333:
165:
384:
378:
158:
458:= 3, there are eight stereoisomers. Among them, there are four pairs of enantiomers: R,R,R and S,S,S; R,R,S and S,S,R; R,S,S and S,R,R; and R,S,R and S,R,S. There are many more pairs of diastereomers, because each of these configurations is a diastereomer with respect to every other configuration excluding its own enantiomer (for example, R,R,R is a diastereomer of R,R,S; R,S,R; and R,S,S). For
36:
339:
149:
142:
430:
If a molecule contains two asymmetric centers, there are up to four possible configurations, and they cannot all be non-superposable mirror images of each other. The possibilities for different isomers continue to multiply as more stereocenters are added to a molecule. In general, the number of
298:
the erythro isomer has two identical substituents on the same side and the threo isomer has them on opposite sides. When drawn as a zig-zag chain, the erythro isomer has two identical substituents on different sides of the plane (anti). The names are derived from the diastereomeric four-carbon
222:
in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is,
277:
priorities. Syn describes groups on the same face while anti describes groups on opposite faces. The concept applies only to the Zigzag projection. The descriptors only describe relative stereochemistry rather than absolute stereochemistry. All isomers are same.
207:. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related)
234:, for instance, are diastereomers. Even though they share the same molar weight, glucose is more stable than galactose. This difference in stability causes galactose to be absorbed slightly faster than glucose in human body.
264:
When the single bond between the two centres is free to rotate, cis/trans descriptors become invalid. Two widely accepted prefixes used to distinguish diastereomers on sp³-hybridised bonds in an open-chain molecule are
1082:
1087:
541:
828:
948:
451:, but possess an internal plane of symmetry allowing it to be superposed on its mirror image. These equivalent configurations cannot be considered diastereomers.
553:
310:. These prefixes are not recommended for use outside of the realm of saccharides because their definitions can lead to conflicting interpretations.
223:
excluding the opposing enantiomer). Diastereomers have different physical properties (unlike most aspects of enantiomers) and often different
860:"Catalytic Enantioselective Desymmetrization of Meso Compounds in Total Synthesis of Natural Products: Towards an Economy of Chiral Reagents"
215:. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.
273:. Masamune proposed the descriptors which work even if the groups are not attached to adjacent carbon atoms. It also works regardless of
941:
230:
Diastereomers differ not only in physical properties but also in chemical reactivity — how a compound reacts with others. Glucose and
914:
605:
274:
119:
1120:
211:
and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are
57:
100:
1105:
934:
537:
53:
72:
1115:
524:
As stated previously, two diastereomers will not have identical chemical properties. This knowledge is harnessed in
79:
958:
46:
1077:
705:"Differentiation and Quantification of Diastereomeric Pairs of Glycosphingolipids using Gas-phase Ion Chemistry"
1182:
1003:
498:
483:
478:
Double bond isomers are always considered diastereomers, not enantiomers. Diastereomerism can also occur at a
86:
68:
1141:
1055:
972:
470:(subsets of the five- and six-carbon sugars) are examples of sets of compounds that differ in this way.
436:
1151:
1136:
987:
462:= 4, there are sixteen stereoisomers, or eight pairs of enantiomers. The four enantiomeric pairs of
1049:
224:
1187:
1110:
1008:
887:
295:
1097:
910:
879:
859:
799:
781:
742:
724:
685:
667:
601:
529:
245:
240:
is the preference for the formation of one or more than one diastereomer over the other in an
505:
1146:
1072:
982:
871:
842:
789:
773:
732:
716:
675:
659:
630:
580:
525:
241:
1156:
1044:
1024:
998:
332:
192:
93:
926:
794:
761:
737:
704:
680:
647:
533:
440:
1176:
1039:
444:
408:
322:
891:
838:
17:
1161:
977:
509:
494:
448:
253:
249:
208:
204:
762:"Origin of High Diastereoselectivity in Reactions of Seven-Membered-Ring Enolates"
164:
720:
833:
479:
439:
centers in the molecule. This holds true except in cases where the molecule has
35:
383:
377:
157:
1029:
219:
883:
837:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
785:
728:
671:
846:
634:
584:
314:
303:
231:
180:
875:
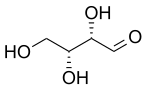
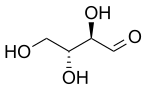
803:
777:
746:
689:
858:
Merad, Jérémy; Candy, Mathieu; Pons, Jean-Marc; Bressy, Cyril (May 2017).
648:"The comparative rates of absorption of sugars from the human intestine"
624:
574:
338:
286:
Two older prefixes still commonly used to distinguish diastereomers are
148:
463:
307:
172:
663:
431:
stereoisomers of a molecule can be determined by calculating 2, where
141:
513:
467:
300:
212:
909:(8th ed.). United States: Cengage Learning. pp. 138–142.
528:
to separate a mixture of enantiomers. This is the principle behind
620:
27:
Molecules which are non-mirror image, non-identical stereoisomers
930:
29:
760:
Lavinda, Olga; Witt, Collin H.; Woerpel, K. A. (2022-03-28).
532:. After preparing the diastereomers, they are separated by
504:
In the case of diastereomerism occurring at a double bond,
248:
is attributed to torsional and steric interactions in the
508:, or entgegen and zusammen (German), is used in notating
131:
542:
stereochemistry of ketonization of enols and enolates
1129:
1096:
1065:
1017:
965:
60:. Unsourced material may be challenged and removed.
766:Angewandte Chemie International Edition in English
1088:Ultraviolet–visible spectroscopy of stereoisomers
703:Chao, Hsi-Chun; McLuckey, Scott A. (2020-10-06).
646:McCance, Robert Alexander; Madders, Kate (1930).
294:. In the case of saccharides, when drawn in the
942:
8:
949:
935:
927:
327:
256:approaching the stereocenter in reaction.
793:
736:
679:
120:Learn how and when to remove this message
819:Eric V. Anslyn, Dennis A. Dougherty 2006
497:give two non-superposable isomers. Many
317:, one of the essential amino acids. The
566:
596:Garrett, R.H.; Grisham, C.M. (2005),
7:
600:, Belmont CA: Thomson, p. 205,
466:and the eight enantiomeric pairs of
135:Diastereomers that are also epimers
58:adding citations to reliable sources
834:Compendium of Chemical Terminology
25:
1083:NMR spectroscopy of stereoisomers
817:Modern physical organic chemistry
554:Cahn–Ingold–Prelog priority rules
1121:Diastereomeric recrystallization
474:Diastereomerism at a double bond
382:
376:
337:
331:
163:
156:
147:
140:
34:
540:. Note also the example of the
162:
155:
146:
139:
45:needs additional citations for
1:
1116:Chiral column chromatography
721:10.1021/acs.analchem.0c02755
501:are diastereomers as well.
447:are molecules that contain
435: = the number of
1204:
1078:Chiral derivatizing agents
959:enantioselective synthesis
313:Another threo compound is
218:Diastereomers differ from
134:
1004:Supramolecular chirality
905:Brown, William (2018).
847:10.1351/goldbook.E02212
635:10.1351/goldbook.E02069
585:10.1351/goldbook.D01679
876:10.1055/s-0036-1589493
778:10.1002/anie.202114183
499:conformational isomers
426:Multiple stereocenters
321:diastereomer of it is
1142:Chiral pool synthesis
1056:Diastereomeric excess
1152:Asymmetric catalysis
1137:Asymmetric induction
709:Analytical Chemistry
598:Biochemistry 3rd ed.
238:Diastereoselectivity
54:improve this article
18:Diastereoselectivity
1050:Enantiomeric excess
715:(19): 13387–13395.
652:Biochemical Journal
225:chemical reactivity
1147:Chiral auxiliaries
1111:Kinetic resolution
1009:Inherent chirality
994:-symmetric ligands
772:(14): e202114183.
576:diastereoisomerism
573:IUPAC "Gold Book"
491:relative positions
296:Fischer projection
199:(sometimes called
1170:
1169:
1106:Recrystallization
1098:Chiral resolution
907:Organic Chemistry
664:10.1042/bj0240795
556:for nomenclature.
538:recrystallization
530:chiral resolution
423:
422:
406:
395:-Allothreonine (2
394:
361:
349:
246:stereoselectivity
189:
188:
183:
175:
130:
129:
122:
104:
16:(Redirected from
1195:
1073:Optical rotation
1018:Chiral molecules
983:Planar chirality
951:
944:
937:
928:
921:
920:
902:
896:
895:
870:(9): 1938–1954.
855:
849:
826:
820:
814:
808:
807:
797:
757:
751:
750:
740:
700:
694:
693:
683:
643:
637:
618:
612:
610:
593:
587:
571:
526:chiral synthesis
404:
392:
386:
380:
359:
347:
341:
335:
328:
242:organic reaction
203:) are a type of
201:diastereoisomers
181:
173:
167:
160:
151:
144:
132:
125:
118:
114:
111:
105:
103:
62:
38:
30:
21:
1203:
1202:
1198:
1197:
1196:
1194:
1193:
1192:
1183:Stereochemistry
1173:
1172:
1171:
1166:
1157:Organocatalysis
1125:
1092:
1061:
1045:Racemic mixture
1013:
999:Axial chirality
993:
966:Chirality types
961:
955:
925:
924:
917:
904:
903:
899:
857:
856:
852:
827:
823:
815:
811:
759:
758:
754:
702:
701:
697:
645:
644:
640:
619:
615:
608:
595:
594:
590:
572:
568:
563:
550:
522:
476:
428:
387:
371:
342:
284:
282:Erythro / threo
262:
252:resulting from
193:stereochemistry
126:
115:
109:
106:
63:
61:
51:
39:
28:
23:
22:
15:
12:
11:
5:
1201:
1199:
1191:
1190:
1185:
1175:
1174:
1168:
1167:
1165:
1164:
1159:
1154:
1149:
1144:
1139:
1133:
1131:
1127:
1126:
1124:
1123:
1118:
1113:
1108:
1102:
1100:
1094:
1093:
1091:
1090:
1085:
1080:
1075:
1069:
1067:
1063:
1062:
1060:
1059:
1053:
1047:
1042:
1037:
1032:
1027:
1021:
1019:
1015:
1014:
1012:
1011:
1006:
1001:
996:
991:
985:
980:
975:
969:
967:
963:
962:
956:
954:
953:
946:
939:
931:
923:
922:
915:
897:
850:
839:erythro, threo
821:
809:
752:
695:
658:(3): 795–804.
638:
613:
606:
588:
565:
564:
562:
559:
558:
557:
549:
546:
534:chromatography
521:
518:
475:
472:
445:meso compounds
427:
424:
421:
420:
389:
388:
373:
372:
344:
343:
283:
280:
261:
258:
244:. In general,
187:
186:
178:
169:
168:
161:
153:
152:
145:
137:
136:
128:
127:
110:September 2021
69:"Diastereomer"
42:
40:
33:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1200:
1189:
1186:
1184:
1181:
1180:
1178:
1163:
1160:
1158:
1155:
1153:
1150:
1148:
1145:
1143:
1140:
1138:
1135:
1134:
1132:
1128:
1122:
1119:
1117:
1114:
1112:
1109:
1107:
1104:
1103:
1101:
1099:
1095:
1089:
1086:
1084:
1081:
1079:
1076:
1074:
1071:
1070:
1068:
1064:
1057:
1054:
1051:
1048:
1046:
1043:
1041:
1040:Meso compound
1038:
1036:
1033:
1031:
1028:
1026:
1023:
1022:
1020:
1016:
1010:
1007:
1005:
1002:
1000:
997:
995:
990:
986:
984:
981:
979:
976:
974:
971:
970:
968:
964:
960:
952:
947:
945:
940:
938:
933:
932:
929:
918:
916:9781305580350
912:
908:
901:
898:
893:
889:
885:
881:
877:
873:
869:
865:
861:
854:
851:
848:
844:
840:
836:
835:
830:
825:
822:
818:
813:
810:
805:
801:
796:
791:
787:
783:
779:
775:
771:
767:
763:
756:
753:
748:
744:
739:
734:
730:
726:
722:
718:
714:
710:
706:
699:
696:
691:
687:
682:
677:
673:
669:
665:
661:
657:
653:
649:
642:
639:
636:
632:
628:
627:
622:
617:
614:
609:
607:0-534-41020-0
603:
599:
592:
589:
586:
582:
578:
577:
570:
567:
560:
555:
552:
551:
547:
545:
543:
539:
535:
531:
527:
519:
517:
515:
511:
507:
502:
500:
496:
492:
490:
486:
481:
473:
471:
469:
465:
461:
457:
452:
450:
449:stereocenters
446:
443:forms. These
442:
438:
434:
425:
418:
414:
410:
409:Allothreonine
402:
398:
391:
390:
385:
379:
375:
374:
369:
365:
362:-Threonine (2
357:
353:
350:-Threonine (2
346:
345:
340:
334:
330:
329:
326:
324:
323:allothreonine
320:
316:
311:
309:
305:
302:
297:
293:
289:
281:
279:
276:
272:
268:
259:
257:
255:
254:electrophiles
251:
247:
243:
239:
235:
233:
228:
226:
221:
216:
214:
210:
209:stereocenters
206:
202:
198:
197:diastereomers
194:
185:
179:
177:
171:
170:
166:
159:
154:
150:
143:
138:
133:
124:
121:
113:
102:
99:
95:
92:
88:
85:
81:
78:
74:
71: –
70:
66:
65:Find sources:
59:
55:
49:
48:
43:This article
41:
37:
32:
31:
19:
1162:Biocatalysis
1035:Diastereomer
1034:
1025:Stereoisomer
988:
978:Stereocenter
957:Concepts in
906:
900:
867:
863:
853:
832:
824:
816:
812:
769:
765:
755:
712:
708:
698:
655:
651:
641:
625:
623:"Gold Book"
616:
597:
591:
575:
569:
523:
520:Applications
510:nomenclature
503:
495:substituents
488:
484:
482:, where the
477:
464:aldopentoses
459:
455:
453:
432:
429:
416:
412:
400:
396:
367:
363:
355:
351:
318:
312:
291:
287:
285:
270:
266:
263:
250:stereocenter
237:
236:
229:
217:
205:stereoisomer
200:
196:
190:
116:
107:
97:
90:
83:
76:
64:
52:Please help
47:verification
44:
480:double bond
468:aldohexoses
220:enantiomers
1177:Categories
1030:Enantiomer
626:enantiomer
561:References
260:Syn / anti
184:-erythrose
80:newspapers
1188:Isomerism
1130:Reactions
973:Chirality
884:0039-7881
864:Synthesis
786:1521-3773
729:0003-2700
672:0264-6021
315:threonine
304:erythrose
232:galactose
1066:Analysis
892:99010495
804:35076978
747:32883073
690:16744419
548:See also
176:-threose
795:8940697
738:7544660
681:1254520
514:alkenes
319:erythro
308:threose
301:aldoses
292:erythro
213:epimers
94:scholar
913:
890:
882:
802:
792:
784:
745:
735:
727:
688:
678:
670:
629:
604:
579:
437:chiral
403:) and
381:
358:) and
336:
96:
89:
82:
75:
67:
888:S2CID
829:IUPAC
621:IUPAC
489:trans
288:threo
101:JSTOR
87:books
1058:(de)
1052:(ee)
911:ISBN
880:ISSN
800:PMID
782:ISSN
743:PMID
725:ISSN
686:PMID
668:ISSN
602:ISBN
454:For
441:meso
306:and
290:and
271:anti
269:and
73:news
872:doi
843:doi
841:".
790:PMC
774:doi
733:PMC
717:doi
676:PMC
660:doi
631:doi
581:doi
536:or
512:of
506:E-Z
493:of
487:vs
485:cis
275:CIP
267:syn
191:In
56:by
1179::
886:.
878:.
868:49
866:.
862:.
831:,
798:.
788:.
780:.
770:61
768:.
764:.
741:.
731:.
723:.
713:92
711:.
707:.
684:.
674:.
666:.
656:24
654:.
650:.
544:.
516:.
419:)
415:,3
411:(2
399:,3
366:,3
354:,3
325:.
227:.
195:,
992:2
989:C
950:e
943:t
936:v
919:.
894:.
874::
845::
806:.
776::
749:.
719::
692:.
662::
633::
611:.
583::
460:n
456:n
433:n
417:R
413:R
407:-
405:D
401:S
397:S
393:L
370:)
368:S
364:R
360:D
356:R
352:S
348:L
182:D
174:D
123:)
117:(
112:)
108:(
98:·
91:·
84:·
77:·
50:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.