176:
232:
34:
20:
415:
358:
191:, the photon energy is converted and increases the molecule's internal energy level. Likewise, when an excited molecule releases energy, it can do so in the form of a photon. Depending on the energy of the photon, this could correspond to a change in vibrational, electronic, or rotational
23:
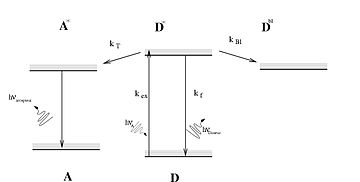
A Jablonski diagram showing the excitation of molecule A to its singlet excited state (A*) followed by intersystem crossing to the triplet state (A) that relaxes to the ground state by phosphorescence. It was used to describe absorption and emission of light by
198:
Radiative transitions involve either the absorption or emission of a photon. As mentioned above, these transitions are denoted with solid arrows with their tails at the initial energy level and their tips at the final energy level.
213:(IC), which occurs when a vibrational state of an electronically excited state can couple to a vibrational state of a lower electronic state. The molecule could then subsequently relax further through vibrational relaxation.
162:
by straight arrows. The vibrational ground states of each electronic state are indicated with thick lines, the higher vibrational states with thinner lines. The diagram is named after the Polish physicist
202:
Nonradiative transitions arise through several different mechanisms, all differently labeled in the diagram. Relaxation of the excited state to its lowest vibrational level is called
399:
456:
188:
392:
236:
206:. This process involves the dissipation of energy from the molecule to its surroundings, and thus it cannot occur for isolated molecules.
51:
295:
224:, intersystem crossing is much more important than in molecules that exhibit only small spin-orbit coupling. ISC can be followed by
117:
273:“Jablonski Diagram.” 2006. In IUPAC Compendium of Chemical Terminoloy, 3rd ed. International Union of Pure and Applied Chemistry.
495:
98:
449:
385:
210:
70:
55:
203:
77:
249:
500:
480:
442:
343:
164:
84:
150:, and also the transitions between them. The states are arranged vertically by energy and grouped horizontally by
44:
175:
151:
66:
338:
485:
306:
Jabłoński, Aleksander "Efficiency of Anti-Stokes
Fluorescence in Dyes" Nature 1933, volume 131, pp. 839-840.
490:
195:. The changes between these levels are called "transitions" and are plotted on the Jablonski diagram.
217:
155:
221:
220:(ISC); this is a transition to a state with a different spin multiplicity. In molecules with large
143:
91:
475:
365:
291:
159:
426:
369:
307:
254:
139:
225:
179:
Jablonski diagram including vibrational levels for absorbance, non-radiative decay, and
231:
469:
422:
192:
180:
131:
33:
19:
274:
147:
414:
311:
357:
174:
27:
326:, Tenth Edition (2020), pp 457-458, W.H. Freeman and Co.
290:, 8th edition (2006), page 494, Oxford University Press.
344:
Consequences of Light
Absorption – The Jablonski Diagram
430:
373:
58:. Unsourced material may be challenged and removed.
339:Florida State University: Jablonski diagram primer
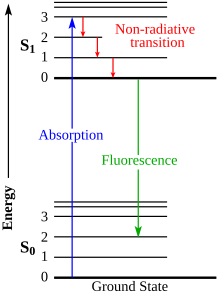
16:Diagram of the electronic states of a molecule
450:
393:
8:
209:A second type of nonradiative transition is
457:
443:
400:
386:
118:Learn how and when to remove this message
230:
18:
275:https://doi.org/10.1351/goldbook.J03360
266:
158:are indicated by squiggly arrows and
7:
411:
409:
354:
352:
56:adding citations to reliable sources
429:. You can help Knowledge (XXG) by
372:. You can help Knowledge (XXG) by
138:is a diagram that illustrates the
14:
237:Förster resonance energy transfer
235:A Jablonski diagram representing
413:
356:
32:
167:who first proposed it in 1933.
43:needs additional citations for
324:Quantitative Chemical Analysis
1:
286:P., Atkins, P., de Paula, J.
517:
408:
351:
322:Harris, D. C. Lucy, C. A.
288:Atkins' Physical Chemistry
156:Nonradiative transitions
496:Molecular physics stubs
250:Franck–Condon principle
425:-related article is a
368:–related article is a
240:
204:vibrational relaxation
184:
25:
234:
178:
160:radiative transitions
22:
218:intersystem crossing
165:Aleksander Jabłoński
52:improve this article
222:spin-orbit coupling
211:internal conversion
67:"Jablonski diagram"
501:Spectroscopy stubs
241:
185:
144:vibrational levels
26:
481:Molecular physics
438:
437:
381:
380:
366:molecular physics
152:spin multiplicity
140:electronic states
136:Jablonski diagram
128:
127:
120:
102:
508:
459:
452:
445:
417:
410:
402:
395:
388:
360:
353:
327:
320:
314:
312:10.1038/131839b0
304:
298:
284:
278:
271:
255:Grotrian diagram
216:A third type is
189:absorbs a photon
187:When a molecule
123:
116:
112:
109:
103:
101:
60:
36:
28:
516:
515:
511:
510:
509:
507:
506:
505:
466:
465:
464:
463:
407:
406:
349:
335:
330:
321:
317:
305:
301:
285:
281:
272:
268:
264:
246:
226:phosphorescence
173:
124:
113:
107:
104:
61:
59:
49:
37:
17:
12:
11:
5:
514:
512:
504:
503:
498:
493:
488:
486:Photochemistry
483:
478:
468:
467:
462:
461:
454:
447:
439:
436:
435:
418:
405:
404:
397:
390:
382:
379:
378:
361:
347:
346:
341:
334:
333:External links
331:
329:
328:
315:
299:
279:
265:
263:
260:
259:
258:
252:
245:
242:
172:
169:
142:and often the
126:
125:
40:
38:
31:
15:
13:
10:
9:
6:
4:
3:
2:
513:
502:
499:
497:
494:
492:
489:
487:
484:
482:
479:
477:
474:
473:
471:
460:
455:
453:
448:
446:
441:
440:
434:
432:
428:
424:
419:
416:
412:
403:
398:
396:
391:
389:
384:
383:
377:
375:
371:
367:
362:
359:
355:
350:
345:
342:
340:
337:
336:
332:
325:
319:
316:
313:
309:
303:
300:
297:
296:0-7167-8759-8
293:
289:
283:
280:
276:
270:
267:
261:
256:
253:
251:
248:
247:
243:
238:
233:
229:
227:
223:
219:
214:
212:
207:
205:
200:
196:
194:
193:energy levels
190:
182:
177:
170:
168:
166:
161:
157:
153:
149:
145:
141:
137:
133:
130:In molecular
122:
119:
111:
100:
97:
93:
90:
86:
83:
79:
76:
72:
69: –
68:
64:
63:Find sources:
57:
53:
47:
46:
41:This article
39:
35:
30:
29:
24:fluorescents.
21:
491:Spectroscopy
431:expanding it
423:spectroscopy
420:
374:expanding it
363:
348:
323:
318:
302:
287:
282:
269:
215:
208:
201:
197:
186:
181:fluorescence
135:
132:spectroscopy
129:
114:
105:
95:
88:
81:
74:
62:
50:Please help
45:verification
42:
257:(for atoms)
171:Transitions
470:Categories
262:References
78:newspapers
108:July 2023
476:Diagrams
244:See also
148:molecule
92:scholar
294:
239:(FRET)
94:
87:
80:
73:
65:
421:This
364:This
146:of a
99:JSTOR
85:books
427:stub
370:stub
292:ISBN
134:, a
71:news
308:doi
54:by
472::
228:.
154:.
458:e
451:t
444:v
433:.
401:e
394:t
387:v
376:.
310::
277:.
183:.
121:)
115:(
110:)
106:(
96:·
89:·
82:·
75:·
48:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.