45:
69:. The ground state of this molecule is bonding and the first excited state is antibonding. This means that when we plot the potential energy of the molecule (i.e. the average electrostatic energy of the two protons and the electron plus the kinetic energy of the latter) as the function of proton-proton separation, the ground state has a minimum but the excited state is repulsive (see Fig. 1a). Normally, the molecule is in the ground state, in one of the lowest vibrational levels (marked by horizontal lines).
170:. The proton TOF spectra revealed three peaks of kinetic energy spaced by a half of the photon energy. As the neutral H atom was taking the other half of the photon energy, this was an unambiguous confirmation of the bond softening process leading to the 1ω, 2ω and 3ω dissociation limits. Such a process which absorbs more than the minimum number of photons is known as above-threshold dissociation.
125:
The top arrow represents one photon absorption, which is a continuous process. In the region of the anticrossing the molecule is in a superposition of the ground and the excited states, continuously exchanging energy with the laser field. As the internuclear separation increases, the molecule absorbs
85:
At high laser intensity absorptions and stimulated emissions of photons are so frequent that the molecule cannot be regarded as a system separate from the laser field; the molecule is "dressed" in photons forming a single system. However, the number of photons in this system varies when photons are
72:
In the presence of light, the molecule may absorb a photon (violet arrow), provided its frequency matches the energy difference between the ground and the excited states. The excited state is unstable and the molecule dissociates within femtoseconds into hydrogen atom and a proton releasing kinetic
23:
by strong laser fields. To make this effect significant, the strength of the electric field in the laser light has to be comparable with the electric field the bonding electron "feels" from the nuclei of the molecule. Such fields are typically in the range of 1–10 V/Å, which corresponds to laser
89:
In the dressed model, photon absorption (and emission) is no longer represented by vertical transitions. As the energy must be conserved, photon absorption occurs at the curve crossings. For example, if the molecule is in the ground electronic state with 10 photons present, it can jump to the
133:
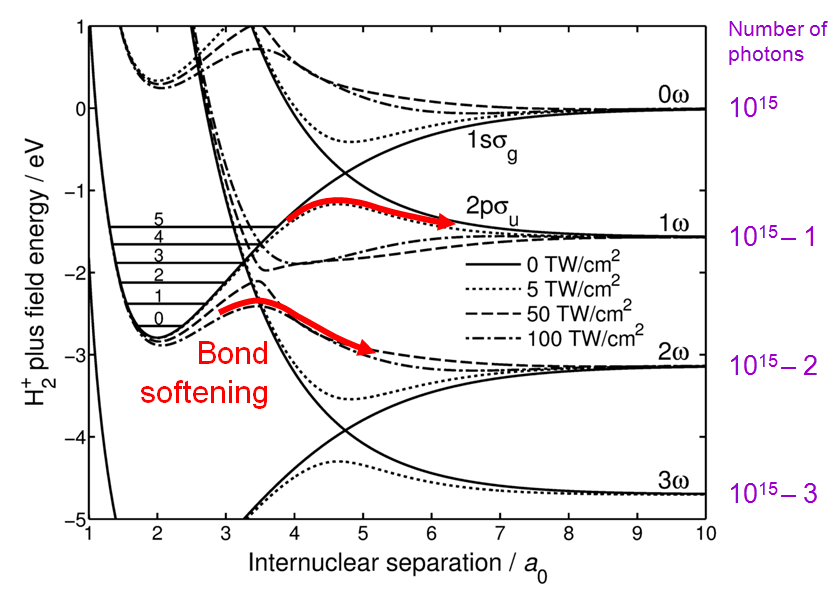
The bottom arrow represents a process initiated at the 3-photon gap. As the system passes through this gap, the 1-photon gap is wide open and the system slides along the top branch of the 1-photon anticrossing. The molecule dissociates to the 2ω limit via absorption of 3 photons followed by
73:
energy (red arrow). This is the usual description of photon absorption, which works well at low intensity. At high intensity, however, the interaction of the light with the molecule is so strong that the potential energy curves become distorted. To take this distortion into account requires
165:
was used to generate intense pulses of about 80 ps duration at the second harmonic of 532 nm. In a vacuum chamber, the pulses were focused on molecular hydrogen under low pressure (about 10 mbar) inducing ionization and dissociation. The kinetic energy of protons was measured in a
86:
absorbed and emitted. Therefore, to plot the energy diagram of the dressed molecule, we need to repeat the energy curves at each number of photons. The number of photons is very large but only a few curve repetitions need to be considered in this very tall ladder, as shown in Fig. 1b.
40:
Theoretical description of bond softening can be traced back to early work on dissociation of diatomic molecules in intense laser fields. While the quantitative description of this process requires quantum mechanics, it can be understood qualitatively using quite simple models.
141:, i.e. they are accurate only for infinitely slow transitions. When the dissociation is fast and the gap is small, a diabatic transition may occur where the system ends up on the other branch of the anticrossing. The probability of such a transition is described by the
51:
At low intensity (a) it is convenient to plot molecular energy curves and indicate photon transitions with vertical arrows. At high intensity (b) it is more appropriate to "dress" the molecular curves in photons and consider photon transitions at the curve
440:
Natan, Adi; Ware, Matthew R.; Prabhudesai, Vaibhav S.; Lev, Uri; Bruner, Barry D.; Heber, Oded; Bucksbaum, Philip H. (2016). "Observation of
Quantum Interferences via Light-Induced Conical Intersections in Diatomic Molecules".
109:
When strong laser field perturbs the molecule, its energy levels are no longer the same as in the absence of the field. To calculate the new energy levels, the perturbation must be included as off-diagonal elements of the
90:
repulsive state absorbing a photon at the curve crossing (violet circle) and dissociate to the 10-1 photon limit (red arrow). This "curve jumping" is in fact continuous and can be explained in terms of avoided crossings.
122:
and the higher the laser intensity, the larger the gap of the anticrossing as shown in Fig. 2. The molecule can dissociate along the lower branch of the anticrossings as indicated by the red arrows.
100:
Curve crossings become anticrossings, which induces bond softening. The distorted curves have been calculated from undistorted ones in Matlab using
Hamiltonian diagonalisation.
94:
173:
A comprehensive review puts the mechanism of bond softening in a broader research context. Anticrossings of diatomic energy curves have many similarities to the
167:
134:
re-emission of 1 photon. (One-step even-photon absorptions and emissions are forbidden by the symmetry of the system.)
93:
514:
509:
194:
Bandrauk, André D.; Sink, Michael L. (1981). "Photodissociation in intense laser fields: Predissociation analogy".
142:
44:
519:
145:. When applied to the dissociation through the 3-photon gap, the formula gives a small probability of the H
126:
energy and the electronic wavefunction evolves to the antibonding state on the femtosecond timescale. The H
115:
264:
Giusti-Suzor, A.; Mies, F.H.; DiMauro, L.F.; Charron, E.; Yang, B. (1995). "Topical review: Dynamics of H
111:
350:
Zavriyev, A.; Bucksbaum, P.H.; Squier, J.; Saline, F. (1993). "Light-Induced
Vibrational Structure in H
460:
414:
367:
316:
277:
238:
203:
174:
158:
98:
Figure 2: Distortion of molecular energy curves dressed in photons for increasing laser intensity.
484:
450:
476:
383:
332:
138:
62:
29:
468:
422:
375:
324:
285:
246:
211:
119:
303:
Bucksbaum, P.H.; Zavriyev, A.; Muller, H.G.; Schumacher, D.W. (1990). "Softening of the H
464:
418:
371:
320:
281:
242:
207:
74:
426:
250:
503:
289:
20:
488:
472:
229:
Sharp, T.E. (1971). "Potential-energy curves for molecular hydrogen and its ions".
162:
149:
molecular ion ending up in the 3ω dissociation limit without emitting any photons.
379:
328:
28:
10–10 W/cm. Nowadays, these intensities are routinely achievable from table-top
25:
49:
Figure 1: Two theoretical models of a molecule interacting with laser field.
480:
387:
336:
215:
402:
455:
92:
403:"Atomic and Molecular Dynamics in Intense Optical Fields"
161:
in 1990 at the time of its experimental observation. A
61:Consider the simplest diatomic molecule, the
8:
177:of energy surfaces in polyatomic molecules.
19:is an effect of reducing the strength of a
157:The "bond softening" phrase was coined by
454:
307:molecular bond in intense laser fields".
43:
186:
81:Dressing in photons at high intensity
7:
401:Sheehy, B.; DiMauro, L. F. (1996).
75:"dressing" the molecule in photons
14:
427:10.1146/annurev.physchem.47.1.463
168:time-of-flight (TOF) spectrometer
130:ion dissociates to the 1ω limit.
118:. In consequence, the crossings
473:10.1103/PhysRevLett.116.143004
1:
251:10.1016/s0092-640x(70)80007-9
137:The anticrossing curves are
380:10.1103/PhysRevLett.70.1077
329:10.1103/physrevlett.64.1883
536:
358:in Intense Laser Fields".
290:10.1088/0953-4075/28/3/006
268:in intense laser fields".
153:Experimental confirmation
57:Low-intensity description
443:Physical Review Letters
120:turn into anticrossings
105:Energy curve distortion
101:
53:
407:Annu. Rev. Phys. Chem
175:conical intersections
96:
47:
143:Landau–Zener formula
465:2016PhRvL.116n3004N
419:1996ARPC...47..463S
372:1993PhRvL..70.1077Z
321:1990PhRvL..64.1883B
282:1995JPhB...28..309G
243:1971AD......2..119S
208:1981JChPh..74.1110B
114:, which has to be
102:
54:
30:Ti:Sapphire lasers
515:Quantum chemistry
510:Molecular physics
315:(16): 1883–1886.
527:
494:
492:
458:
437:
431:
430:
398:
392:
391:
366:(8): 1077–1080.
347:
341:
340:
300:
294:
293:
261:
255:
254:
226:
220:
219:
216:10.1063/1.441217
191:
535:
534:
530:
529:
528:
526:
525:
524:
500:
499:
498:
497:
439:
438:
434:
400:
399:
395:
360:Phys. Rev. Lett
357:
353:
349:
348:
344:
309:Phys. Rev. Lett
306:
302:
301:
297:
267:
263:
262:
258:
228:
227:
223:
193:
192:
188:
183:
155:
148:
129:
107:
83:
66:
59:
38:
12:
11:
5:
533:
531:
523:
522:
520:Photochemistry
517:
512:
502:
501:
496:
495:
449:(14): 143004.
432:
393:
355:
351:
342:
304:
295:
276:(3): 309–339.
265:
256:
221:
185:
184:
182:
179:
159:Phil Bucksbaum
154:
151:
146:
127:
106:
103:
82:
79:
64:
58:
55:
37:
34:
17:Bond softening
13:
10:
9:
6:
4:
3:
2:
532:
521:
518:
516:
513:
511:
508:
507:
505:
490:
486:
482:
478:
474:
470:
466:
462:
457:
452:
448:
444:
436:
433:
428:
424:
420:
416:
412:
408:
404:
397:
394:
389:
385:
381:
377:
373:
369:
365:
361:
346:
343:
338:
334:
330:
326:
322:
318:
314:
310:
299:
296:
291:
287:
283:
279:
275:
271:
260:
257:
252:
248:
244:
240:
236:
232:
225:
222:
217:
213:
209:
205:
201:
197:
196:J. Chem. Phys
190:
187:
180:
178:
176:
171:
169:
164:
160:
152:
150:
144:
140:
135:
131:
123:
121:
117:
113:
104:
99:
95:
91:
87:
80:
78:
76:
70:
68:
56:
50:
46:
42:
35:
33:
31:
27:
22:
21:chemical bond
18:
446:
442:
435:
410:
406:
396:
363:
359:
345:
312:
308:
298:
273:
269:
259:
234:
230:
224:
199:
195:
189:
172:
163:Nd:YAG laser
156:
136:
132:
124:
116:diagonalised
108:
97:
88:
84:
71:
60:
48:
39:
16:
15:
413:: 463–494.
237:: 119–169.
231:Atomic Data
202:(2): 1110.
112:Hamiltonian
26:intensities
504:Categories
456:1511.05626
270:J. Phys. B
181:References
52:crossings.
139:adiabatic
481:27104704
388:10054280
337:10041519
489:1710720
461:Bibcode
415:Bibcode
368:Bibcode
317:Bibcode
278:Bibcode
239:Bibcode
204:Bibcode
487:
479:
386:
335:
36:Theory
485:S2CID
451:arXiv
354:and D
493:>
477:PMID
384:PMID
333:PMID
469:doi
447:116
423:doi
376:doi
325:doi
286:doi
247:doi
212:doi
67:ion
506::
483:.
475:.
467:.
459:.
445:.
421:.
411:47
409:.
405:.
382:.
374:.
364:70
362:.
331:.
323:.
313:64
311:.
284:.
274:28
272:.
245:.
233:.
210:.
200:74
198:.
77:.
32:.
491:.
471::
463::
453::
429:.
425::
417::
390:.
378::
370::
356:2
352:2
339:.
327::
319::
305:2
292:.
288::
280::
266:2
253:.
249::
241::
235:2
218:.
214::
206::
147:2
128:2
65:2
63:H
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.