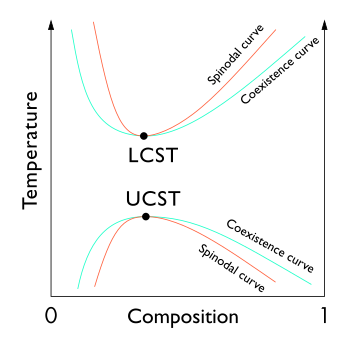234:
20:
59:
may coexist. Equivalently, it is the boundary between the set of conditions in which it is thermodynamically favorable for the system to be fully mixed and the set of conditions in which it is thermodynamically favorable for it to
130:
171:
32:
88:
In binary (two component) mixtures, the binodal can be determined at a given temperature by drawing a tangent line to the free energy.
438:
77:
508:
433:
164:
620:
448:
503:
248:
630:
110:
157:
655:
554:
184:
676:
549:
309:
574:
564:
314:
493:
253:
129:
Redhi, Gyanisavan
Govindsamy (2003). "5.7 Fitting Mathematical Equations to the Binodal Curve Data".
468:
360:
350:
263:
218:
615:
544:
378:
65:
645:
640:
610:
569:
458:
410:
395:
288:
258:
600:
223:
61:
590:
443:
180:
114:
388:
383:
340:
273:
268:
68:
of all solution components is equal in each phase. The extremum of a binodal curve in
56:
40:
670:
625:
605:
528:
488:
423:
355:
278:
650:
523:
518:
513:
478:
428:
345:
559:
453:
365:
233:
69:
498:
473:
400:
370:
304:
283:
107:
19:
149:
635:
463:
73:
24:
16:
Graphical curve that denotes a point at which two distinct phases coexist
483:
418:
335:
330:
204:
64:. In general, the binodal is defined by the condition at which the
23:
A phase diagram displaying binodal (coexistence) curves as well as
213:
199:
28:
153:
138:(Ph.D.). University of Natal, Durban, South Africa. p. 123
209:
132:
THERMODYNAMICS OF LIQUID MIXTURES CONTAINING CARBOXYLIC ACIDS
583:
537:
409:
323:
297:
241:
192:
55:, denotes the condition at which two distinct
165:
8:
172:
158:
150:
108:http://old.iupac.org/goldbook/BT07273.pdf
18:
102:
100:
96:
7:
14:
232:
106:IUPAC binodal curve definition
35:critical solution temperatures.
72:coincides with the one of the
1:
621:Macroscopic quantum phenomena
631:Order and disorder (physics)
693:
230:
656:Thermo-dielectric effect
555:Enthalpy of vaporization
249:Bose–Einstein condensate
76:curve and is known as a
62:separate into two phases
550:Enthalpy of sublimation
565:Latent internal energy
315:Color-glass condensate
36:
375:Magnetically ordered
22:
254:Fermionic condensate
47:, also known as the
469:Chemical ionization
361:Programmable matter
351:Quantum spin liquid
219:Supercritical fluid
616:Leidenfrost effect
545:Enthalpy of fusion
310:Quark–gluon plasma
113:2017-05-17 at the
66:chemical potential
37:
664:
663:
646:Superheated vapor
641:Superconductivity
611:Equation of state
459:Flash evaporation
411:Phase transitions
396:String-net liquid
289:Photonic molecule
259:Degenerate matter
49:coexistence curve
684:
601:Compressed fluid
236:
181:States of matter
174:
167:
160:
151:
147:
145:
143:
137:
118:
117:accessed 2/20/13
104:
692:
691:
687:
686:
685:
683:
682:
681:
667:
666:
665:
660:
591:Baryonic matter
579:
533:
504:Saturated fluid
444:Crystallization
405:
379:Antiferromagnet
319:
293:
237:
228:
188:
178:
141:
139:
135:
128:
126:
121:
115:Wayback Machine
105:
98:
94:
86:
17:
12:
11:
5:
690:
688:
680:
679:
677:Thermodynamics
669:
668:
662:
661:
659:
658:
653:
648:
643:
638:
633:
628:
623:
618:
613:
608:
603:
598:
593:
587:
585:
581:
580:
578:
577:
572:
570:Trouton's rule
567:
562:
557:
552:
547:
541:
539:
535:
534:
532:
531:
526:
521:
516:
511:
506:
501:
496:
491:
486:
481:
476:
471:
466:
461:
456:
451:
446:
441:
439:Critical point
436:
431:
426:
421:
415:
413:
407:
406:
404:
403:
398:
393:
392:
391:
386:
381:
373:
368:
363:
358:
353:
348:
343:
341:Liquid crystal
338:
333:
327:
325:
321:
320:
318:
317:
312:
307:
301:
299:
295:
294:
292:
291:
286:
281:
276:
274:Strange matter
271:
269:Rydberg matter
266:
261:
256:
251:
245:
243:
239:
238:
231:
229:
227:
226:
221:
216:
207:
202:
196:
194:
190:
189:
179:
177:
176:
169:
162:
154:
125:
124:External links
122:
120:
119:
95:
93:
90:
85:
84:Binary systems
82:
78:critical point
41:thermodynamics
15:
13:
10:
9:
6:
4:
3:
2:
689:
678:
675:
674:
672:
657:
654:
652:
649:
647:
644:
642:
639:
637:
634:
632:
629:
627:
626:Mpemba effect
624:
622:
619:
617:
614:
612:
609:
607:
606:Cooling curve
604:
602:
599:
597:
594:
592:
589:
588:
586:
582:
576:
573:
571:
568:
566:
563:
561:
558:
556:
553:
551:
548:
546:
543:
542:
540:
536:
530:
529:Vitrification
527:
525:
522:
520:
517:
515:
512:
510:
507:
505:
502:
500:
497:
495:
494:Recombination
492:
490:
489:Melting point
487:
485:
482:
480:
477:
475:
472:
470:
467:
465:
462:
460:
457:
455:
452:
450:
447:
445:
442:
440:
437:
435:
434:Critical line
432:
430:
427:
425:
424:Boiling point
422:
420:
417:
416:
414:
412:
408:
402:
399:
397:
394:
390:
387:
385:
382:
380:
377:
376:
374:
372:
369:
367:
364:
362:
359:
357:
356:Exotic matter
354:
352:
349:
347:
344:
342:
339:
337:
334:
332:
329:
328:
326:
322:
316:
313:
311:
308:
306:
303:
302:
300:
296:
290:
287:
285:
282:
280:
277:
275:
272:
270:
267:
265:
262:
260:
257:
255:
252:
250:
247:
246:
244:
240:
235:
225:
222:
220:
217:
215:
211:
208:
206:
203:
201:
198:
197:
195:
191:
186:
182:
175:
170:
168:
163:
161:
156:
155:
152:
148:
134:
133:
123:
116:
112:
109:
103:
101:
97:
91:
89:
83:
81:
79:
75:
71:
67:
63:
58:
54:
53:binodal curve
50:
46:
42:
34:
30:
26:
21:
651:Superheating
595:
524:Vaporization
519:Triple point
514:Supercooling
479:Lambda point
429:Condensation
346:Time crystal
324:Other states
264:Quantum Hall
140:. Retrieved
131:
127:
87:
52:
48:
44:
38:
33:upper (UCST)
29:lower (LCST)
27:curves, and
560:Latent heat
509:Sublimation
454:Evaporation
389:Ferromagnet
384:Ferrimagnet
366:Dark matter
298:High energy
70:temperature
575:Volatility
538:Quantities
499:Regelation
474:Ionization
449:Deposition
401:Superglass
371:Antimatter
305:QCD matter
284:Supersolid
279:Superfluid
242:Low energy
92:References
671:Category
636:Spinodal
584:Concepts
464:Freezing
111:Archived
74:spinodal
25:spinodal
596:Binodal
484:Melting
419:Boiling
336:Crystal
331:Colloid
142:22 July
45:binodal
224:Plasma
205:Liquid
57:phases
43:, the
214:Vapor
200:Solid
193:State
136:(PDF)
185:list
144:2018
31:and
210:Gas
51:or
39:In
673::
212:/
99:^
80:.
187:)
183:(
173:e
166:t
159:v
146:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.