278:
135:
126:
22:
483:
644:
The formation of dicoronylene in hydrocracking reactors is a serious problem because its low solubility make it precipitate in any cooler part of the reactor flow path. This causes plugging of flow lines that require periodic shutdown and removal of the reddish deposits. Dicoronylene is also a
199:
700:
Goddard, Richard; Haenel, Matthias W.; Herndon, William C.; Krueger, Carl; Zander, Maximilian (1995). "Crystallization of Large Planar
Polycyclic Aromatic Hydrocarbons: The Molecular and Crystal Structures of Hexabenzo[bc,ef,hi,kl,no,qr]coronene and
614:
molecules fuse. It is estimated that catalytical hydrocracking produces several hundred metric tons of dicoronylene worldwide per year, making it the most prevalent large PAH. In this process, the analogous 18-ring PAH formed from coronene and ovalene
301:
InChI=1S/C48H20/c1-5-23-9-13-27-17-31-33-19-29-15-11-25-7-3-22-4-8-26-12-16-30-20-34(46(33)48-43(29)39(25)36(22)40(26)44(30)48)32-18-28-14-10-24-6-2-21(1)35-37(23)41(27)47(45(31)32)42(28)38(24)35/h1-20H
311:
InChI=1/C48H20/c1-5-23-9-13-27-17-31-33-19-29-15-11-25-7-3-22-4-8-26-12-16-30-20-34(46(33)48-43(29)39(25)36(22)40(26)44(30)48)32-18-28-14-10-24-6-2-21(1)35-37(23)41(27)47(45(31)32)42(28)38(24)35/h1-20H
599:. After these were extracted and identified, a reddish residue remained, which was sparingly soluble in organic solvents. Elemental analysis indicated that it was most likely the condensed dimer of
496:
678:
Dicoronylene has been studied as a model for interstellar PAHs. Its large size and planarity have also shown promise as a chromatographic separation material.
327:
1177:
675:. Unlike coronene, dicoronylene has symmetrical fluorescence excitation and emission spectra. It is virtually insoluble in most solvents.
292:
105:
559:
Due to its large size and limited availability, the organic chemistry of dicoronylene is little known. Dicoronylene does undergo a
770:
523:
761:
43:
86:
39:
503:
58:
1079:
256:
65:
32:
560:
335:
C1=CC2=C3C4=C1C=CC5=C4C6=C(C=C5)C=C7C8=CC9=C1C4=C(C=CC5=C4C4=C(C=C5)C=CC5=CC(=C8C1=C54)C1=C7C6=C3C(=C1)C=C2)C=C9
912:
72:
572:
668:
54:
973:
968:
958:
953:
948:
943:
866:
147:
273:
896:
165:
1014:
754:
575:
on the ends of the bay region, making a new six-membered ring. Heating removes the anhydride as
1075:
851:
1065:
861:
856:
710:
564:
428:
350:
219:
175:
79:
277:
1115:
1110:
1024:
993:
927:
922:
815:
646:
576:
474:
125:
1171:
747:
661:
660:
shows masses of dicoronylene and the condensed trimer, tetramer, and pentamer in the
607:
134:
1009:
891:
876:
835:
810:
739:
672:
588:
567:
on one or both of the central bay regions on either side of the bridging ring. The
519:
456:
244:
1034:
1019:
917:
794:
568:
409:
21:
963:
932:
820:
415:
372:
210:
1156:
1146:
1141:
1070:
978:
886:
830:
664:
of the black product. These larger coronene condensates are black in color.
653:
606:
Dicoronylene was later discovered to occur as a by-product of the catalytic
1151:
1105:
1095:
1085:
1055:
1039:
1029:
983:
871:
825:
784:
657:
611:
600:
592:
730:
The
Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons
714:
1120:
1100:
1090:
789:
596:
393:
231:
587:
Dicoronylene was first observed in the solid residue produced in coal
988:
881:
473:
Except where otherwise noted, data are given for materials in their
1136:
636:) is also formed in 1% to 20% proportions. It is purple in color.
198:
188:
649:
formed on hydrocracking catalysts, which reduces their activity.
548:
743:
15:
449:α = 90°, β = 90.24(1)°, γ = 90°
261:
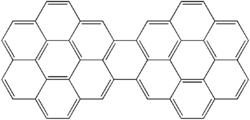
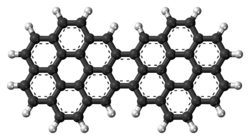
526:. It has 15 rings and is a brick-red solid. Its formula is
579:
gas and gives the corresponding 16-ring and 17-ring PAHs.
491:
610:
used in petroleum processing. It is formed when two
1129:
1048:
1002:
905:
844:
803:
777:
46:. Unsourced material may be challenged and removed.
547:. Dicoronylene sublimes under high vacuum, 0.001
243:
701:Benzo[1,2,3-bc:4,5,6-b'c']dicoronene".
174:
755:
8:
671:and these solutions have a greenish yellow
762:
748:
740:
591:. This residue contained large amounts of
276:
218:
117:
106:Learn how and when to remove this message
703:Journal of the American Chemical Society
687:
332:
297:
272:
667:Dicoronylene is moderately soluble in
695:
693:
691:
304:Key: QOPWDVCEMZLGPK-UHFFFAOYSA-N
7:
44:adding citations to reliable sources
314:Key: QOPWDVCEMZLGPK-UHFFFAOYAN
234:
14:
1178:Polycyclic aromatic hydrocarbons
771:Polycyclic aromatic hydrocarbons
481:
362:
133:
124:
20:
524:polycyclic aromatic hydrocarbon
477:(at 25 °C , 100 kPa).
31:needs additional citations for
571:of maleic anhydride forms two
551:, between 250 °C and 300 °C.
356:
1:
442: = 0.3832(1) nm,
438: = 1.0376(1) nm,
1194:
471:
446: = 3.1914(3) nm
402:
343:
323:
288:
158:
146:
141:
132:
123:
937:-Benzopyrene(Olympicene)
728:Fetzer, J. C. (2000).
669:1,2,4-trichlorobenzene
913:Benzacephenanthrylene
561:Diels–Alder reaction
148:Preferred IUPAC name
40:improve this article
897:Tricyclobutabenzene
715:10.1021/ja00106a004
573:carbon–carbon bonds
380: g·mol
120:
732:. New York: Wiley.
504:Infobox references
398:1.57 g/cm (calc.)
118:
1165:
1164:
1076:Hexabenzocoronene
959:Benzofluoranthene
954:Benzofluoranthene
949:Benzofluoranthene
944:Benzofluoranthene
867:Benzophenanthrene
522:for a very large
512:Chemical compound
510:
509:
257:CompTox Dashboard
200:Interactive image
116:
115:
108:
90:
1185:
1066:Diindenoperylene
974:Dibenzanthracene
969:Dibenzanthracene
964:trans-Bicalicene
764:
757:
750:
741:
734:
733:
725:
719:
718:
697:
635:
634:
633:
625:
624:
565:maleic anhydride
546:
545:
544:
536:
535:
494:
488:
485:
484:
429:Lattice constant
379:
364:
358:
351:Chemical formula
281:
280:
265:
263:
247:
236:
222:
202:
178:
137:
128:
121:
111:
104:
100:
97:
91:
89:
48:
24:
16:
1193:
1192:
1188:
1187:
1186:
1184:
1183:
1182:
1168:
1167:
1166:
1161:
1130:General classes
1125:
1044:
998:
901:
840:
799:
773:
768:
738:
737:
727:
726:
722:
699:
698:
689:
684:
645:constituent of
642:
632:
629:
628:
627:
623:
620:
619:
618:
616:
585:
557:
543:
540:
539:
538:
534:
531:
530:
529:
527:
513:
506:
501:
500:
499: ?)
490:
486:
482:
478:
464:
450:
447:
431:
421:
412:
377:
367:
361:
353:
339:
336:
331:
330:
319:
316:
315:
312:
306:
305:
302:
296:
295:
284:
266:
259:
250:
237:
225:
205:
192:
181:
168:
154:
153:
152:Benzodicoronene
112:
101:
95:
92:
49:
47:
37:
25:
12:
11:
5:
1191:
1189:
1181:
1180:
1170:
1169:
1163:
1162:
1160:
1159:
1154:
1149:
1144:
1139:
1133:
1131:
1127:
1126:
1124:
1123:
1118:
1116:Trinaphthylene
1113:
1111:Superphenalene
1108:
1103:
1098:
1093:
1088:
1083:
1073:
1068:
1063:
1058:
1052:
1050:
1046:
1045:
1043:
1042:
1037:
1032:
1027:
1025:Dibenzopyrenes
1022:
1017:
1012:
1006:
1004:
1000:
999:
997:
996:
994:Tetraphenylene
991:
986:
981:
976:
971:
966:
961:
956:
951:
946:
941:
940:
939:
930:
925:
915:
909:
907:
903:
902:
900:
899:
894:
889:
884:
879:
874:
869:
864:
859:
854:
852:Benzanthracene
848:
846:
842:
841:
839:
838:
833:
828:
823:
818:
816:Acenaphthylene
813:
807:
805:
801:
800:
798:
797:
792:
787:
781:
779:
775:
774:
769:
767:
766:
759:
752:
744:
736:
735:
720:
686:
685:
683:
680:
641:
638:
630:
621:
584:
581:
577:carbon dioxide
556:
553:
541:
532:
511:
508:
507:
502:
480:
479:
475:standard state
472:
469:
468:
465:
455:
452:
451:
448:
434:
432:
427:
424:
423:
419:
413:
408:
405:
404:
400:
399:
396:
390:
389:
386:
382:
381:
375:
369:
368:
365:
359:
354:
349:
346:
345:
341:
340:
338:
337:
334:
326:
325:
324:
321:
320:
318:
317:
313:
310:
309:
307:
303:
300:
299:
291:
290:
289:
286:
285:
283:
282:
274:DTXSID40348352
269:
267:
255:
252:
251:
249:
248:
240:
238:
230:
227:
226:
224:
223:
215:
213:
207:
206:
204:
203:
195:
193:
186:
183:
182:
180:
179:
171:
169:
164:
161:
160:
156:
155:
151:
150:
144:
143:
139:
138:
130:
129:
114:
113:
55:"Dicoronylene"
28:
26:
19:
13:
10:
9:
6:
4:
3:
2:
1190:
1179:
1176:
1175:
1173:
1158:
1155:
1153:
1150:
1148:
1145:
1143:
1140:
1138:
1135:
1134:
1132:
1128:
1122:
1119:
1117:
1114:
1112:
1109:
1107:
1104:
1102:
1099:
1097:
1094:
1092:
1089:
1087:
1084:
1081:
1077:
1074:
1072:
1069:
1067:
1064:
1062:
1059:
1057:
1054:
1053:
1051:
1047:
1041:
1038:
1036:
1033:
1031:
1028:
1026:
1023:
1021:
1018:
1016:
1015:Benzoperylene
1013:
1011:
1008:
1007:
1005:
1001:
995:
992:
990:
987:
985:
982:
980:
977:
975:
972:
970:
967:
965:
962:
960:
957:
955:
952:
950:
947:
945:
942:
938:
936:
931:
929:
926:
924:
921:
920:
919:
916:
914:
911:
910:
908:
904:
898:
895:
893:
890:
888:
885:
883:
880:
878:
875:
873:
870:
868:
865:
863:
862:Benzofluorene
860:
858:
857:Benzofluorene
855:
853:
850:
849:
847:
843:
837:
834:
832:
829:
827:
824:
822:
819:
817:
814:
812:
809:
808:
806:
802:
796:
793:
791:
788:
786:
783:
782:
780:
776:
772:
765:
760:
758:
753:
751:
746:
745:
742:
731:
724:
721:
716:
712:
708:
704:
696:
694:
692:
688:
681:
679:
676:
674:
670:
665:
663:
662:mass spectrum
659:
655:
650:
648:
639:
637:
613:
609:
608:hydrocracking
604:
602:
598:
594:
590:
582:
580:
578:
574:
570:
566:
562:
554:
552:
550:
525:
521:
517:
505:
498:
493:
476:
470:
466:
462:
458:
457:Formula units
454:
453:
445:
441:
437:
433:
430:
426:
425:
417:
414:
411:
407:
406:
401:
397:
395:
392:
391:
387:
384:
383:
376:
374:
371:
370:
355:
352:
348:
347:
342:
333:
329:
322:
308:
298:
294:
287:
279:
275:
271:
270:
268:
258:
254:
253:
246:
242:
241:
239:
233:
229:
228:
221:
217:
216:
214:
212:
209:
208:
201:
197:
196:
194:
190:
185:
184:
177:
173:
172:
170:
167:
163:
162:
157:
149:
145:
140:
136:
131:
127:
122:
119:Dicoronylene
110:
107:
99:
88:
85:
81:
78:
74:
71:
67:
64:
60:
57: –
56:
52:
51:Find sources:
45:
41:
35:
34:
29:This article
27:
23:
18:
17:
1061:Dicoronylene
1060:
1010:Anthanthrene
934:
892:Triphenylene
877:Fluoranthene
836:Phenanthrene
811:Acenaphthene
729:
723:
706:
702:
677:
673:fluorescence
666:
651:
643:
605:
589:gasification
586:
558:
520:trivial name
516:Dicoronylene
515:
514:
460:
443:
439:
435:
159:Identifiers
102:
93:
83:
76:
69:
62:
50:
38:Please help
33:verification
30:
1035:Triangulene
1020:Corannulene
928:Benzopyrene
923:Benzopyrene
918:Benzopyrene
795:Naphthalene
569:double bond
410:Space group
385:Appearance
344:Properties
1080:Hexa-cata-
821:Anthracene
682:References
640:Properties
583:Occurrence
416:monoclinic
403:Structure
373:Molar mass
211:ChemSpider
187:3D model (
176:98570-53-7
166:CAS Number
96:March 2023
66:newspapers
1157:Phenacene
1147:Cyclacene
1142:Circulene
1071:Heptacene
979:Pentacene
887:Tetracene
831:Phenalene
709:: 30–41.
654:pyrolysis
555:Structure
388:dark red
1172:Category
1152:Helicene
1106:Sumanene
1096:Rubicene
1086:Kekulene
1056:Coronene
1049:7+ rings
1040:Zethrene
1030:Hexacene
984:Perylene
872:Chrysene
826:Fluorene
785:Butalene
658:coronene
652:Thermal
612:coronene
601:coronene
593:coronene
1121:Truxene
1101:Rubrene
1091:Ovalene
1003:6 rings
906:5 rings
845:4 rings
804:3 rings
790:Azulene
778:2 rings
597:ovalene
518:is the
497:what is
495: (
394:Density
378:596.688
232:PubChem
80:scholar
989:Picene
882:Pyrene
492:verify
489:
328:SMILES
245:636081
220:551959
142:Names
82:
75:
68:
61:
53:
1137:Acene
563:with
293:InChI
189:JSmol
87:JSTOR
73:books
647:coke
595:and
549:torr
418:, P2
59:news
711:doi
707:117
656:of
603:.
422:/c
262:EPA
235:CID
42:by
1174::
705:.
690:^
631:22
622:56
542:20
533:48
467:2
366:20
360:48
1082:)
1078:(
935:H
933:6
763:e
756:t
749:v
717:.
713::
626:H
617:C
615:(
537:H
528:C
487:N
463:)
461:Z
459:(
444:c
440:b
436:a
420:1
363:H
357:C
264:)
260:(
191:)
109:)
103:(
98:)
94:(
84:·
77:·
70:·
63:·
36:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.