kilogram), or mg/L CaCO
2452:
CO2 in seawater : equilibrium, kinetics, isotopes
2317:
Creative
Commons Attribution 4.0 International License
2236:
Creative Commons Attribution 4.0 International License
1437:
into solution. Ca will not influence alkalinity, but
389:
294:
2187:. 2nd Ed. Long Grove, Illinois: Waveland Press, Inc.
1568:
In the ocean, alkalinity is completely dominated by
63:
2750:, A. G. Dickson & C. Goyet, eds. ORNL/CDIAC-74.
2449:Zeebe, Richard E.; Wolf-Gladrow, Dieter A. (2001).
1979:
1977:
1262:), thus it increases alkalinity by two per mole of
2585:"Home : Woods Hole Oceanographic Institution"
2028:The Geochemistry of Natural Waters, Second Edition
2025:
862:
328:
2537:
2535:
2382:Chemical oceanography and the marine carbon cycle
2169:: CS1 maint: DOI inactive as of September 2024 (
1657:S), which eventually escape into the atmosphere.
1040:, the measured total alkalinity is set equal to:
165:Although alkalinity is primarily a term used by
2486:"Ocean Acidification: The Other CO 2 Problem"
1962:. United States Environment Protection Agency
8:
933:in solution. In most Earth surface waters
339:Certain ions, including Na, K, Ca, Mg, Cl,
244:ion donors. For that work, he received the
2284:Annual Review of Environment and Resources
2203:Annual Review of Environment and Resources
91:, which is an absolute measurement on the
2385:. Cambridge: Cambridge University Press.
2295:
2214:
1837:
1835:
1281:Alkalinity is typically reported as mg/L
846:
835:
819:
806:
801:
784:
768:
763:
752:
736:
731:
715:
707:
691:
683:
667:
662:
646:
641:
622:
617:
609:
587:
582:
571:
555:
547:
531:
526:
509:
490:
485:
477:
458:
453:
442:
420:
403:
390:
388:
318:
301:
293:
2414:Stumm, Werner; Morgan, James J. (1996).
1622:, results in increasing absorption of CO
1116:equivalence point, the dissolution of CO
2710:Chapter 12: Cross sectoral perspectives
2032:. Englewood Cliffs, NJ: Prentice Hall.
1782:
1669:Global temporal and spatial variability
95:scale. Alkalinity is the strength of a
2379:Emerson, Steven; Hedges, John (2008).
2162:
1986:Environmental Science & Technology
1616:carbon dioxide level in the atmosphere
1088:interactions that occur in seawater.)
1012:acids are common acid titrants, while
2374:
2372:
2297:10.1146/annurev-environ-012320-083019
2216:10.1146/annurev-environ-012320-083019
1425:and its dissociation will add Ca and
250:Svante Arrhenius#Ionic disassociation
7:
2510:10.1146/annurev.marine.010908.163834
2019:
2017:
2015:
1877:
1875:
1873:
1733:local ocean acidification mitigation
990:
1695:is often inversely proportional to
1564:Effects of climate change on oceans
985:. For example, the addition of CO
53:
1931:10.1016/b978-0-12-409548-9.09397-0
1771:Global Ocean Data Analysis Project
843:
839:
836:
816:
812:
803:
788:
785:
760:
756:
753:
733:
712:
708:
688:
684:
659:
655:
652:
643:
614:
610:
579:
575:
572:
552:
548:
519:
516:
510:
482:
478:
450:
446:
443:
236:in solution, and defined acids as
14:
2420:(3rd ed.). New York: Wiley.
87:. It should not be confused with
34:Sea surface alkalinity (from the
2310:
2229:
1719:This section is an excerpt from
1493:Another way of writing this is:
1380:Only at high (basic) pH values:
2490:Annual Review of Marine Science
1714:Interventions to add alkalinity
1576:plus a small contribution from
438:
158:plus a small contribution from
853:
832:
826:
798:
792:
781:
775:
749:
743:
728:
722:
704:
698:
680:
674:
638:
632:
606:
597:
568:
562:
544:
538:
523:
513:
506:
500:
474:
465:
439:
425:
417:
408:
400:
323:
315:
306:
298:
1:
2773:, available as a stand-alone
2079:10.1016/j.marchem.2007.01.006
1917:Mattson, M. D. (2014-01-01),
1820:. Water Research Center. 2014
1546:Changes to oceanic alkalinity
1412:Dissolution of carbonate rock
213:of Anderson College, now the
2755:Carbonate system calculators
2612:. 2011-10-16. Archived from
2587:. 2012-03-14. Archived from
2118:10.1016/0198-0149(81)90121-7
1864:10.1016/0304-4203(92)90047-E
1632:Ocean alkalinity enhancement
146:, particularly those on non-
2610:"WOCE Global Data Resource"
2092:Dickson, A.G. (June 1981).
1919:"Alkalinity of Freshwater☆"
1317:Addition (or removal) of CO
1305:Carbon dioxide interactions
941:rocks and other geological
64:
2860:
1738:technology readiness level
1718:
1549:
79:') is the capacity of
18:
2024:Drever, James I. (1988).
1796:Dictionary.com Unabridged
1556:pH § pH in the ocean
215:University of Strathclyde
211:Wilhelm (William) Dittmar
131:(milligrams per liter of
2844:Water quality indicators
2183:Benjamin. Mark M. 2015.
1620:carbon dioxide emissions
246:Nobel Prize in Chemistry
240:ion donors and bases as
19:Not to be confused with
2715:13 October 2022 at the
2565:iridl.ldeo.columbia.edu
2455:. Amsterdam: Elsevier.
2144:(inactive 2024-09-12),
1766:Dealkalization of water
1697:sea surface temperature
1552:Ocean § Alkalinity
1100:which is converted to H
1024:are common indicators.
223:Johan Georg Forchhammer
864:
330:
42:
2834:Chemical oceanography
2726:2 August 2022 at the
2264:9 August 2021 at the
2142:10.2105/smww.2882.023
1818:"What is alkalinity?"
1542:with the atmosphere.
1028:Theoretical treatment
865:
331:
219:Challenger expedition
173:, it is also used by
115:with an acid such as
33:
935:carbonate alkalinity
913:Detailed description
905:, which is equal to
903:carbonate alkalinity
405:conservative cations
387:
292:
107:. It is measured by
2839:Acid–base chemistry
2502:2009ARMS....1..169D
2253:5 June 2022 at the
2248:Annex VII: Glossary
2110:1981DSRA...28..609D
2071:2007MarCh.106..287W
1992:(18): 10302–10311.
1885:Marine geochemistry
1856:1992MarCh..40...49D
1639:Aerobic respiration
1628:ocean acidification
1560:Ocean acidification
773:
672:
630:
595:
536:
498:
463:
422:conservative anions
209:In 1884, Professor
2769:2011-10-14 at the
2561:"dataset: GEOSECS"
1960:"Total Alkalinity"
1352:At all pH values:
860:
858:
759:
658:
613:
578:
522:
481:
449:
326:
256:Simplified summary
248:in 1903. See also
179:temporary hardness
75:'ashes of the
43:
2679:978-0-309-08761-2
2639:www.pmel.noaa.gov
2462:978-0-08-052922-6
2392:978-0-511-64987-5
2346:978-0-309-08761-2
1998:10.1021/es401046s
1940:978-0-12-409548-9
1895:978-1-118-34909-0
1663:sulfide oxidation
1647:sulfate reduction
1022:bromocresol green
1000:, endpoints, and
991:see example below
923:equivalence point
423:
406:
321:
304:
133:calcium carbonate
74:
62:
2851:
2807:(also available
2781:spreadsheet, or
2731:
2706:
2700:
2699:
2658:
2649:
2648:
2646:
2645:
2631:
2625:
2624:
2622:
2621:
2606:
2600:
2599:
2597:
2596:
2581:
2575:
2574:
2572:
2571:
2557:
2551:
2548:
2542:
2539:
2530:
2529:
2481:
2475:
2474:
2446:
2440:
2439:
2411:
2405:
2404:
2376:
2367:
2366:
2325:
2319:
2314:
2309:
2299:
2275:
2269:
2244:
2238:
2233:
2228:
2218:
2194:
2188:
2181:
2175:
2174:
2168:
2160:
2159:
2158:
2128:
2122:
2121:
2089:
2083:
2082:
2065:(1–2): 287–300.
2059:Marine Chemistry
2050:
2044:
2043:
2031:
2021:
2010:
2009:
1981:
1972:
1971:
1969:
1967:
1956:
1950:
1949:
1948:
1947:
1914:
1908:
1907:
1879:
1868:
1867:
1844:Marine Chemistry
1839:
1830:
1829:
1827:
1825:
1814:
1808:
1807:
1805:
1804:
1787:
1756:Base (chemistry)
1610:
1609:
1608:
1512:
1511:
1510:
1489:
1488:
1487:
1477:
1476:
1475:
1448:
1447:
1446:
1436:
1435:
1434:
1406:
1405:
1404:
1394:
1393:
1392:
1375:
1374:
1373:
1344:
1343:
1342:
1332:
1331:
1330:
1273:
1272:
1271:
1257:
1256:
1255:
1237:
1236:
1235:
1227:
1226:
1216:
1215:
1214:
1201:
1200:
1199:
1191:
1190:
1180:
1179:
1178:
1147:
1146:
1145:
908:
900:
899:
898:
888:
887:
886:
875:total alkalinity
869:
867:
866:
861:
859:
852:
851:
850:
825:
824:
823:
811:
810:
791:
774:
772:
767:
742:
741:
740:
721:
720:
719:
697:
696:
695:
673:
671:
666:
651:
650:
631:
629:
621:
596:
594:
586:
561:
560:
559:
537:
535:
530:
499:
497:
489:
464:
462:
457:
434:
424:
421:
407:
404:
393:
378:
377:
376:
362:
361:
360:
350:
349:
348:
335:
333:
332:
327:
322:
319:
305:
302:
283:
282:
281:
271:
270:
269:
230:Svante Arrhenius
121:milliequivalents
69:
67:
57:
55:
25:base (chemistry)
2859:
2858:
2854:
2853:
2852:
2850:
2849:
2848:
2824:
2823:
2771:Wayback Machine
2757:
2740:
2735:
2734:
2728:Wayback Machine
2717:Wayback Machine
2707:
2703:
2680:
2660:
2659:
2652:
2643:
2641:
2633:
2632:
2628:
2619:
2617:
2608:
2607:
2603:
2594:
2592:
2583:
2582:
2578:
2569:
2567:
2559:
2558:
2554:
2549:
2545:
2540:
2533:
2483:
2482:
2478:
2463:
2448:
2447:
2443:
2428:
2413:
2412:
2408:
2393:
2378:
2377:
2370:
2347:
2327:
2326:
2322:
2277:
2276:
2272:
2266:Wayback Machine
2255:Wayback Machine
2245:
2241:
2196:
2195:
2191:
2185:Water Chemistry
2182:
2178:
2161:
2156:
2154:
2152:
2133:2320 alkalinity
2130:
2129:
2125:
2091:
2090:
2086:
2052:
2051:
2047:
2040:
2023:
2022:
2013:
1983:
1982:
1975:
1965:
1963:
1958:
1957:
1953:
1945:
1943:
1941:
1916:
1915:
1911:
1896:
1881:
1880:
1871:
1841:
1840:
1833:
1823:
1821:
1816:
1815:
1811:
1802:
1800:
1789:
1788:
1784:
1779:
1761:Biological pump
1747:
1742:
1741:
1724:
1716:
1694:
1687:
1671:
1656:
1652:
1643:Denitrification
1625:
1614:The increasing
1607:
1604:
1603:
1602:
1600:
1590:
1566:
1548:
1536:
1532:
1529:+ 2 H ⇌ Ca + CO
1528:
1520:
1509:
1506:
1505:
1504:
1502:
1500:
1486:
1483:
1482:
1481:
1479:
1474:
1471:
1470:
1469:
1467:
1465:
1461:
1457:
1445:
1442:
1441:
1440:
1438:
1433:
1430:
1429:
1428:
1426:
1424:
1419:
1414:
1403:
1400:
1399:
1398:
1396:
1391:
1388:
1387:
1386:
1384:
1372:
1369:
1368:
1367:
1365:
1363:
1359:
1348:
1341:
1338:
1337:
1336:
1334:
1329:
1326:
1325:
1324:
1322:
1320:
1315:
1313:
1307:
1301:divided by 2).
1300:
1292:
1288:
1277:
1270:
1267:
1266:
1265:
1263:
1261:
1254:
1251:
1250:
1249:
1247:
1234:
1231:
1230:
1229:
1225:
1222:
1221:
1220:
1218:
1213:
1210:
1209:
1208:
1206:
1198:
1195:
1194:
1193:
1189:
1186:
1185:
1184:
1182:
1177:
1174:
1173:
1172:
1170:
1164:
1155:
1151:
1144:
1141:
1140:
1139:
1137:
1132:
1127:
1123:
1119:
1115:
1111:
1107:
1103:
1099:
1095:
1079:
1075:
1071:
1067:
1063:
1059:
1055:
1051:
1047:
1030:
988:
980:
967:conjugate bases
915:
906:
897:
894:
893:
892:
890:
885:
882:
881:
880:
878:
857:
856:
842:
815:
802:
732:
711:
687:
642:
551:
432:
431:
385:
384:
375:
372:
371:
370:
368:
359:
356:
355:
354:
352:
347:
344:
343:
342:
340:
290:
289:
280:
277:
276:
275:
273:
268:
265:
264:
263:
261:
258:
207:
200:
194:
130:
105:conjugate bases
97:buffer solution
28:
17:
12:
11:
5:
2857:
2855:
2847:
2846:
2841:
2836:
2826:
2825:
2822:
2821:
2812:
2786:
2756:
2753:
2752:
2751:
2739:
2738:External links
2736:
2733:
2732:
2701:
2678:
2670:10.17226/26278
2650:
2626:
2601:
2576:
2552:
2543:
2531:
2496:(1): 169–192.
2476:
2461:
2441:
2426:
2406:
2391:
2368:
2345:
2337:10.17226/26278
2320:
2270:
2239:
2189:
2176:
2150:
2123:
2104:(6): 609–623.
2084:
2045:
2038:
2011:
1973:
1951:
1939:
1909:
1894:
1869:
1850:(1–2): 49–63.
1831:
1809:
1781:
1780:
1778:
1775:
1774:
1773:
1768:
1763:
1758:
1753:
1746:
1743:
1728:carbon removal
1725:
1717:
1715:
1712:
1692:
1685:
1670:
1667:
1654:
1650:
1623:
1605:
1593:
1592:
1588:
1547:
1544:
1539:
1538:
1534:
1530:
1526:
1518:
1514:
1513:
1507:
1498:
1491:
1490:
1484:
1472:
1463:
1459:
1455:
1443:
1431:
1422:
1417:
1416:Addition of CO
1413:
1410:
1409:
1408:
1401:
1389:
1378:
1377:
1370:
1361:
1357:
1346:
1339:
1327:
1318:
1314:
1311:
1310:Addition of CO
1308:
1306:
1303:
1298:
1290:
1286:
1275:
1268:
1259:
1252:
1244:
1243:
1239:
1238:
1232:
1223:
1211:
1203:
1202:
1196:
1187:
1175:
1167:
1166:
1162:
1158:
1157:
1153:
1149:
1142:
1130:
1125:
1121:
1117:
1113:
1109:
1105:
1101:
1097:
1093:
1082:
1081:
1077:
1073:
1069:
1065:
1061:
1057:
1053:
1049:
1045:
1029:
1026:
1014:phenolpthalein
986:
978:
927:stoichiometric
914:
911:
895:
883:
871:
870:
855:
849:
845:
841:
838:
834:
831:
828:
822:
818:
814:
809:
805:
800:
797:
794:
790:
787:
783:
780:
777:
771:
766:
762:
758:
755:
751:
748:
745:
739:
735:
730:
727:
724:
718:
714:
710:
706:
703:
700:
694:
690:
686:
682:
679:
676:
670:
665:
661:
657:
654:
649:
645:
640:
637:
634:
628:
625:
620:
616:
612:
608:
605:
602:
599:
593:
590:
585:
581:
577:
574:
570:
567:
564:
558:
554:
550:
546:
543:
540:
534:
529:
525:
521:
518:
515:
512:
508:
505:
502:
496:
493:
488:
484:
480:
476:
473:
470:
467:
461:
456:
452:
448:
445:
441:
437:
435:
433:
430:
427:
419:
416:
413:
410:
402:
399:
396:
394:
392:
373:
357:
345:
337:
336:
325:
317:
314:
311:
308:
300:
297:
278:
266:
257:
254:
228:Also in 1884,
206:
203:
196:
190:
171:oceanographers
128:
15:
13:
10:
9:
6:
4:
3:
2:
2856:
2845:
2842:
2840:
2837:
2835:
2832:
2831:
2829:
2820:
2819:Matlab script
2816:
2813:
2810:
2806:
2802:
2798:
2794:
2790:
2787:
2784:
2780:
2776:
2772:
2768:
2765:
2762:
2761:
2760:
2754:
2749:
2745:
2742:
2741:
2737:
2729:
2725:
2722:
2718:
2714:
2711:
2705:
2702:
2697:
2693:
2689:
2685:
2681:
2675:
2671:
2667:
2663:
2657:
2655:
2651:
2640:
2636:
2630:
2627:
2616:on 2011-10-16
2615:
2611:
2605:
2602:
2591:on 2012-03-14
2590:
2586:
2580:
2577:
2566:
2562:
2556:
2553:
2547:
2544:
2538:
2536:
2532:
2527:
2523:
2519:
2515:
2511:
2507:
2503:
2499:
2495:
2491:
2487:
2480:
2477:
2472:
2468:
2464:
2458:
2454:
2453:
2445:
2442:
2437:
2433:
2429:
2427:0-471-51184-6
2423:
2419:
2418:
2410:
2407:
2402:
2398:
2394:
2388:
2384:
2383:
2375:
2373:
2369:
2364:
2360:
2356:
2352:
2348:
2342:
2338:
2334:
2330:
2324:
2321:
2318:
2313:
2307:
2303:
2298:
2293:
2290:(1): 83–112.
2289:
2285:
2281:
2274:
2271:
2267:
2263:
2260:
2256:
2252:
2249:
2243:
2240:
2237:
2232:
2226:
2222:
2217:
2212:
2209:(1): 83–112.
2208:
2204:
2200:
2193:
2190:
2186:
2180:
2177:
2172:
2166:
2153:
2151:9780875532998
2147:
2143:
2139:
2135:
2134:
2127:
2124:
2119:
2115:
2111:
2107:
2103:
2099:
2095:
2088:
2085:
2080:
2076:
2072:
2068:
2064:
2060:
2056:
2049:
2046:
2041:
2039:0-13-351396-3
2035:
2030:
2029:
2020:
2018:
2016:
2012:
2007:
2003:
1999:
1995:
1991:
1987:
1980:
1978:
1974:
1961:
1955:
1952:
1942:
1936:
1932:
1928:
1924:
1920:
1913:
1910:
1905:
1901:
1897:
1891:
1887:
1886:
1878:
1876:
1874:
1870:
1865:
1861:
1857:
1853:
1849:
1845:
1838:
1836:
1832:
1819:
1813:
1810:
1799:(Online). n.d
1798:
1797:
1792:
1786:
1783:
1776:
1772:
1769:
1767:
1764:
1762:
1759:
1757:
1754:
1752:
1749:
1748:
1744:
1739:
1734:
1729:
1722:
1713:
1711:
1709:
1705:
1700:
1698:
1689:
1682:
1679:
1676:
1668:
1666:
1664:
1660:
1659:Nitrification
1648:
1644:
1640:
1635:
1633:
1629:
1621:
1617:
1612:
1598:
1586:
1585:
1584:
1581:
1579:
1575:
1571:
1565:
1561:
1557:
1553:
1545:
1543:
1524:
1523:
1522:
1496:
1495:
1494:
1453:
1452:
1451:
1411:
1383:
1382:
1381:
1355:
1354:
1353:
1350:
1309:
1304:
1302:
1296:
1284:
1279:
1241:
1240:
1205:
1204:
1169:
1168:
1160:
1159:
1136:
1135:
1134:
1089:
1087:
1043:
1042:
1041:
1039:
1035:
1027:
1025:
1023:
1019:
1015:
1011:
1007:
1003:
999:
996:A variety of
994:
992:
984:
976:
972:
971:organic acids
968:
964:
960:
956:
952:
948:
944:
940:
936:
932:
928:
924:
920:
912:
910:
904:
876:
847:
829:
820:
807:
795:
778:
769:
764:
746:
737:
725:
716:
701:
692:
677:
668:
663:
647:
635:
626:
623:
618:
603:
600:
591:
588:
583:
565:
556:
541:
532:
527:
503:
494:
491:
486:
471:
468:
459:
454:
436:
428:
414:
411:
397:
395:
383:
382:
381:
366:
312:
309:
295:
288:
287:
286:
255:
253:
251:
247:
243:
239:
235:
231:
226:
224:
220:
216:
212:
204:
202:
199:
193:
188:
184:
180:
176:
172:
168:
163:
161:
157:
153:
149:
145:
140:
138:
134:
126:
122:
118:
114:
110:
106:
102:
98:
94:
90:
86:
85:acidification
82:
78:
72:
66:
60:
51:
47:
40:
37:
32:
26:
22:
2758:
2747:
2743:DOE (1994) "
2708:IPCC (2022)
2704:
2661:
2642:. Retrieved
2638:
2629:
2618:. Retrieved
2614:the original
2604:
2593:. Retrieved
2589:the original
2579:
2568:. Retrieved
2564:
2555:
2546:
2493:
2489:
2479:
2451:
2444:
2416:
2409:
2381:
2328:
2323:
2287:
2283:
2273:
2246:IPCC, 2021:
2242:
2206:
2202:
2192:
2184:
2179:
2155:, retrieved
2132:
2126:
2101:
2097:
2087:
2062:
2058:
2048:
2027:
1989:
1985:
1964:. Retrieved
1954:
1944:, retrieved
1925:, Elsevier,
1922:
1912:
1884:
1847:
1843:
1822:. Retrieved
1812:
1801:. Retrieved
1794:
1785:
1751:Alkali soils
1732:
1701:
1690:
1683:
1680:
1672:
1636:
1613:
1594:
1582:
1567:
1540:
1515:
1492:
1415:
1379:
1351:
1345:). Adding CO
1316:
1282:
1280:
1245:
1090:
1083:
1031:
1006:Hydrochloric
995:
961:, dissolved
916:
902:
874:
872:
365:conservative
364:
338:
259:
227:
208:
197:
191:
177:to describe
175:hydrologists
167:limnologists
164:
141:
99:composed of
45:
44:
1574:bicarbonate
1501:+ H ⇌ Ca +
1466:→ 2 Ca + 2
1148:+ H → B(OH)
1034:groundwater
1032:In typical
929:sum of the
201:emissions.
156:bicarbonate
39:climatology
2828:Categories
2775:executable
2644:2023-03-28
2620:2023-03-28
2595:2023-03-28
2570:2023-03-28
2157:2022-12-01
1946:2023-01-09
1824:5 February
1803:2018-09-30
1777:References
1675:weathering
1550:See also:
1161:OH + H → H
1018:methyl red
1002:indicators
965:, and the
943:weathering
187:wastewater
144:freshwater
103:and their
101:weak acids
83:to resist
46:Alkalinity
2793:R package
2696:245089649
2518:1941-1405
2471:246683387
2401:558876135
2363:245089649
2306:225741986
2225:225741986
1904:781078031
1618:, due to
1591:= + 2 +
1570:carbonate
955:phosphate
951:hydroxide
939:carbonate
830:−
796:−
779:−
770:−
747:−
726:−
717:−
669:−
627:−
592:−
557:−
533:−
495:−
460:−
415:∑
412:−
398:∑
313:∑
296:∑
242:hydroxide
238:hydronium
152:carbonate
148:limestone
109:titrating
59:romanized
2801:Mac OS X
2767:Archived
2724:Archived
2713:Archived
2688:35533244
2664:. 2022.
2635:"CARINA"
2526:21141034
2436:31754493
2355:35533244
2331:. 2022.
2262:Archived
2251:Archived
2165:citation
2006:23883395
1791:"alkali"
1745:See also
1181:+ 2 H →
1086:ion pair
1038:seawater
1010:sulfuric
998:titrants
983:basicity
959:silicate
183:rainfall
113:solution
89:basicity
77:saltwort
2797:Windows
2789:seacarb
2785:script.
2498:Bibcode
2106:Bibcode
2067:Bibcode
1966:6 March
1852:Bibcode
1704:GEOSECS
1333:and/or
1297:of CaCO
975:acetate
973:(e.g.,
963:ammonia
921:to the
303:cations
205:History
137:titrant
73:
65:al-qaly
61::
54:القلوية
2783:MATLAB
2764:CO2SYS
2694:
2686:
2676:
2524:
2516:
2469:
2459:
2434:
2424:
2399:
2389:
2361:
2353:
2343:
2304:
2223:
2148:
2036:
2004:
1937:
1902:
1892:
1578:borate
1562:, and
1454:2 CaCO
1395:+ H ⇌
1242:+ H →
1217:+ H →
1020:, and
947:borate
351:, and
320:anions
195:and NO
160:borate
50:Arabic
48:(from
36:GLODAP
21:alkali
2805:Linux
2779:Excel
2692:S2CID
2359:S2CID
2302:S2CID
2257:. In
2221:S2CID
1726:Some
1597:ocean
1407:+ 2 H
1138:B(OH)
931:bases
919:acids
363:are "
125:liter
81:water
2817:, a
2815:CSYS
2809:here
2803:and
2795:for
2791:, a
2684:PMID
2674:ISBN
2522:PMID
2514:ISSN
2467:OCLC
2457:ISBN
2432:OCLC
2422:ISBN
2397:OCLC
2387:ISBN
2351:PMID
2341:ISBN
2171:link
2146:ISBN
2034:ISBN
2002:PMID
1968:2013
1935:ISBN
1900:OCLC
1890:ISBN
1826:2018
1708:WOCE
1661:and
1653:or H
1645:and
1572:and
1525:CaCO
1497:CaCO
1421:CaCO
1364:O ⇌
1285:CaCO
1008:and
993:).
889:and
234:ions
169:and
154:and
123:per
111:the
71:lit.
2719:in
2666:doi
2506:doi
2333:doi
2292:doi
2211:doi
2138:doi
2114:doi
2075:doi
2063:106
1994:doi
1927:doi
1860:doi
1630:).
1580:.
1533:+ H
1503:HCO
1468:HCO
1458:+ H
1385:HCO
1376:+ H
1366:HCO
1360:+ H
1323:HCO
1207:HPO
1152:+ H
1078:sws
1064:+ 2
1052:+ 2
1036:or
969:of
907:+ 2
879:HCO
369:HCO
262:HCO
185:or
142:In
117:HCl
23:or
2830::
2799:,
2777:,
2746:,"
2690:.
2682:.
2672:.
2653:^
2637:.
2563:.
2534:^
2520:.
2512:.
2504:.
2492:.
2488:.
2465:.
2430:.
2395:.
2371:^
2357:.
2349:.
2339:.
2300:.
2288:45
2286:.
2282:.
2219:.
2207:45
2205:.
2201:.
2167:}}
2163:{{
2112:.
2102:28
2100:.
2096:.
2073:.
2061:.
2057:.
2014:^
2000:.
1990:47
1988:.
1976:^
1933:,
1921:,
1898:.
1872:^
1858:.
1848:40
1846:.
1834:^
1793:.
1601:CO
1558:,
1554:,
1480:SO
1478:+
1462:SO
1439:CO
1427:CO
1397:CO
1356:CO
1335:CO
1295:MW
1283:as
1278:.
1264:CO
1248:CO
1228:PO
1192:PO
1171:PO
1129:CO
1104:CO
1080:−
1076:−
1072:+
1068:+
1060:+
1056:+
1048:=
1016:,
957:,
953:,
949:,
891:CO
353:NO
341:SO
274:CO
252:.
162:.
139:.
93:pH
68:,
56:,
52::
41:).
2811:)
2698:.
2668::
2647:.
2623:.
2598:.
2573:.
2528:.
2508::
2500::
2494:1
2473:.
2438:.
2403:.
2365:.
2335::
2308:.
2294::
2227:.
2213::
2173:)
2140::
2120:.
2116::
2108::
2081:.
2077::
2069::
2042:.
2008:.
1996::
1970:.
1929::
1906:.
1866:.
1862::
1854::
1828:.
1806:.
1740:.
1723:.
1693:T
1686:T
1655:2
1651:2
1624:2
1606:3
1589:T
1587:A
1537:O
1535:2
1531:2
1527:3
1519:2
1508:3
1499:3
1485:4
1473:3
1464:4
1460:2
1456:3
1444:3
1432:3
1423:3
1418:2
1402:3
1390:3
1371:3
1362:2
1358:2
1347:2
1340:3
1328:3
1319:2
1312:2
1299:3
1291:3
1287:3
1276:T
1269:3
1260:2
1253:3
1233:4
1224:2
1219:H
1212:4
1197:4
1188:2
1183:H
1176:4
1165:O
1163:2
1156:O
1154:2
1150:3
1143:4
1131:2
1126:2
1122:2
1118:2
1114:2
1110:2
1106:3
1102:2
1098:2
1094:2
1074:T
1070:T
1066:T
1062:T
1058:T
1054:T
1050:T
1046:T
1044:A
987:2
979:3
896:3
884:3
854:]
848:2
844:O
840:N
837:H
833:[
827:]
821:4
817:O
813:P
808:3
804:H
799:[
793:]
789:F
786:H
782:[
776:]
765:4
761:O
757:S
754:H
750:[
744:]
738:+
734:H
729:[
723:]
713:S
709:H
705:[
702:+
699:]
693:3
689:H
685:N
681:[
678:+
675:]
664:4
660:O
656:i
653:S
648:3
644:H
639:[
636:+
633:]
624:3
619:4
615:O
611:P
607:[
604:2
601:+
598:]
589:2
584:4
580:O
576:P
573:H
569:[
566:+
563:]
553:H
549:O
545:[
542:+
539:]
528:4
524:)
520:H
517:O
514:(
511:B
507:[
504:+
501:]
492:2
487:3
483:O
479:C
475:[
472:2
469:+
466:]
455:3
451:O
447:C
444:H
440:[
429:=
426:)
418:(
409:)
401:(
374:3
358:3
346:4
324:)
316:(
310:=
307:)
299:(
279:3
267:3
198:x
192:x
129:3
27:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.