122:
68:
271:
Magnus, Philip; Turnbull, Rachel (2006). "Thermal and Acid-Catalyzed
Hofmann–Martius Rearrangement of 3-N-Aryl-2-oxindoles into 3-(Arylamino)-2-oxindoles".
121:
67:
329:
156:
90:
145:
98:
97:
centers around dissociation of the reactant with the positively charged organic residue R attacking the aniline ring in a
133:
129:
44:
48:
36:
79:
151:
86:
85:
The reaction is also known to work for aryl ethers and two conceptually related reactions are the
94:
288:
242:"XV.—Intramolecular rearrangement of the alkylarylamines: Formation of 4-amino-n-butylbenzene"
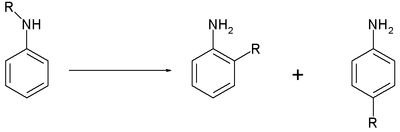
59:
32:
17:
280:
253:
222:
191:
51:
323:
226:
195:
292:
257:
55:
310:
40:
284:
241:
210:
179:
74:
When the catalyst is a metal halide the reaction is also called the
54:-alkylated aniline. The reaction requires heat, and the
104:
In one study this rearrangement was applied to a 3-N(CH
313:at 80 °C gives 30% 2-o (ortho) and 37% 2-p (para)
215:Berichte der Deutschen Chemischen Gesellschaft
184:Berichte der Deutschen Chemischen Gesellschaft
8:
128:The reaction is named after German chemists
240:Reilly, J.; Hickinbottom, W. J. (1920).
180:"Methylirung der Phenylgruppe im Anilin"
178:Hofmann, A. W.; Martius, C. A. (1871).
170:
7:
211:"Umwandlung des Anilins in Toluidin"
25:
76:Reilly–Hickinbottom rearrangement
120:
66:
1:
29:Hofmann–Martius rearrangement
18:Hofmann-Martius rearrangement
346:
157:Fischer–Hepp rearrangement
134:Carl Alexander von Martius
130:August Wilhelm von Hofmann
91:Fischer–Hepp rearrangement
39:converting an N-alkylated
146:Friedel–Crafts alkylation
99:Friedel–Crafts alkylation
227:10.1002/cber.18720050241
196:10.1002/cber.18710040271
330:Rearrangement reactions
209:Hofmann, A. W. (1872).
37:rearrangement reaction
43:to the corresponding
258:10.1039/ct9201700103
82:and Joseph Reilly).
80:Wilfred Hickinbottom
152:Fries rearrangement
87:Fries rearrangement
95:reaction mechanism
285:10.1021/ol061191z
148:-like reactions:
60:hydrochloric acid
33:organic chemistry
16:(Redirected from
337:
314:
303:
297:
296:
268:
262:
261:
237:
231:
230:
206:
200:
199:
175:
124:
70:
58:is an acid like
21:
345:
344:
340:
339:
338:
336:
335:
334:
320:
319:
318:
317:
304:
300:
273:Organic Letters
270:
269:
265:
239:
238:
234:
208:
207:
203:
177:
176:
172:
167:
142:
115:
111:
107:
23:
22:
15:
12:
11:
5:
343:
341:
333:
332:
322:
321:
316:
315:
298:
279:(16): 3497–9.
263:
232:
221:(2): 720–722.
201:
169:
168:
166:
163:
162:
161:
160:
159:
154:
141:
138:
126:
125:
116:)-2-oxindole:
113:
109:
105:
72:
71:
24:
14:
13:
10:
9:
6:
4:
3:
2:
342:
331:
328:
327:
325:
312:
308:
302:
299:
294:
290:
286:
282:
278:
274:
267:
264:
259:
255:
251:
247:
243:
236:
233:
228:
224:
220:
216:
212:
205:
202:
197:
193:
189:
185:
181:
174:
171:
164:
158:
155:
153:
150:
149:
147:
144:
143:
139:
137:
135:
131:
123:
119:
118:
117:
102:
100:
96:
92:
88:
83:
81:
78:(named after
77:
69:
65:
64:
63:
61:
57:
53:
50:
46:
42:
38:
34:
30:
19:
306:
301:
276:
272:
266:
249:
246:J. Chem. Soc
245:
235:
218:
214:
204:
187:
183:
173:
127:
103:
84:
75:
73:
28:
26:
252:: 103–137.
190:(2): 742.
165:References
47:and / or
324:Category
305:heating
293:16869644
140:See also
89:and the
56:catalyst
311:toluene
41:aniline
291:
93:. Its
45:ortho
35:is a
289:PMID
132:and
52:aryl
49:para
27:The
309:in
281:doi
254:doi
250:117
223:doi
192:doi
108:)(C
31:in
326::
287:.
275:.
248:.
244:.
217:.
213:.
186:.
182:.
136:.
101:.
62:.
307:1
295:.
283::
277:8
260:.
256::
229:.
225::
219:5
198:.
194::
188:4
114:5
112:H
110:6
106:3
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.