122:
213:. Glucosylceramide is a major constituent of skin lipids, where it is essential for lamellar body formation in the stratum corneum and to maintain the water permeability barrier of the skin. Glucosylceramide is the only glycosphingolipid common to plants, fungi and animals. It is usually considered to be the principal glycosphingolipid in plants. It is a major component of the outer layer of the plasma membrane. Galactosylceramides have not been found in plants.
250:
sugar and the hydroxy and amide groups of the sphingosine base of the ceramide. These hydrogen bonds within the cerebrosides result in the molecules having a high transition temperature and compact alignment. Monoglycosylceramides in conjunction with cholesterol are prevalent in the lipid-raft micro domain, which are important sites in the binding of proteins, and enzyme-receptor interactions.
25:
249:
The melting point of cerebrosides is considerably greater than physiological body temperature, >37.0 °C, giving glycolipids a paracrystalline, similar to liquid crystal structure. Cerebroside molecules are able form up to eight intermolecular hydrogen bonds between the polar hydrogens of the
240:
The biosynthesis of monoglycosylceramides requires a direct transfer of the carbohydrate moiety from a sugar-nucleotide, such as uridine 5-diphosphate(UDP)-galactose, or UDP-glucose to the ceramide unit. The glycosyl-transferase catalyzed reaction results in an inversion of the glycosidic bond
267:
Analysis of monoglycosylceramides can be done by high-resolution thin-layer chromatography, high-performance liquid chromatography (HPLC), and mass spectrometry. Reversed-phase HPLC is now the standard method for separation of molecular species, often after benzoylation, enabling lipids to be
196:
Galactosylceramide is the principal glycosphingolipid in brain tissue. Galactosylceramides are present in all nervous tissues, and can compose up to 2% dry weight of grey matter and 12% of white matter. They are major constituents of
258:
Degradation of glycosphingolipids occurs in the lysosome, which contains digestive enzymes in animal cells. The lysosome breaks down the glycosphingolipid to its primary components, fatty acids, sphingosine, and saccharide.
241:
stereochemistry, changing from α →β. Synthesis of galactosylceramide, and glucosylceramide occurs on the lumenal surface of the endoplasmic reticulum, and on the cytosolic side of the early Golgi membranes respectively.
228:
formation or remyelination. The sugar moiety is linked glycosidically to the C-1 hydroxyl group of ceramide, such as in lactosylceramide. Cerebrosides containing a sulfuric ester (
465:
232:) group, known as sulfatides, also occur in the myelin sheath of nerves. These compounds are preferably named as sulfates of the parent glycosphingolipid.
193:
bonded glycosidically to the terminal OH group of ceramide are defined as cerebrosides. Sphingosine is the main long-chain base present in ceramide.
487:
177:(a.k.a. galactosylceramides). Galactocerebrosides are typically found in neural tissue, while glucocerebrosides are found in other tissues.
108:
460:
46:
352:
Yanagihara, T; Cumings, JN (1969). "Fatty acid composition of cerebrosides and cerebroside sulphatides in cerebral oedema".
89:
61:
42:
480:
313:"Cerebroside synthesis as a measure of the rate of remyelination following cuprizone-induced demyelination in brain"
68:
35:
454:
75:
690:
516:
473:
613:
421:
57:
287:. Clinical features include acroparaesthesia (tingling, pins and needles sensation in the extremities)
711:
277:
311:
Jurevics, H; Hostettler, J; Muse, ED; Sammond, DW; Matsushima, GK; Toews, AD; Morell, P (May 2001).
121:
651:
537:
377:
174:
284:
584:
512:
369:
334:
140:
632:
549:
542:
361:
324:
198:
170:
82:
450:
496:
291:
190:
161:
with a single sugar residue at the 1-hydroxyl moiety. The sugar residue can be either
705:
624:
329:
312:
217:
151:
508:
396:
206:
126:
381:
662:
571:
559:
24:
504:
643:
605:
166:
338:
373:
670:
524:
186:
158:
365:
229:
210:
162:
201:. Glucosylceramide is found at low levels in animal cells such as the
225:
221:
202:
148:
144:
500:
469:
283:
a) Ceramide trihexoside (globotriaosylceramide) accumulation –
594:
589:
579:
216:
Monogalactosylceramide is the largest single component of the
18:
290:
b) Galactocerebroside (galactosylceramidase) accumulation –
224:. Cerebroside synthesis can therefore give a measurement of
189:. Monoglycosyl and oligoglycosylceramides having a mono or
280:. The corresponding defects for galactocerebrosides are:
426:
National
Institute of Neurological Disorders and Stroke
276:
A defect in the degradation of glucocerebrosides is
683:
660:
641:
622:
603:
569:
558:
523:
49:. Unsourced material may be challenged and removed.
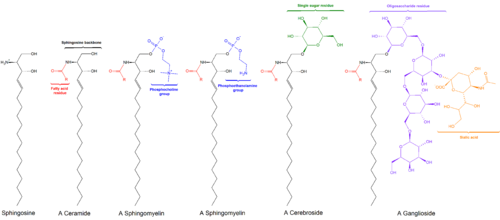
185:The fundamental structure of a cerebroside is
481:
8:
169:; the two major types are therefore called
566:
488:
474:
466:
453:at the U.S. National Library of Medicine
328:
143:which are important components of animal
109:Learn how and when to remove this message
461:The Lipid Library: Monoglycosylceramides
120:
303:
7:
47:adding citations to reliable sources
268:detected by UV spectrophotometry.
209:, and nervous tissues, especially
14:
395:Harvey, James (18 January 2021).
330:10.1046/j.1471-4159.2001.00310.x
23:
428:. National Institutes of Health
173:(a.k.a. glucosylceramides) and
34:needs additional citations for
1:
728:
317:Journal of Neurochemistry
455:Medical Subject Headings
691:Sphingosine-1-phosphate
517:metabolic intermediates
129:
125:General structures of
614:Globotriaosylceramide
354:Acta Neuropathologica
137:monoglycosylceramides
124:
43:improve this article
16:Lipid classification
245:Physical properties
175:galactocerebrosides
652:Galactocerebroside
538:Galactocerebroside
513:glycosphingolipids
366:10.1007/BF00685311
157:They consist of a
141:glycosphingolipids
130:
699:
698:
679:
678:
397:"Acroparesthesia"
278:Gaucher's disease
263:Chemical analysis
171:glucocerebrosides
139:) are a group of
119:
118:
111:
93:
719:
633:Glucocerebroside
567:
550:Lactosylceramide
543:Glucocerebroside
490:
483:
476:
467:
438:
437:
435:
433:
422:"Krabbe Disease"
418:
412:
411:
409:
407:
392:
386:
385:
349:
343:
342:
332:
308:
199:oligodendrocytes
114:
107:
103:
100:
94:
92:
51:
27:
19:
727:
726:
722:
721:
720:
718:
717:
716:
702:
701:
700:
695:
675:
656:
637:
618:
599:
562:
554:
519:
497:Glycoconjugates
494:
447:
442:
441:
431:
429:
420:
419:
415:
405:
403:
394:
393:
389:
351:
350:
346:
310:
309:
305:
300:
285:Fabry's disease
274:
272:Role in disease
265:
256:
247:
238:
183:
115:
104:
98:
95:
52:
50:
40:
28:
17:
12:
11:
5:
725:
723:
715:
714:
704:
703:
697:
696:
694:
693:
687:
685:
681:
680:
677:
676:
674:
673:
667:
665:
658:
657:
655:
654:
648:
646:
639:
638:
636:
635:
629:
627:
620:
619:
617:
616:
610:
608:
601:
600:
598:
597:
592:
587:
582:
576:
574:
564:
556:
555:
553:
552:
547:
546:
545:
540:
529:
527:
521:
520:
495:
493:
492:
485:
478:
470:
464:
463:
458:
446:
445:External links
443:
440:
439:
413:
387:
344:
323:(4): 1067–76.
302:
301:
299:
296:
292:Krabbe disease
273:
270:
264:
261:
255:
252:
246:
243:
237:
234:
191:polysaccharide
182:
179:
117:
116:
31:
29:
22:
15:
13:
10:
9:
6:
4:
3:
2:
724:
713:
710:
709:
707:
692:
689:
688:
686:
682:
672:
669:
668:
666:
664:
659:
653:
650:
649:
647:
645:
640:
634:
631:
630:
628:
626:
625:sphingomyelin
621:
615:
612:
611:
609:
607:
602:
596:
593:
591:
588:
586:
583:
581:
578:
577:
575:
573:
568:
565:
561:
557:
551:
548:
544:
541:
539:
536:
535:
534:
531:
530:
528:
526:
522:
518:
514:
510:
509:sphingolipids
506:
502:
498:
491:
486:
484:
479:
477:
472:
471:
468:
462:
459:
456:
452:
449:
448:
444:
427:
423:
417:
414:
402:
398:
391:
388:
383:
379:
375:
371:
367:
363:
359:
355:
348:
345:
340:
336:
331:
326:
322:
318:
314:
307:
304:
297:
295:
293:
288:
286:
281:
279:
271:
269:
262:
260:
253:
251:
244:
242:
235:
233:
231:
227:
223:
219:
218:myelin sheath
214:
212:
208:
204:
200:
194:
192:
188:
180:
178:
176:
172:
168:
164:
160:
155:
153:
150:
146:
142:
138:
134:
128:
127:sphingolipids
123:
113:
110:
102:
91:
88:
84:
81:
77:
74:
70:
67:
63:
60: –
59:
58:"Cerebroside"
55:
54:Find sources:
48:
44:
38:
37:
32:This article
30:
26:
21:
20:
532:
451:Cerebrosides
430:. Retrieved
425:
416:
404:. Retrieved
400:
390:
360:(1): 62–67.
357:
353:
347:
320:
316:
306:
289:
282:
275:
266:
257:
248:
239:
215:
207:erythrocytes
195:
184:
156:
136:
133:Cerebrosides
132:
131:
105:
96:
86:
79:
72:
65:
53:
41:Please help
36:verification
33:
712:Glycolipids
663:sphingosine
572:ganglioside
560:Ganglioside
533:Cerebroside
505:glycolipids
401:Radiopaedia
298:References
254:Catabolism
149:nerve cell
69:newspapers
644:sulfatide
606:globoside
236:Synthesis
181:Structure
167:galactose
152:membranes
706:Category
671:Ceramide
525:Ceramide
432:3 August
406:3 August
339:11359872
187:ceramide
159:ceramide
99:May 2014
563:pathway
374:4303520
230:sulfate
211:neurons
163:glucose
83:scholar
515:, and
503:, and
501:lipids
457:(MeSH)
382:244169
380:
372:
337:
226:myelin
222:nerves
203:spleen
145:muscle
85:
78:
71:
64:
56:
684:Other
642:From
623:From
604:From
570:From
378:S2CID
90:JSTOR
76:books
511:and
434:2023
408:2023
370:PMID
335:PMID
147:and
62:news
661:To
595:GD2
590:GM3
585:GM2
580:GM1
362:doi
325:doi
220:of
165:or
45:by
708::
507::
499:,
424:.
399:.
376:.
368:.
358:12
356:.
333:.
321:77
319:.
315:.
294:.
205:,
154:.
489:e
482:t
475:v
436:.
410:.
384:.
364::
341:.
327::
135:(
112:)
106:(
101:)
97:(
87:·
80:·
73:·
66:·
39:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.