84:
198:
57:
112:
The term is also used to describe a situation where an enantiomeric excess lies far below the observational horizon, but is still relevant, e.g. in highly enantiosensitive, self-amplifying reactions.
239:
32:
is non-measurable. The underlying reason for the lack of rotation is the specific electronic properties of the molecule. The term was introduced by
232:
83:
56:
263:
225:
128:
Chiral
Discrimination of Cryptochiral Saturated Quaternary and Tertiary Hydrocarbons by Asymmetric Autocatalysis
258:
25:
65:
77:
45:
162:
131:
102:
50:
29:
209:
76:
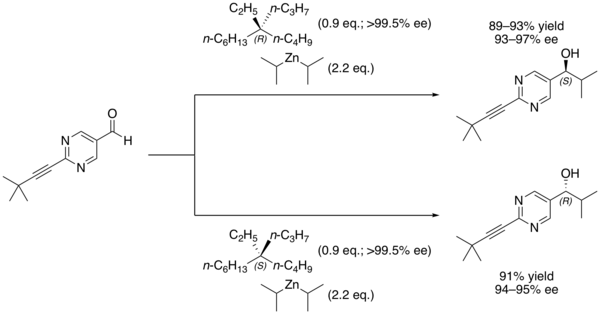
performed in the presence of 5-ethyl-5-propylundecane forms a secondary alcohol with a high
139:
205:
73:
17:
252:
91:
69:
197:
68:
of another chemical whose stereochemical nature can be measured. For example, the
64:
It is still possible to distinguish between the two enantiomers by using them in
90:
Even a slight enantiomeric excess of the alkane is rapidly amplified due to the
33:
106:
130:
Kawasaki, T.; Tanaka, H.; Tsutsumi, T.; Kasahara, T.; Sato, I.; Soai, K.
157:
Struijk, MP Peerlings, HWI Meijer, EW Polymer
Preprints 37(2), 497–498 (
181:
98:
143:
53:, but neither enantiomeric form has any observable optical rotation:
40:
109:
having lobes of different sizes attached to a central core.
82:
72:
of 2-(3,3-dimethylbut-1-ynyl)pyrimidine-5-carbaldehyde with
55:
80:
based on the major enantiomer of the alkane that was used.
213:
43:
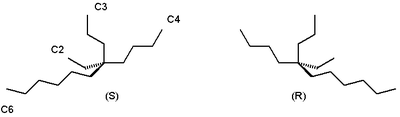
5-ethyl-5-propylundecane found in certain species of
233:
176:, Kurt Mislow, Collect. Czech. Chem. Commun.
8:
174:Absolute Asymmetric Synthesis: A Commentary
240:
226:
120:
60:Enantiomers of 5-ethyl-5-propylundecane
28:in which a molecule is chiral but its
7:
194:
192:
182:https://doi.org/10.1135/cccc20030849
212:. You can help Knowledge (XXG) by
14:
196:
97:Cryptochirality also occurs in
155:Cryptochirality and dendrimers
1:
101:systems growing from chiral
280:
191:
94:nature of this reaction.
49:is chiral at its central
138:; 128(18); 6032–6033.
87:
61:
264:Stereochemistry stubs
86:
59:
24:is a special case of
66:asymmetric synthesis
78:enantiomeric excess
88:
62:
46:Phaseolus vulgaris
221:
220:
144:10.1021/ja061429e
132:J. Am. Chem. Soc.
105:, for example in
51:quaternary carbon
39:For example, the
30:specific rotation
271:
242:
235:
228:
200:
193:
184:
171:
165:
152:
146:
125:
279:
278:
274:
273:
272:
270:
269:
268:
259:Stereochemistry
249:
248:
247:
246:
206:stereochemistry
189:
187:
180:, 68, 849-864,
172:
168:
153:
149:
126:
122:
118:
74:diisopropylzinc
22:cryptochirality
18:stereochemistry
12:
11:
5:
277:
275:
267:
266:
261:
251:
250:
245:
244:
237:
230:
222:
219:
218:
201:
186:
185:
166:
147:
119:
117:
114:
13:
10:
9:
6:
4:
3:
2:
276:
265:
262:
260:
257:
256:
254:
243:
238:
236:
231:
229:
224:
223:
217:
215:
211:
208:article is a
207:
202:
199:
195:
190:
183:
179:
175:
170:
167:
164:
160:
156:
151:
148:
145:
141:
137:
133:
129:
124:
121:
115:
113:
110:
108:
104:
100:
95:
93:
92:autocatalytic
85:
81:
79:
75:
71:
70:Soai reaction
67:
58:
54:
52:
48:
47:
42:
37:
35:
31:
27:
23:
19:
214:expanding it
203:
188:
177:
173:
169:
158:
154:
150:
135:
127:
123:
111:
96:
89:
63:
44:
38:
21:
15:
34:Kurt Mislow
253:Categories
116:References
107:dendrimers
103:initiators
99:polymeric
36:in 1977.
26:chirality
163:Article
41:alkane
204:This
210:stub
178:2003
159:1996
136:2006
140:doi
16:In
255::
161:)
134:;
20:,
241:e
234:t
227:v
216:.
142::
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.