1722:
35:
1487:
26:
279:
186:
1244:
814:
1855:
986:
1261:
Cyclophosphazenes such as hexachlorophosphazene are distinguished by notable stability and equal P–N bond lengths which, in many such cyclic molecules, would imply delocalization or even aromaticity. To account for these features, early bonding models starting from the mid-1950s invoked a delocalised
1945:
Among these, the best structurally characterised are the 1:1 adducts with aluminium trichloride or with gallium trichloride; they are found with the Al/Ga atom bound to a N and assume a more prominently distorted chair conformation compared to the free hexachlorophosphazene. The adducts also exhibit
1506:
at approximately 60 °C affords the pure trimer free of the tetramer. Reaction conditions such as temperature may also be tuned to maximise the yield of the trimer at the expense of the other possible products; nonetheless, commercial samples of hexachlorophosphazene usually contain appreciable
1247:
Depictions of P–N bonding in a general cyclotriphosphazene: left, a representation of alternating single and double P–N bonds (does not account for equal bond lengths), used as a matter of convention; middle, the earlier proposed delocalised ring system (discredited due to infeasibility of P
1878:
The nitrogen centres of hexachlorophosphazene are weakly basic, and this Lewis base behaviour has been suggested to play a role in the polymerisation mechanism. Specifically, hexachlorophosphazene has been reported to form adducts of various stoichiometries with Lewis acids
1721:
1089:
Occasionally, commercial or suggested practical applications have been reported, too, utilising hexachlorophosphazene as a precursor chemical. Derivatives of noted interest include the hexalkoxyphosphazene lubricants obtained from
1486:
822:
794:
2484:"Structural investigations of phosphorus–nitrogen compounds. 7. Relationships between physical properties, electron densities, reaction mechanisms and hydrogen-bonding motifs of N3P3Cl(6 − n)(NHBu t ) n derivatives"
1854:
1213:
spectroscopy is the usual method for assaying hexachlorophosphazene and its reactions. Hexachlorophosphazene exhibits a single resonance at 20.6 ppm as all P environments are chemically equivalent.
2074:
species), resulting from the nucleophilic hexasubstitution of the hexachlorophosphazene P atoms, have attracted interest for their high thermal and chemical stability as well as their low
880:
2097:
properties have been investigated. Some of them appear promising for future applications as fibre- or membrane-forming high performance materials, since they combine transparency,
999:
872:
25:
1951:
34:
1428:(HCl) side product. Since ammonium chloride is insoluble in chlorinated solvents, workup is facilitated. For the reaction under such conditions, the following
2078:. Certain hexalkoxyphosphazenes (such as the hexa-phenoxy derivative) have been put to commercial use as fireproof materials and high temperature lubricants.
328:
2482:
Bartlett, Stewart W.; Coles, Simon J.; Davies, David B.; Hursthouse, Michael B.; i̇Bişogˇlu, Hanife; Kiliç, Adem; Shaw, Robert A.; Ün, İlker (2006).
1146:
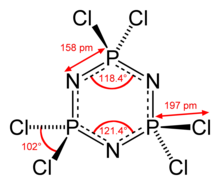
atoms bonded to each phosphorus atom. Hexachlorophosphazene molecule contains six equivalent P–N bonds, for which the adjacent P–N distances are 157
876:
1955:
1210:
123:
2553:
2357:
693:
1053:
1799:. 250 °C induces polymerisation. The tetramer also polymerises in this manner, although more slowly. The conversion is a type of
2766:
2746:
2464:
293:
2805:
2790:
1862:
This polydichlorophosphazene product is the starting material for a wide class of polymeric compounds, collectively known as
1166:
775:
424:
2594:
Heston, Amy J.; Panzner, Matthew J.; Youngs, Wiley J.; Tessier, Claire A. (2005). "Lewis Acid
Adducts of [PCl2N]3".
2128:, where the polyphosphazenes confer fire resistance, imperviousness to oils, and flexibility even at very low temperatures.
1787:
is due to the combined steric effects and oxygen lone pair π-backdonation (which deactivates already substituted P atoms).
2729:
Nielsen, Morris L.; Cranford, Garland (2007) . "Trimeric
Phosphonitrile Chloride and Tetrameric Phosphonitrile Chloride".
1285:
contribution is negligible, invalidating the earlier hypothesis. Instead, a charge separated model is generally accepted.
1086:. There is large academic interest in the compound relating to the phosphorus-nitrogen bonding and phosphorus reactivity.
864:
1006:
2795:
2075:
850:
236:
1490:
The three major cyclophosphazene products resulting from the reaction of phosphorus pentachloride and ammonium chloride
1252:
participation); right, the most accurate description to current knowledge, where the majority of the bonding is ionic
2810:
2785:
2638:"A nitrogen and phosphorus enriched pyridine bridged inorganic–organic hybrid material for supercapacitor application"
2062:
rings. Solid films of the trimer and tetramer will not undergo any chemical change under such irradiation conditions.
1800:
1384:
Modern syntheses are based on the developments by Schenk and Römer who used ammonium chloride in place of ammonia and
257:
1480:
2001:
in chloroform, though for this application the tetramer octachlorotetraphosphazene usually proves more effective.
2137:
Discovery of cyclophosphazenes: Liebig-Wöhler, Briefwechsel vol. 1, 63; Ann. Chem. (Liebig), vol. 11 (1834), 146.
2082:
1823:
1730:
1417:
1300:
1281:
Starting from the late 1980s, more modern calculations and the lack of spectroscopic evidence reveal that the P 3
1091:
935:
193:
1570:
1516:
1324:
1184:
950:
720:
664:
1476:
2159:"Syntheses and structures of cyclic and short-chain linear phosphazenes bearing 4-phenylphenoxy side groups"
1925:
1684:
868:
745:
528:
1858:
Hexachlorotriphosphazene ROP and subsequent nucleophilic substitution for desired polyphosphazene synthesis
813:
181:
2157:
Allcock, Harry R.; Ngo, Dennis C.; Parvez, Masood; Whittle, Robert R.; Birdsall, William J. (1991-03-01).
2147:
2141:
945:
143:
2815:
2349:
1804:
1734:
1589:
1232:
1217:
1199:
1069:
930:
836:
806:
47:
1098:, or chemically resistant inorganic polymers with desirable thermal and mechanical properties known as
1916:
1574:
884:
453:
2637:
1807:, but is overall not very well understood. Prolonged heating of the polymer at higher temperatures (
1725:
A SN2 substitution at hexachlorotriphosphazene. A trigonal bipyramidal transition state is proposed.
1898:
1625:
1540:
1395:
1346:
1320:
274:
89:
2665:
2220:
2044:
1947:
1880:
1609:
1503:
1495:
1374:
1065:
1022:
658:
601:
2155:
Example of hexalkoxyphosphazene synthesis from hexachlorophosphazene and structure description:
1288:
According to this description, the P–N bond is viewed as a very polarised one (between notional
1224:
stretches. Other bands are found at 860 and 500–600 cm, respectively assigned to ring and ν
856:
2459:
Mark, J. E.; Allcock, H. R.; West, R. “Inorganic
Polymers” Prentice Hall, Englewood, NJ: 1992.
2800:
2762:
2742:
2657:
2611:
2559:
2549:
2505:
2460:
2419:
2411:
2353:
2286:
2212:
2178:
2086:
1967:
1934:
1889:
1578:
1561:
1425:
1394:. By replacing ammonia with ammonium chloride allows the reaction to proceed without a strong
1350:
1316:
1243:
566:
2734:
2696:
2649:
2603:
2495:
2403:
2341:
2278:
2204:
2170:
1994:
1812:
1784:
1385:
1231:
Hexachlorophosphazene and many of its derivatives have been characterized by single crystal
1026:
616:
541:
438:
351:
163:
888:
245:
2110:
2040:
1907:
1863:
1759:
1688:
1515:
The mechanism of the above reaction has not been resolved, but it has been suggested that
1120:
1099:
1049:
940:
278:
185:
99:
1950:
behaviour in solution for temperatures down to −60 °C, which can be monitored with
892:
2125:
2102:
2098:
2010:
1990:
derivatives, postulated to go through a cyclophosphazene pyridinium salt intermediate.
1987:
1304:
1270:
1155:
1103:
977:
842:
2779:
2669:
2094:
1819:
1429:
1421:
413:
403:
174:
2224:
900:
2761:
Holleman, A. F.; Wiberg, E. "Inorganic
Chemistry" Academic Press: San Diego, 2001.
2266:
1998:
1966:
Hexachlorophosphazene has also found applications in research by enabling aromatic
1741:
by alkoxide proceeds via displacement of chloride at separate phosphorus centers:
1715:
1708:
1586:
1582:
1413:
1183:
ring in hexachlorophosphazene deviates from planarity and is slightly ruffled (see
860:
641:
572:
225:
2267:"Regio- and stereochemical control in substitution reactions of cyclophosphazenes"
1843:~ 15000. It was first observed in the late 19th century and its form after chain
1388:
1079:
955:
907:
580:
479:
2738:
2545:
Phosphorus-nitrogen compounds ; cyclic, linear, and high polymeric systems
1866:. Substitution of the chloride groups by other nucleophilic groups, especially
2500:
2483:
2189:
Novel hexalkoxyphosphazene synthesis not starting from hexachlorophosphazene:
2106:
1844:
1378:
1362:
1139:
1057:
1034:
501:
475:
449:
372:
154:
2700:
2661:
2563:
2415:
2290:
2216:
2182:
1296:), with sufficient ionic character to account for most of the bond strength.
74:
2,2,4,4,6,6-hexachloro-2,2,4,4,6,6-hexahydro-1,3,5,2,4,6-triazatriphosphorine
2121:
2090:
1848:
1349:
to yield a new substance that could be washed with cold water to remove the
1147:
2615:
2509:
2423:
2543:
2208:
1536:(tetrachlorophosphonium hexachlorophosphate(V)) and the reaction proceeds
1971:
1867:
1738:
1711:
1691:
1499:
1391:
1370:
1366:
1143:
1135:
1095:
1061:
1037:
1031:
965:
2282:
2192:
2174:
2013:
react forming clear liquids identified as alkyl-substituted derivatives
2653:
2146:. American Chemical Journal, vol. 17, p. 275.H. N. Stokes (1896),
2071:
1552:
1335:
1083:
960:
393:
212:
194:
2607:
2407:
2158:
2117:
1983:
673:
505:
134:
2714:
R. Klement (1963). "Phosphonitrilic
Chlorides". In G. Brauer (ed.).
2684:
2391:
2390:
Chaplin, Adrian B.; Harrison, John A.; Dyson, Paul J. (2005-11-01).
976:
Except where otherwise noted, data are given for materials in their
2081:
Polyphosphazenes obtained from polymerised hexachlorophosphazene (
1242:
680:
122:
112:
1479:), 3 (the trimer hexachlorotriphosphazene), and 4 (the tetramer
1319:
in 1834. In that report he describes experiments conducted with
896:
1220:, the 1370 and 1218 cm vibrational bands are assigned to ν
1870:
as laid out above, yields numerous characterised derivatives.
1315:
The synthesis of hexachlorophosphazene was first reported by
2140:
First reports on their polymerisation: H. N. Stokes (1895),
1853:
1720:
1485:
1299:
The rest (~15%) of the bond strength may be attributed to a
262:
2193:"A Novel Synthesis of Hexasubstituted Cyclotriphosphazenes"
408:
112 to 114 °C (234 to 237 °F; 385 to 387 K)
51:
2,2,4,4,6,6-Hexachloro-1,3,5,2λ,4λ,6λ-triazatriphosphinine
2149:
2152:
American
Chemical Journal, vol. 18 issue 8, p. 629.
1507:
amounts of octachlorotetraphosphazene, even up to 40%.
994:
2191:
Ye, Chengfeng; Zhang, Zefu; Liu, Weimin (2002-01-01).
2009:
Both the trimer and tetramer in hydrocarbon solutions
2392:"Revisiting the Electronic Structure of Phosphazenes"
1803:(ROP). The ROP mechanism is found to be catalysed by
2733:. Inorganic Syntheses. Vol. 6. pp. 94–97.
2488:
2718:. Vol. 1. NY, NY: Academic Press. p. 575.
2716:
2116:Polyphosphazene-based components have been used in
1624:and subsequent HCl elimination, creates a growing
1307:into π-accepting σ* molecular orbitals on the P.
1078:(phosphazyl dichloride). Its classification as a
224:
2636:Dhiman, Nisha; Mohanty, Paritosh (2019-10-28).
2039:= 3, 4. Such reactions proceed under prolonged
302:InChI=1S/Cl6N3P3/c1-10(2)7-11(3,4)9-12(5,6)8-10
98:
2085:) have garnered attention within the field of
1847:has been called "inorganic rubber" due to its
1150:. This is characteristically shorter than the
312:InChI=1/Cl6N3P3/c1-10(2)7-11(3,4)9-12(5,6)8-10
1494:Purification by sublimation gives mainly the
8:
1154:. 177 pm P–N bonds in the valence saturated
2336:
2334:
2332:
2330:
2328:
2326:
2324:
2322:
2320:
2070:The hexalkoxyphosphazenes (especially the
1707:Hexachlorophosphazene reacts readily with
277:
184:
162:
17:
2499:
2318:
2316:
2314:
2312:
2310:
2308:
2306:
2304:
2302:
2300:
244:
2163:Journal of the American Chemical Society
2056:
2050:
2029:
2023:
2016:
1937:
1928:
1919:
1910:
1901:
1892:
1883:
1833:
1829:
1775:
1771:
1767:
1755:
1751:
1747:
1671:
1667:
1663:
1659:
1655:
1646:
1642:
1638:
1634:
1619:
1605:
1601:
1597:
1564:
1555:
1546:
1531:
1527:
1519:
1475:can usually take values of 2 (the dimer
1457:
1453:
1449:
1441:
1408:
1401:
1361:) coproduct. The new compound contained
1356:
1341:
1330:
1262:π system arising from the overlap of N 2
1194:
1190:
1179:
1175:
1165:symmetry, and each phosphorus center is
1130:
1126:
1074:
1044:
1040:
363:
359:
2238:
333:
298:
273:
175:
2455:
2453:
2047:) illumination without affecting the
1687:leads to the formation of one of the
305:Key: UBIJTWDKTYCPMQ-UHFFFAOYSA-N
142:
7:
2631:
2629:
2627:
2625:
2589:
2587:
2585:
2583:
2581:
2579:
2577:
2575:
2573:
2537:
2535:
2533:
2531:
2529:
2527:
2525:
2523:
2521:
2519:
2477:
2475:
2473:
2451:
2449:
2447:
2445:
2443:
2441:
2439:
2437:
2435:
2433:
2385:
2383:
2381:
2379:
2377:
2375:
2373:
2371:
2369:
2265:Allen, Christopher W. (1991-03-01).
2260:
2258:
2256:
2254:
2252:
2250:
2248:
2246:
2244:
2242:
2143:On the chloronitrides of phosphorus
1993:The compound may also be used as a
1056:backbone consisting of alternating
315:Key: UBIJTWDKTYCPMQ-UHFFFAOYAJ
215:
1303:interaction: the N lone pairs can
14:
1795:Heating hexachlorophosphazene to
336:N1=P(N=P(N=P1(Cl)Cl)(Cl)Cl)(Cl)Cl
1986:, resulting in 4,4'-substituted
1616:which through further attack of
984:
812:
33:
24:
1933:, but no isolable product with
1818:The structure of the inorganic
1082:highlights its relationship to
980:(at 25 °C , 100 kPa).
62:Phosphonitrilic chloride trimer
1202:species is completely planar.
1169:with a Cl–P–Cl angle of 101°.
1110:Structure and characterisation
1094:of hexachlorophosphazene with
1064:atoms, and can be viewed as a
776:Occupational safety and health
1:
1997:reagent for the synthesis of
1115:Bond lengths and conformation
68:Hexachlorocyclotriphosphazene
2548:. New York: Academic Press.
2076:glass transition temperature
1551:(tetrachlorophosphonium) by
1305:donate some electron density
1801:ring-opening polymerisation
1791:Ring-opening polymerisation
1524:is found in its ionic form
1119:Hexachlorophosphazene is a
71:Triphosphonitrilic chloride
2832:
2739:10.1002/9780470132371.ch28
1811:. 350 °C) will cause
1481:octachlorotetraphosphazene
1377:. It was sensitive toward
1142:atoms, and two additional
1106:of hexachlorophosphazene.
418:decomposes (above 167 °C)
2501:10.1107/S0108768106000851
2346:Chemistry of the Elements
2344:; Earnshaw, Alan (1997).
2083:poly(dichlorophosphazene)
2005:Photochemical degradation
1824:Poly(dichlorophosphazene)
1418:1,1,2,2-tetrachloroethane
1301:negative hyperconjugation
1092:nucleophilic substitution
974:
936:Poly(dichlorophosphazene)
916:
793:
773:
768:
686:
559:
344:
324:
289:
82:
56:
46:
41:
32:
23:
2701:10.1002/jlac.18340110202
2685:"Nachtrag der Redaction"
2642:New Journal of Chemistry
2197:Synthetic Communications
1477:tetrachlorodiphosphazene
1325:phosphorus pentachloride
1206:Characterisation methods
1161:The molecule possesses D
951:Tetrasulfur tetranitride
851:Precautionary statements
721:Enthalpy of vaporization
65:Hexachlorotriphosphazene
2806:Phosphorus heterocycles
2542:Allcock, H. R. (1972).
746:Enthalpy of sublimation
529:Magnetic susceptibility
1859:
1826:) comprises a linear –
1726:
1491:
1253:
1134:core with alternating
946:Trithiazyl trichloride
630: = 12.83 Å,
626: = 13.87 Å,
519:50.7 wt % (60 °C)
516:38.9 wt % (40 °C)
513:27.7 wt % (20 °C)
493:53.7 wt % (60 °C)
490:36.8 wt % (40 °C)
487:22.3 wt % (20 °C)
467:39.2 wt % (60 °C)
464:35.6 wt % (40 °C)
461:24.5 wt % (20 °C)
19:Hexachlorophosphazene
2791:Nitrogen heterocycles
2350:Butterworth-Heinemann
2342:Greenwood, Norman N.
2209:10.1081/SCC-120002003
2101:flexibility, tunable
1857:
1754:+ 3 NaOR → (NPCl(OR))
1724:
1685:intramolecular attack
1585:) creates a reactive
1489:
1424:, which tolerate the
1277:Modern bonding models
1246:
1233:X-ray crystallography
1200:hexafluorophosphazene
1070:hypothetical compound
1048:. The molecule has a
1019:Hexachlorophosphazene
931:Hexafluorophosphazene
2111:desirable properties
2109:, and various other
1982:-dialkylanilines or
1398:associated with the
1198:ring in the related
1187:). By contrast, the
454:carbon tetrachloride
2796:Inorganic compounds
2731:Inorganic Syntheses
2648:(42): 16670–16675.
2596:Inorganic Chemistry
2396:Inorganic Chemistry
2283:10.1021/cr00002a002
2175:10.1021/ja00007a041
1541:nucleophilic attack
1511:Formation mechanism
1396:exothermic reaction
667:(slightly ruffled)
634: = 6.09 Å
439:Solubility in water
432:60 °C at 0.05 Torr
398:1.98 g/mL at 25 °C
380: g·mol
20:
2811:Six-membered rings
2786:Chlorine compounds
2683:J. Liebig (1834).
2654:10.1039/C9NJ03976G
2087:inorganic polymers
1970:reactions between
1860:
1770:+ 3 NaOR → (NP(OR)
1727:
1683:until an eventual
1504:vacuum sublimation
1492:
1375:elemental analysis
1373:, on the basis of
1323:. They found that
1254:
1185:chair conformation
1102:produced from the
1023:inorganic compound
1007:Infobox references
917:Related compounds
18:
2608:10.1021/ic050974y
2602:(19): 6518–6520.
2555:978-0-323-14751-4
2408:10.1021/ic0511266
2402:(23): 8407–8417.
2359:978-0-08-037941-8
1703:Substitution at P
1426:hydrogen chloride
1351:ammonium chloride
1015:Chemical compound
1013:
1012:
923:Related compounds
837:Hazard statements
567:Crystal structure
388:colourless solid
258:CompTox Dashboard
124:Interactive image
2823:
2770:
2759:
2753:
2752:
2726:
2720:
2719:
2711:
2705:
2704:
2680:
2674:
2673:
2633:
2620:
2619:
2591:
2568:
2567:
2539:
2514:
2513:
2503:
2479:
2468:
2457:
2428:
2427:
2387:
2364:
2363:
2348:(2nd ed.).
2338:
2295:
2294:
2271:Chemical Reviews
2262:
2228:
2186:
2169:(7): 2628–2634.
2061:
2034:
1995:peptide coupling
1962:Coupling reagent
1940:
1931:
1922:
1913:
1904:
1895:
1886:
1864:polyphosphazenes
1838:
1813:depolymerisation
1785:regioselectivity
1779:
1762:
1675:
1650:
1623:
1612:
1568:
1558:
1550:
1535:
1522:
1467:
1411:
1404:
1360:
1344:
1333:
1295:
1291:
1197:
1182:
1133:
1100:polyphosphazenes
1077:
1047:
1027:chemical formula
997:
991:
988:
987:
902:
898:
894:
890:
886:
882:
878:
874:
870:
866:
862:
858:
844:
816:
760:
735:
710:
687:Thermochemistry
617:Lattice constant
594:
593:
542:Refractive index
379:
366:
352:Chemical formula
282:
281:
266:
264:
248:
228:
217:
196:
188:
177:
166:
146:
126:
102:
37:
28:
21:
2831:
2830:
2826:
2825:
2824:
2822:
2821:
2820:
2776:
2775:
2774:
2773:
2760:
2756:
2749:
2728:
2727:
2723:
2713:
2712:
2708:
2682:
2681:
2677:
2635:
2634:
2623:
2593:
2592:
2571:
2556:
2541:
2540:
2517:
2481:
2480:
2471:
2458:
2431:
2389:
2388:
2367:
2360:
2340:
2339:
2298:
2264:
2263:
2240:
2235:
2190:
2156:
2134:
2132:Further reading
2126:shock absorbers
2068:
2060:
2054:
2048:
2033:
2027:
2021:
2014:
2011:photochemically
2007:
1964:
1939:
1935:
1930:
1926:
1921:
1917:
1912:
1908:
1903:
1899:
1894:
1890:
1885:
1881:
1876:
1837:
1831:
1827:
1793:
1777:
1773:
1769:
1765:
1757:
1753:
1749:
1745:
1705:
1700:
1673:
1669:
1665:
1661:
1657:
1653:
1648:
1644:
1640:
1636:
1632:
1621:
1617:
1607:
1603:
1599:
1595:
1566:
1562:
1557:
1553:
1548:
1544:
1533:
1529:
1525:
1521:
1517:
1513:
1461:
1455:
1451:
1443:
1436:
1410:
1406:
1403:
1399:
1358:
1354:
1343:
1339:
1332:
1328:
1313:
1293:
1289:
1279:
1259:
1241:
1227:
1223:
1208:
1196:
1192:
1188:
1181:
1177:
1173:
1164:
1132:
1128:
1124:
1123:, containing a
1121:cyclic molecule
1117:
1112:
1076:
1072:
1046:
1042:
1029:
1016:
1009:
1004:
1003:
1002: ?)
993:
989:
985:
981:
970:
941:Polyphosphazene
924:
853:
839:
825:
809:
786:
761:
758:
752:
748:
736:
733:
727:
723:
711:
708:
702:
698:
695:
694:Std enthalpy of
676:
661:
659:Molecular shape
649:
635:
619:
610:
604:
592:
589:
588:
587:
583:
569:
552:
550:
535:−149×10 cm/mol
532:
522:
496:
470:
441:
429:
426:
377:
365:
361:
357:
354:
340:
337:
332:
331:
320:
317:
316:
313:
307:
306:
303:
297:
296:
285:
267:
260:
251:
231:
218:
206:
169:
149:
129:
116:
105:
92:
78:
77:
52:
12:
11:
5:
2829:
2827:
2819:
2818:
2813:
2808:
2803:
2798:
2793:
2788:
2778:
2777:
2772:
2771:
2754:
2747:
2721:
2706:
2675:
2621:
2569:
2554:
2515:
2494:(2): 321–329.
2469:
2429:
2365:
2358:
2296:
2277:(2): 119–135.
2237:
2236:
2234:
2231:
2230:
2229:
2203:(2): 203–209.
2187:
2153:
2138:
2133:
2130:
2107:hydrophobicity
2103:hydrophilicity
2067:
2064:
2006:
2003:
1988:phenylpyridine
1963:
1960:
1875:
1874:Lewis basicity
1872:
1792:
1789:
1781:
1780:
1763:
1704:
1701:
1699:
1696:
1681:
1680:
1651:
1614:
1613:
1512:
1509:
1469:
1468:
1381:by hot water.
1347:exothermically
1312:
1309:
1278:
1275:
1258:
1257:Early analyses
1255:
1240:
1237:
1225:
1221:
1207:
1204:
1162:
1116:
1113:
1111:
1108:
1104:polymerisation
1014:
1011:
1010:
1005:
983:
982:
978:standard state
975:
972:
971:
969:
968:
963:
958:
953:
948:
943:
938:
933:
927:
925:
922:
919:
918:
914:
913:
912:Non-flammable
910:
904:
903:
881:P305+P351+P338
873:P303+P361+P353
869:P301+P330+P331
854:
849:
846:
845:
840:
835:
832:
831:
826:
821:
818:
817:
810:
805:
802:
801:
791:
790:
789:mild irritant
787:
784:
781:
780:
771:
770:
766:
765:
762:
756:
750:
744:
741:
740:
737:
731:
725:
719:
716:
715:
714:−812.4 kJ/mol
712:
706:
700:
692:
689:
688:
684:
683:
677:
672:
669:
668:
662:
657:
654:
653:
650:
640:
637:
636:
622:
620:
615:
612:
611:
608:
605:
600:
597:
596:
590:
584:
579:
576:
575:
570:
565:
562:
561:
557:
556:
555:1.62 (589 nm)
553:
548:
540:
537:
536:
533:
527:
524:
523:
521:
520:
517:
514:
510:
508:
498:
497:
495:
494:
491:
488:
484:
482:
472:
471:
469:
468:
465:
462:
458:
456:
446:
445:
442:
437:
434:
433:
430:
423:
420:
419:
416:
410:
409:
406:
400:
399:
396:
390:
389:
386:
382:
381:
375:
369:
368:
355:
350:
347:
346:
342:
341:
339:
338:
335:
327:
326:
325:
322:
321:
319:
318:
314:
311:
310:
308:
304:
301:
300:
292:
291:
290:
287:
286:
284:
283:
270:
268:
256:
253:
252:
250:
249:
241:
239:
233:
232:
230:
229:
221:
219:
211:
208:
207:
205:
204:
200:
198:
190:
189:
179:
171:
170:
168:
167:
159:
157:
151:
150:
148:
147:
139:
137:
131:
130:
128:
127:
119:
117:
110:
107:
106:
104:
103:
95:
93:
88:
85:
84:
80:
79:
76:
75:
72:
69:
66:
63:
59:
58:
54:
53:
50:
44:
43:
39:
38:
30:
29:
13:
10:
9:
6:
4:
3:
2:
2828:
2817:
2814:
2812:
2809:
2807:
2804:
2802:
2799:
2797:
2794:
2792:
2789:
2787:
2784:
2783:
2781:
2768:
2767:0-12-352651-5
2764:
2758:
2755:
2750:
2748:9780470132371
2744:
2740:
2736:
2732:
2725:
2722:
2717:
2710:
2707:
2702:
2698:
2694:
2690:
2686:
2679:
2676:
2671:
2667:
2663:
2659:
2655:
2651:
2647:
2643:
2639:
2632:
2630:
2628:
2626:
2622:
2617:
2613:
2609:
2605:
2601:
2597:
2590:
2588:
2586:
2584:
2582:
2580:
2578:
2576:
2574:
2570:
2565:
2561:
2557:
2551:
2547:
2546:
2538:
2536:
2534:
2532:
2530:
2528:
2526:
2524:
2522:
2520:
2516:
2511:
2507:
2502:
2497:
2493:
2489:
2485:
2478:
2476:
2474:
2470:
2466:
2465:0-13-465881-7
2462:
2456:
2454:
2452:
2450:
2448:
2446:
2444:
2442:
2440:
2438:
2436:
2434:
2430:
2425:
2421:
2417:
2413:
2409:
2405:
2401:
2397:
2393:
2386:
2384:
2382:
2380:
2378:
2376:
2374:
2372:
2370:
2366:
2361:
2355:
2351:
2347:
2343:
2337:
2335:
2333:
2331:
2329:
2327:
2325:
2323:
2321:
2319:
2317:
2315:
2313:
2311:
2309:
2307:
2305:
2303:
2301:
2297:
2292:
2288:
2284:
2280:
2276:
2272:
2268:
2261:
2259:
2257:
2255:
2253:
2251:
2249:
2247:
2245:
2243:
2239:
2232:
2226:
2222:
2218:
2214:
2210:
2206:
2202:
2198:
2194:
2188:
2184:
2180:
2176:
2172:
2168:
2164:
2160:
2154:
2151:
2150:
2145:
2144:
2139:
2136:
2135:
2131:
2129:
2127:
2123:
2119:
2114:
2112:
2108:
2104:
2100:
2096:
2095:thermoplastic
2092:
2088:
2084:
2079:
2077:
2073:
2065:
2063:
2059:
2053:
2046:
2042:
2038:
2032:
2026:
2020:
2012:
2004:
2002:
2000:
1999:oligopeptides
1996:
1991:
1989:
1985:
1981:
1977:
1973:
1969:
1961:
1959:
1957:
1953:
1949:
1943:
1941:
1932:
1923:
1914:
1905:
1896:
1887:
1873:
1871:
1869:
1865:
1856:
1852:
1850:
1846:
1845:cross-linking
1842:
1839:chain, where
1836:
1825:
1821:
1820:chloropolymer
1816:
1814:
1810:
1806:
1802:
1798:
1790:
1788:
1786:
1783:The observed
1764:
1761:
1744:
1743:
1742:
1740:
1736:
1732:
1723:
1719:
1717:
1713:
1710:
1702:
1697:
1695:
1693:
1690:
1686:
1679:
1652:
1631:
1630:
1629:
1628:intermediate
1627:
1611:
1594:
1593:
1592:
1591:
1588:
1584:
1580:
1576:
1572:
1569:
1559:
1542:
1539:
1523:
1510:
1508:
1505:
1501:
1497:
1488:
1484:
1482:
1478:
1474:
1465:
1460:
1447:
1439:
1435:
1434:
1433:
1431:
1430:stoichiometry
1427:
1423:
1422:chlorobenzene
1419:
1416:solvents are
1415:
1397:
1393:
1390:
1387:
1382:
1380:
1376:
1372:
1368:
1364:
1352:
1348:
1337:
1326:
1322:
1318:
1310:
1308:
1306:
1302:
1297:
1286:
1284:
1276:
1274:
1272:
1269:
1265:
1256:
1251:
1245:
1238:
1236:
1234:
1229:
1219:
1214:
1212:
1205:
1203:
1201:
1186:
1170:
1168:
1159:
1157:
1153:
1149:
1145:
1141:
1137:
1122:
1114:
1109:
1107:
1105:
1101:
1097:
1093:
1087:
1085:
1081:
1071:
1067:
1063:
1059:
1055:
1051:
1039:
1036:
1033:
1028:
1024:
1020:
1008:
1001:
996:
979:
973:
967:
964:
962:
959:
957:
954:
952:
949:
947:
944:
942:
939:
937:
934:
932:
929:
928:
926:
921:
920:
915:
911:
909:
906:
905:
855:
852:
848:
847:
841:
838:
834:
833:
830:
827:
824:
820:
819:
815:
811:
808:
804:
803:
799:
797:
792:
788:
783:
782:
778:
777:
772:
767:
763:
755:
747:
743:
742:
738:
730:
722:
718:
717:
713:
705:
697:
691:
690:
685:
682:
678:
675:
674:Dipole moment
671:
670:
666:
663:
660:
656:
655:
651:
647:
643:
642:Formula units
639:
638:
633:
629:
625:
621:
618:
614:
613:
606:
603:
599:
598:
585:
582:
578:
577:
574:
571:
568:
564:
563:
558:
554:
547:
543:
539:
538:
534:
530:
526:
525:
518:
515:
512:
511:
509:
507:
503:
500:
499:
492:
489:
486:
485:
483:
481:
477:
474:
473:
466:
463:
460:
459:
457:
455:
451:
448:
447:
443:
440:
436:
435:
431:
428:
422:
421:
417:
415:
414:Boiling point
412:
411:
407:
405:
404:Melting point
402:
401:
397:
395:
392:
391:
387:
384:
383:
376:
374:
371:
370:
356:
353:
349:
348:
343:
334:
330:
323:
309:
299:
295:
288:
280:
276:
275:DTXSID4061331
272:
271:
269:
259:
255:
254:
247:
243:
242:
240:
238:
235:
234:
227:
223:
222:
220:
214:
210:
209:
202:
201:
199:
197:
192:
191:
187:
183:
180:
178:
176:ECHA InfoCard
173:
172:
165:
161:
160:
158:
156:
153:
152:
145:
144:ChEMBL2022081
141:
140:
138:
136:
133:
132:
125:
121:
120:
118:
114:
109:
108:
101:
97:
96:
94:
91:
87:
86:
81:
73:
70:
67:
64:
61:
60:
55:
49:
45:
40:
36:
31:
27:
22:
16:
2816:Phosphazenes
2757:
2730:
2724:
2715:
2709:
2692:
2688:
2678:
2645:
2641:
2599:
2595:
2544:
2491:
2487:
2399:
2395:
2345:
2274:
2270:
2200:
2196:
2166:
2162:
2148:
2142:
2115:
2080:
2069:
2066:Applications
2057:
2051:
2036:
2030:
2024:
2018:
2008:
1992:
1979:
1975:
1965:
1944:
1877:
1861:
1840:
1834:
1817:
1808:
1796:
1794:
1782:
1735:substitution
1731:nucleophilic
1728:
1709:alkali metal
1706:
1682:
1677:
1615:
1590:intermediate
1587:nucleophilic
1583:side product
1571:dissociation
1537:
1514:
1493:
1472:
1470:
1463:
1458:
1445:
1437:
1414:chlorocarbon
1383:
1314:
1298:
1287:
1282:
1280:
1267:
1263:
1260:
1249:
1230:
1215:
1209:
1171:
1160:
1151:
1118:
1088:
1018:
1017:
828:
795:
785:Main hazards
774:
764:76.2 kJ/mol
753:
739:55.2 kJ/mol
728:
703:
645:
631:
627:
623:
573:orthorhombic
545:
83:Identifiers
57:Other names
15:
2695:: 139–150.
2091:elastomeric
2045:mercury arc
1974:and either
1849:elastomeric
1805:Lewis acids
1641:] → [Cl
1581:(the major
1575:Elimination
1389:chlorinated
1218:IR spectrum
1167:tetrahedral
1158:analogues.
1156:phosphazane
1080:phosphazene
1054:unsaturated
956:Polythiazyl
908:Flash point
823:Signal word
779:(OHS/OSH):
602:Point group
586:62 (Pnma, D
581:Space group
480:cyclohexane
444:decomposes
425:Sublimation
385:Appearance
345:Properties
182:100.012.160
2780:Categories
2689:Ann. Pharm
2233:References
2122:fuel lines
1851:behaviour.
1766:(NPCl(OR))
1666:] → HN=PCl
1637:+ [PCl
1604:] → HN=PCl
1600:+ [PCl
1412:. Typical
1379:hydrolysis
1317:von Liebig
1140:phosphorus
1058:phosphorus
807:Pictograms
560:Structure
502:Solubility
476:Solubility
450:Solubility
427:conditions
373:Molar mass
246:7VR28MTM9D
155:ChemSpider
111:3D model (
90:CAS Number
48:IUPAC name
2670:208761169
2662:1369-9261
2564:838102247
2416:0020-1669
2291:0009-2665
2217:0039-7911
2183:0002-7863
1948:fluxional
1868:alkoxides
1828:(N=P(−Cl)
1822:product (
1712:alkoxides
1698:Reactions
1692:oligomers
1674:+ HCl + H
1658:+ [Cl
1530:][PCl
1432:applies:
1311:Synthesis
1096:alkoxides
1025:with the
877:P304+P340
798:labelling
696:formation
203:213-376-8
195:EC Number
2801:Nitrides
2616:16156607
2510:16552166
2424:16270979
2225:97319633
2099:backbone
2035:, where
1972:pyridine
1968:coupling
1778:+ 3 NaCl
1739:chloride
1618:[PCl
1608:+ HCl +
1545:[PCl
1526:[PCl
1500:tetramer
1392:solvents
1345:) react
1271:orbitals
1144:chlorine
1136:nitrogen
1062:nitrogen
966:Borazine
769:Hazards
531:(χ)
100:940-71-6
2118:O-rings
2072:aryloxy
1662:P−N=PCl
1649:] + HCl
1645:P−N=PCl
1626:acyclic
1563:[NH
1502:. Slow
1452:→ (NPCl
1440:[NH
1355:[NH
1336:ammonia
1266:and P 3
1239:Bonding
1084:benzene
1068:of the
1000:what is
998: (
961:Benzene
394:Density
367:
213:PubChem
2765:
2745:
2668:
2660:
2614:
2562:
2552:
2508:
2463:
2422:
2414:
2356:
2289:
2223:
2215:
2181:
2089:. The
1984:indole
1716:amides
1689:cyclic
1670:−N=PCl
1633:HN=PCl
1560:(from
1496:trimer
1471:where
1444:]Cl +
1369:, and
1334:) and
1321:Wöhler
1216:In it
1066:trimer
1050:cyclic
1021:is an
995:verify
992:
829:Danger
757:sublim
506:xylene
378:347.64
329:SMILES
226:220225
164:190959
135:ChEMBL
42:Names
2666:S2CID
2221:S2CID
2015:(NPCl
1956:P-NMR
1746:(NPCl
1386:inert
1211:P-NMR
1073:N≡PCl
665:chair
358:(NPCl
294:InChI
113:JSmol
2763:ISBN
2743:ISBN
2658:ISSN
2612:PMID
2560:OCLC
2550:ISBN
2506:PMID
2461:ISBN
2420:PMID
2412:ISSN
2354:ISBN
2287:ISSN
2213:ISSN
2179:ISSN
2124:and
2093:and
1954:and
1927:VOCl
1918:TaCl
1900:GaCl
1891:AlBr
1882:AlCl
1760:NaCl
1758:+ 3
1733:poly
1729:The
1714:and
1678:etc.
1498:and
1292:and
1226:P–Cl
1172:The
1138:and
1060:and
901:P501
897:P405
893:P363
889:P321
885:P310
865:P280
861:P264
857:P260
843:H314
237:UNII
2735:doi
2697:doi
2650:doi
2604:doi
2496:doi
2404:doi
2279:doi
2205:doi
2171:doi
2167:113
2105:or
2041:UVC
1936:BCl
1737:of
1579:HCl
1577:of
1573:).
1567:]Cl
1543:of
1538:via
1518:PCl
1483:).
1466:HCl
1448:PCl
1420:or
1407:PCl
1359:]Cl
1329:PCl
1222:P–N
796:GHS
732:vap
707:298
504:in
478:in
452:in
263:EPA
216:CID
2782::
2741:.
2693:11
2691:.
2687:.
2664:.
2656:.
2646:43
2644:.
2640:.
2624:^
2610:.
2600:44
2598:.
2572:^
2558:.
2518:^
2504:.
2492:62
2490:.
2486:.
2472:^
2432:^
2418:.
2410:.
2400:44
2398:.
2394:.
2368:^
2352:.
2299:^
2285:.
2275:91
2273:.
2269:.
2241:^
2219:.
2211:.
2201:32
2199:.
2195:.
2177:.
2165:.
2161:.
2120:,
2113:.
2017:2−
1958:.
1942:.
1924:,
1915:,
1909:SO
1906:,
1897:,
1888:,
1832:−)
1815:.
1809:ca
1797:ca
1718:.
1694:.
1676:,
1654:NH
1596:NH
1554:NH
1462:+
1400:NH
1371:Cl
1365:,
1340:NH
1273:.
1235:.
1228:.
1163:3h
1152:ca
1148:pm
1052:,
1038:Cl
899:,
895:,
891:,
887:,
883:,
879:,
875:,
871:,
867:,
863:,
859:,
800::
749:(Δ
724:(Δ
699:(Δ
679:0
652:4
609:3h
595:)
591:2h
2769:.
2751:.
2737::
2703:.
2699::
2672:.
2652::
2618:.
2606::
2566:.
2512:.
2498::
2467:.
2426:.
2406::
2362:.
2293:.
2281::
2227:.
2207::
2185:.
2173::
2058:n
2055:N
2052:n
2049:P
2043:(
2037:n
2031:n
2028:)
2025:x
2022:R
2019:x
1980:N
1978:,
1976:N
1952:N
1938:3
1929:3
1920:5
1911:3
1902:3
1893:3
1884:3
1841:n
1835:n
1830:2
1776:3
1774:)
1772:2
1768:3
1756:3
1752:3
1750:)
1748:2
1672:3
1668:2
1664:3
1660:3
1656:3
1647:3
1643:3
1639:4
1635:3
1622:]
1620:4
1610:H
1606:3
1602:4
1598:3
1565:4
1556:3
1549:]
1547:4
1534:]
1532:6
1528:4
1520:5
1473:n
1464:n
1459:n
1456:)
1454:2
1450:5
1446:n
1442:4
1438:n
1409:5
1405:/
1402:3
1367:N
1363:P
1357:4
1353:(
1342:3
1338:(
1331:5
1327:(
1294:N
1290:P
1283:d
1268:d
1264:p
1250:d
1248:3
1195:3
1193:N
1191:3
1189:P
1180:3
1178:N
1176:3
1174:P
1131:3
1129:N
1127:3
1125:P
1075:2
1045:3
1043:)
1041:2
1035:P
1032:N
1030:(
990:N
759:)
754:H
751:f
734:)
729:H
726:f
709:)
704:H
701:f
681:D
648:)
646:Z
644:(
632:c
628:b
624:a
607:D
551:)
549:D
546:n
544:(
364:3
362:)
360:2
265:)
261:(
115:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.