751:
observed effects (mortality, tail deformation), nor did the effects occur at earlier time points. The study has been regarded as invalid by the Danish
Environmental Protection Agency, which has evaluated icaridin prior to its approval under the EU Biocidal Product Regulation. The reasons for rejection were the testing of a mixture of undisclosed composition, the use of a non-standard test organism, the lack of analytical verification of actual test concentrations, and the fact that the test solution was never renewed with the 25 days of study duration.
795:
286:
211:
55:
46:
507:
743:
four days of exposure. Because the widely used LC50 test for assessing a chemical's environmental toxicity is based on mortality within four days, the authors suggested that icaridin would be incorrectly deemed as "safe" under the test protocol. However, icaridin was also non-toxic in a 21-day reproduction test on the water flea
785:
mosquitoes suggests icaridin does not strongly activate their olfactory receptor neurons, but instead reduces the volatility of the odorants with which it is mixed. By reducing their volatility, icaridin effectively "masks" odorants attractive to mosquitoes on the skin, preventing them from reaching
742:
A 2018 study found that a commercial repellent product containing 20% icaridin, in what the authors described as "conservative exposure doses", is highly toxic to larval salamanders, a major predator of mosquito larvae. The study observed high larval salamander mortality occurring delayed after the
750:
Since only the icaridin content of the tested repellent product is known, the observed effects cannot be readily attributed to icaridin. Furthermore, the effects of the repellent product showed no dose-response relationship, i.e., there was neither an increase of the magnitude or severity of the
550:
which can be used directly on skin or clothing. It has broad efficacy against various arthropods such as mosquitos, ticks, gnats, flies and fleas, and is almost colorless and odorless. A study performed in 2010 showed that picaridin spray and cream at the 20% concentration provided 12 hours of
644:
Icaridin has been reported to be as effective as DEET at a 20% concentration without the irritation associated with DEET. According to the WHO, icaridin “demonstrates excellent repellent properties comparable to, and often superior to, those of the standard DEET.”
1253:
624:
Having been sold in Europe since 1998, on 23 July 2020, icaridin was approved again by the EU Commission for use in repellent products. The approval entered into force on 1 February 2022 and is valid for ten years.
555:, icaridin does not dissolve plastics, synthetics or sealants, is odorless and non-greasy and presents a lower risk of toxicity when used with sunscreen, as it may reduce skin absorption of both compounds.
825:
Commercial products containing icaridin include Cutter
Advanced, Muskol, Repeltec, Skin So Soft Bug Guard Plus, Sawyer Picaridin Insect Repellent, Off! FamilyCare, Autan, Smidge, PiActive and MOK.O.
1479:
776:) revealed that icaridin binds to the DEET-binding site in two distinct orientations and also to a second binding site (sIC-binding site) located at the C-terminal region of the AgamOBP1.
520:
1104:"Commission Implementing Regulation (EU) 2020/1086 of 23 July 2020 approving icaridin as an existing active substance for use in biocidal products of product-type 19"
335:
954:
1324:
696:
641:
and concluded that they are equally preferred mosquito repellents, noting that 50% DEET offers longer protection but is not available in some countries.
637:
are the most effective insect repellents available. A 2018 systematic review found no consistent performance difference between icaridin and DEET in
914:
Efficacy Test of KBR 3023 (Picaridin; Icaridin) - Based
Personal Insect Repellents (20% Cream and 20% Spray) with Ticks Under Laboratory Conditions
1187:"Field evaluation of picaridin repellents reveals differences in repellent sensitivity between Southeast Asian vectors of malaria and arboviruses"
1103:
654:
in 2016 as among the most effective insect repellents when used at a 20% concentration. Icaridin was earlier reported to be effective by
1236:
1587:"The crystal structure of the AgamOBP1•Icaridin complex reveals alternative binding modes and stereo-selective repellent recognition"
563:
300:
1756:
839:
700:
1268:"Field evaluation of repellent formulations containing deet and picaridin against mosquitoes in Northern Territory, Australia"
1066:
148:
527:
1771:
243:
723:
264:
1776:
913:
937:
794:
1796:
1791:
567:
1078:
1504:
1457:
1435:
1062:
206:
761:
168:
1702:
978:"Percutaneous penetration and pharmacodynamics: Wash-in and wash-off of sunscreen and insect repellent"
664:
retests in 2006 gave as result that a 7% solution of icaridin offered little or no protection against
1786:
1658:
1539:
977:
759:
In 2014, a potential odorant receptor for icaridin (and DEET), CquiOR136•CquiOrco, was suggested for
73:
1781:
858:
708:
704:
281:
114:
1624:
1409:
1013:
781:
734:
Icaridin can cause mild to moderate eye irritation on contact and is slightly toxic if ingested.
1173:
772:
odorant binding protein 1 (AgamOBP1). The crystal structure of AgamOBP1•icaridin complex (PDB:
1362:"High mortality in aquatic predators of mosquito larvae caused by exposure to insect repellent"
1684:
1616:
1567:
1391:
1289:
1218:
1155:
1147:
1125:
1005:
997:
773:
1338:
1031:
1674:
1666:
1606:
1598:
1557:
1547:
1505:"Opinion on the application for approval of the active substance Icaridin, Product type: 19"
1381:
1373:
1279:
1240:
1208:
1198:
1185:
Van Roey K, Sokny M, Denis L, Van den Broeck N, Heng S, Siv S, et al. (December 2014).
1137:
1126:"Mosquito repellents for the traveller: does picaridin provide longer protection than DEET?"
989:
656:
650:
547:
477:
451:
358:
188:
124:
252:
719:
1662:
1611:
1586:
1543:
617:
AG and its subsidiary
Saltigo GmbH were spun off from Bayer and the product was renamed
285:
210:
1679:
1646:
1585:
Drakou CE, Tsitsanou KE, Potamitis C, Fessas D, Zervou M, Zographos SE (January 2017).
1562:
1527:
1386:
1361:
1213:
1186:
671:
638:
498:
684:(vector of West Nile virus), while a 15% solution was good for about one hour against
54:
1765:
440:
430:
199:
1746:
1628:
1320:
1017:
309:
InChI=1S/C12H23NO3/c1-3-10(2)16-12(15)13-8-5-4-6-11(13)7-9-14/h10-11,14H,3-9H2,1-2H3
803:
675:
591:
319:
InChI=1/C12H23NO3/c1-3-10(2)16-12(15)13-8-5-4-6-11(13)7-9-14/h10-11,14H,3-9H2,1-2H3
232:
993:
1727:
1203:
852:
1532:
Proceedings of the
National Academy of Sciences of the United States of America
1284:
1267:
1670:
1602:
849:
845:
579:
462:
389:
179:
1703:"Insect Repellent Solutions | Repeltec | AFFIX Labs | Finland"
1151:
1001:
888:
1752:
Choosing and Using Insect
Repellents - National Pesticide Information Center
1647:"Commonly Used Insect Repellents Hide Human Odors from Anopheles Mosquitoes"
1552:
1049:
814:
807:
1688:
1620:
1571:
1395:
1377:
1293:
1222:
1159:
1009:
806:: one where the hydroxyethyl chain attaches to the ring, and one where the
1142:
715:
587:
583:
17:
817:. The commercial material contains a mixture of all four stereoisomers.
605:
among others. The compound was developed by the German chemical company
45:
1645:
Afify A, Betz JF, Riabinina O, Lahondère C, Potter CJ (November 2019).
941:
614:
468:
420:
219:
28:
1360:
Almeida RM, Han BA, Reisinger AJ, Kagemann C, Rosi EJ (October 2018).
159:
1747:
Picaridin
General Fact Sheet - National Pesticide Information Center
1416:. ScienceDaily. Cary Institute of Ecosystem Studies. 31 October 2018
1410:"Widely used mosquito repellent proves lethal to larval salamanders"
1256:(link recreated from Wayback Machine Internet Archive - 19 May 2019)
1254:- Consumer Reports Confirms Effectiveness Of New Alternative To Deet
497:
Except where otherwise noted, data are given for materials in their
1751:
864:
793:
768:
Recent crystal and solution studies showed that icaridin binds to
680:
666:
606:
147:
137:
1728:
Zika virus FAQ: What is it, and what are the risks as it spreads?
834:
634:
552:
410:
571:
1174:
http://jddonline.com/articles/dermatology/S1545961604P0059X/1
1706:
269:
53:
44:
570:(WHO), but the official name that has been approved by the
1528:"Mosquito odorant receptor for DEET and methyl jasmonate"
1266:
Frances SP, Waterson DG, Beebe NW, Cooper RD (May 2004).
955:"Picaridin vs DEET: Which Is the Best Insect Repellent?"
515:
1526:
Xu P, Choo YM, De La Rosa A, Leal WS (November 2014).
660:(7% solution) and the Australian Army (20% solution).
855:
that can be applied to clothing to help prevent bites
699:
recommends using repellents based on icaridin, DEET,
78:
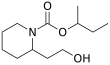
Butan-2-yl 2-(2-hydroxyethyl)piperidine-1-carboxylate
1237:"Mosquito Repellents That Best Protect Against Zika"
1341:. National Pesticide Information Center. March 2009
678:) and a protection time of about 2.5 hours against
867:, another substituted-piperidine insect repellent
747:and a 32-day early life-stage test in zebrafish.
99:-Butyl 2-(2-hydroxyethyl)piperidine-1-carboxylate
1124:Goodyer, Larry; Schofield, Steven (2018-05-01).
231:
1172:Journal of Drugs in Dermatology (Jan-Feb 2004)
648:Icaridin-based products have been evaluated by
123:
1306:"Insect repellents: which keep bugs at bay?"
8:
582:family, along with many pharmaceuticals and
93:Hydroxyethyl isobutyl piperidine carboxylate
1325:Centers for Disease Control and Prevention
957:. Appalachian Mountain Club. 4 August 2023
697:Centers for Disease Control and Prevention
284:
209:
187:
33:
1678:
1610:
1561:
1551:
1385:
1310:, June 2006, vol 71 (issue 6), p. 6.
1283:
1212:
1202:
1141:
251:
1050:"Bayer Completes Spin Off of Lanxess AG"
919:(Report). LANXESS Corporation. p. 9
786:the olfactory receptors to some extent.
714:, PMD) for effective protection against
435:−170 °C (−274 °F; 103 K)
877:
340:
305:
280:
976:Rodriguez J, Maibach HI (2016-01-02).
445:296 °C (565 °F; 569 K)
200:
1640:
1638:
1036:National Pesticide information Center
609:in the 1980s and was given the name
312:Key: QLHULAHOXSSASE-UHFFFAOYSA-N
167:
7:
1591:Cellular and Molecular Life Sciences
1321:"Traveler's Health: Avoid bug bites"
883:
881:
982:Journal of Dermatological Treatment
322:Key: QLHULAHOXSSASE-UHFFFAOYAQ
222:
912:Carroll, Scott P. (5 April 2010).
25:
564:International Nonproprietary Name
551:protection against ticks. Unlike
1480:"ECHA - Information on biocides"
1458:"ECHA - Information on biocides"
1436:"ECHA - Information on biocides"
1339:"Picaridin Technical Fact Sheet"
1191:PLOS Neglected Tropical Diseases
1063:Saltigo renames insect repellant
1032:"Picaridin Technical Fact Sheet"
840:Ethyl butylacetylaminopropionate
701:ethyl butylacetylaminopropionate
505:
376:
370:
1067:Chemical & Engineering News
501:(at 25 °C , 100 kPa).
813:attaches to the oxygen of the
578:. The chemical is part of the
379:
364:
1:
1272:Journal of Medical Entomology
994:10.3109/09546634.2015.1050350
1204:10.1371/journal.pntd.0003326
1243:, April, 2016. 30 May 2018.
724:eastern equine encephalitis
40:
1813:
1285:10.1603/0022-2585-41.3.414
1130:Journal of Travel Medicine
26:
1671:10.1016/j.cub.2019.09.007
1603:10.1007/s00018-016-2335-6
798:Stereoisomers of icaridin
568:World Health Organization
495:
351:
343:O=C(OC(C)CC)N1C(CCO)CCCC1
331:
296:
107:
84:
72:
67:
39:
1079:"Icaridin - an overview"
27:Not to be confused with
1553:10.1073/pnas.1417244111
1726:Cha, Ariana Eunjung. "
1378:10.1098/rsbl.2018.0526
802:Icaridin contains two
799:
762:Culex quinquefasciatus
688:and 4.8 hours against
58:
49:
797:
726:and other illnesses.
57:
48:
1657:(21): 3669–3680.e5.
1136:(Suppl_1): S10–S15.
738:Environmental impact
703:(IR3535), or oil of
597:Trade names include
74:Preferred IUPAC name
1772:Household chemicals
1733:. January 21, 2016.
1731:The Washington Post
1663:2019CBio...29E3669A
1544:2014PNAS..11116592X
1538:(46): 16592–16597.
859:p-Menthane-3,8-diol
821:Commercial products
755:Mechanism of action
562:was proposed as an
452:Solubility in water
397: g·mol
36:
1507:. 10 December 2019
1460:. pp. 220–229
1438:. pp. 231–245
1143:10.1093/jtm/tay005
1052:. 31 January 2005.
944:on August 9, 2011.
800:
782:Anopheles coluzzii
712:-menthane-3,8-diol
695:The United States
528:Infobox references
59:
50:
34:
1777:Insect repellents
770:Anopheles gambiae
594:its spicy taste.
536:Chemical compound
534:
533:
405:colorless liquid
265:CompTox Dashboard
149:Interactive image
63:
62:
16:(Redirected from
1804:
1797:Sec-Butyl esters
1792:Primary alcohols
1734:
1724:
1718:
1717:
1715:
1714:
1705:. Archived from
1699:
1693:
1692:
1682:
1642:
1633:
1632:
1614:
1582:
1576:
1575:
1565:
1555:
1523:
1517:
1516:
1514:
1512:
1501:
1495:
1494:
1492:
1491:
1476:
1470:
1469:
1467:
1465:
1454:
1448:
1447:
1445:
1443:
1432:
1426:
1425:
1423:
1421:
1406:
1400:
1399:
1389:
1372:(10): 20180526.
1357:
1351:
1350:
1348:
1346:
1335:
1329:
1328:
1317:
1311:
1308:Consumer Reports
1304:
1298:
1297:
1287:
1263:
1257:
1251:
1245:
1244:
1241:Consumer Reports
1233:
1227:
1226:
1216:
1206:
1182:
1176:
1170:
1164:
1163:
1145:
1121:
1115:
1114:
1112:
1110:
1100:
1094:
1093:
1091:
1089:
1075:
1069:
1060:
1054:
1053:
1046:
1040:
1039:
1028:
1022:
1021:
973:
967:
966:
964:
962:
951:
945:
940:. Archived from
935:
929:
928:
926:
924:
918:
909:
903:
902:
900:
899:
885:
705:lemon eucalyptus
662:Consumer Reports
657:Consumer Reports
651:Consumer Reports
548:insect repellent
542:, also known as
518:
512:
509:
508:
478:Refractive index
396:
381:
378:
372:
366:
359:Chemical formula
289:
288:
273:
271:
255:
235:
224:
213:
202:
191:
171:
151:
127:
41:
37:
21:
1812:
1811:
1807:
1806:
1805:
1803:
1802:
1801:
1762:
1761:
1743:
1738:
1737:
1725:
1721:
1712:
1710:
1701:
1700:
1696:
1651:Current Biology
1644:
1643:
1636:
1584:
1583:
1579:
1525:
1524:
1520:
1510:
1508:
1503:
1502:
1498:
1489:
1487:
1478:
1477:
1473:
1463:
1461:
1456:
1455:
1451:
1441:
1439:
1434:
1433:
1429:
1419:
1417:
1408:
1407:
1403:
1366:Biology Letters
1359:
1358:
1354:
1344:
1342:
1337:
1336:
1332:
1319:
1318:
1314:
1305:
1301:
1265:
1264:
1260:
1252:
1248:
1235:
1234:
1230:
1184:
1183:
1179:
1171:
1167:
1123:
1122:
1118:
1108:
1106:
1102:
1101:
1097:
1087:
1085:
1077:
1076:
1072:
1061:
1057:
1048:
1047:
1043:
1030:
1029:
1025:
975:
974:
970:
960:
958:
953:
952:
948:
936:
932:
922:
920:
916:
911:
910:
906:
897:
895:
887:
886:
879:
874:
831:
823:
792:
757:
740:
732:
730:Adverse effects
720:West Nile virus
718:that carry the
631:
537:
530:
525:
524:
523: ?)
514:
510:
506:
502:
488:
486:
454:
394:
384:
375:
369:
361:
347:
344:
339:
338:
327:
324:
323:
320:
314:
313:
310:
304:
303:
292:
274:
267:
258:
238:
225:
194:
174:
154:
141:
130:
117:
103:
102:
80:
79:
32:
23:
22:
15:
12:
11:
5:
1810:
1808:
1800:
1799:
1794:
1789:
1784:
1779:
1774:
1764:
1763:
1760:
1759:
1757:EPA fact sheet
1754:
1749:
1742:
1741:External links
1739:
1736:
1735:
1719:
1694:
1634:
1597:(2): 319–338.
1577:
1518:
1496:
1484:echa.europa.eu
1471:
1449:
1427:
1401:
1352:
1330:
1312:
1299:
1278:(3): 414–417.
1258:
1246:
1228:
1177:
1165:
1116:
1095:
1070:
1055:
1041:
1023:
968:
946:
930:
904:
876:
875:
873:
870:
869:
868:
862:
856:
843:
837:
830:
827:
822:
819:
791:
788:
756:
753:
739:
736:
731:
728:
630:
627:
590:, which gives
535:
532:
531:
526:
504:
503:
499:standard state
496:
493:
492:
489:
484:
476:
473:
472:
465:
459:
458:
457:0.82 g/100 mL
455:
450:
447:
446:
443:
437:
436:
433:
427:
426:
423:
417:
416:
413:
407:
406:
403:
399:
398:
392:
386:
385:
382:
373:
367:
362:
357:
354:
353:
349:
348:
346:
345:
342:
334:
333:
332:
329:
328:
326:
325:
321:
318:
317:
315:
311:
308:
307:
299:
298:
297:
294:
293:
291:
290:
277:
275:
263:
260:
259:
257:
256:
248:
246:
240:
239:
237:
236:
228:
226:
218:
215:
214:
204:
196:
195:
193:
192:
184:
182:
176:
175:
173:
172:
164:
162:
156:
155:
153:
152:
144:
142:
135:
132:
131:
129:
128:
120:
118:
113:
110:
109:
105:
104:
101:
100:
94:
91:
87:
86:
82:
81:
77:
76:
70:
69:
65:
64:
61:
60:
51:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1809:
1798:
1795:
1793:
1790:
1788:
1785:
1783:
1780:
1778:
1775:
1773:
1770:
1769:
1767:
1758:
1755:
1753:
1750:
1748:
1745:
1744:
1740:
1732:
1729:
1723:
1720:
1709:on 2022-08-16
1708:
1704:
1698:
1695:
1690:
1686:
1681:
1676:
1672:
1668:
1664:
1660:
1656:
1652:
1648:
1641:
1639:
1635:
1630:
1626:
1622:
1618:
1613:
1608:
1604:
1600:
1596:
1592:
1588:
1581:
1578:
1573:
1569:
1564:
1559:
1554:
1549:
1545:
1541:
1537:
1533:
1529:
1522:
1519:
1506:
1500:
1497:
1485:
1481:
1475:
1472:
1459:
1453:
1450:
1437:
1431:
1428:
1415:
1411:
1405:
1402:
1397:
1393:
1388:
1383:
1379:
1375:
1371:
1367:
1363:
1356:
1353:
1340:
1334:
1331:
1326:
1322:
1316:
1313:
1309:
1303:
1300:
1295:
1291:
1286:
1281:
1277:
1273:
1269:
1262:
1259:
1255:
1250:
1247:
1242:
1238:
1232:
1229:
1224:
1220:
1215:
1210:
1205:
1200:
1197:(12): e3326.
1196:
1192:
1188:
1181:
1178:
1175:
1169:
1166:
1161:
1157:
1153:
1149:
1144:
1139:
1135:
1131:
1127:
1120:
1117:
1105:
1099:
1096:
1084:
1083:ScienceDirect
1080:
1074:
1071:
1068:
1064:
1059:
1056:
1051:
1045:
1042:
1037:
1033:
1027:
1024:
1019:
1015:
1011:
1007:
1003:
999:
995:
991:
987:
983:
979:
972:
969:
956:
950:
947:
943:
939:
934:
931:
915:
908:
905:
894:
893:npic.orst.edu
890:
884:
882:
878:
871:
866:
863:
860:
857:
854:
851:
847:
844:
841:
838:
836:
833:
832:
828:
826:
820:
818:
816:
812:
810:
805:
804:stereocenters
796:
789:
787:
784:
783:
777:
775:
771:
766:
764:
763:
754:
752:
748:
746:
745:Daphnia magna
737:
735:
729:
727:
725:
721:
717:
713:
711:
706:
702:
698:
693:
691:
687:
683:
682:
677:
673:
669:
668:
663:
659:
658:
653:
652:
646:
642:
640:
639:field studies
636:
633:Icaridin and
629:Effectiveness
628:
626:
622:
620:
616:
612:
608:
604:
600:
595:
593:
589:
585:
581:
577:
573:
569:
566:(INN) to the
565:
561:
556:
554:
549:
545:
541:
529:
522:
517:
500:
494:
490:
483:
479:
475:
474:
470:
467:752 g/100mL (
466:
464:
461:
460:
456:
453:
449:
448:
444:
442:
441:Boiling point
439:
438:
434:
432:
431:Melting point
429:
428:
424:
422:
419:
418:
414:
412:
409:
408:
404:
401:
400:
393:
391:
388:
387:
363:
360:
356:
355:
350:
341:
337:
330:
316:
306:
302:
295:
287:
283:
282:DTXSID0034227
279:
278:
276:
266:
262:
261:
254:
250:
249:
247:
245:
242:
241:
234:
230:
229:
227:
221:
217:
216:
212:
208:
205:
203:
201:ECHA InfoCard
198:
197:
190:
186:
185:
183:
181:
178:
177:
170:
169:ChEMBL2104314
166:
165:
163:
161:
158:
157:
150:
146:
145:
143:
139:
134:
133:
126:
122:
121:
119:
116:
112:
111:
106:
98:
95:
92:
89:
88:
83:
75:
71:
66:
56:
52:
47:
43:
42:
38:
30:
19:
1730:
1722:
1711:. Retrieved
1707:the original
1697:
1654:
1650:
1594:
1590:
1580:
1535:
1531:
1521:
1509:. Retrieved
1499:
1488:. Retrieved
1483:
1474:
1462:. Retrieved
1452:
1440:. Retrieved
1430:
1418:. Retrieved
1414:Science News
1413:
1404:
1369:
1365:
1355:
1343:. Retrieved
1333:
1315:
1307:
1302:
1275:
1271:
1261:
1249:
1231:
1194:
1190:
1180:
1168:
1133:
1129:
1119:
1107:. Retrieved
1098:
1086:. Retrieved
1082:
1073:
1058:
1044:
1035:
1026:
988:(1): 11–18.
985:
981:
971:
959:. Retrieved
949:
942:the original
933:
921:. Retrieved
907:
896:. Retrieved
892:
824:
808:
801:
780:
779:Research on
778:
769:
767:
760:
758:
749:
744:
741:
733:
709:
707:(containing
694:
689:
685:
679:
676:dengue fever
670:mosquitoes (
665:
661:
655:
649:
647:
643:
632:
623:
618:
610:
602:
598:
596:
592:black pepper
575:
559:
557:
543:
539:
538:
481:
108:Identifiers
96:
85:Other names
1787:Piperidines
1486:. p. 3
1420:12 December
1088:29 December
889:"Picaridin"
853:insecticide
613:. In 2005,
402:Appearance
352:Properties
207:100.102.177
125:119515-38-7
1782:Carbamates
1766:Categories
1713:2024-08-08
1490:2020-08-31
898:2020-03-29
872:References
850:pyrethroid
846:Permethrin
765:mosquito.
716:mosquitoes
580:piperidine
463:Solubility
425:1.07 g/cm
390:Molar mass
253:N51GQX0837
180:ChemSpider
136:3D model (
115:CAS Number
1511:31 August
1464:31 August
1442:31 August
1152:1708-8305
1109:31 August
1002:0954-6634
938:Picaridin
815:carbamate
790:Chemistry
621:in 2008.
584:alkaloids
560:picaridin
558:The name
544:picaridin
415:odorless
35:Icaridin
18:Picaridin
1689:31630950
1629:12211128
1621:27535661
1612:11107575
1572:25349401
1396:30381452
1345:6 August
1294:15185943
1223:25522134
1160:29718433
1018:40319483
1010:26811157
961:7 August
923:8 August
842:(IR3535)
829:See also
619:Saltidin
611:Bayrepel
603:Saltidin
599:Bayrepel
588:piperine
586:such as
576:icaridin
546:, is an
540:Icaridin
90:KBR 3023
1680:6832857
1659:Bibcode
1563:4246313
1540:Bibcode
1387:6227861
1214:4270489
615:Lanxess
521:what is
519: (
491:1.4717
469:acetone
421:Density
395:229.320
220:PubChem
29:Icariin
1687:
1677:
1627:
1619:
1609:
1570:
1560:
1394:
1384:
1292:
1221:
1211:
1158:
1150:
1016:
1008:
1000:
811:-butyl
672:vector
516:verify
513:
336:SMILES
233:125098
189:111359
160:ChEMBL
68:Names
1625:S2CID
1014:S2CID
917:(PDF)
865:SS220
861:(PMD)
690:Culex
686:Aedes
681:Culex
667:Aedes
607:Bayer
301:InChI
138:JSmol
1685:PMID
1617:PMID
1568:PMID
1513:2020
1466:2020
1444:2020
1422:2018
1392:PMID
1347:2023
1290:PMID
1219:PMID
1156:PMID
1148:ISSN
1111:2020
1090:2023
1006:PMID
998:ISSN
963:2023
925:2024
848:, a
835:DEET
774:5EL2
635:DEET
601:and
553:DEET
411:Odor
244:UNII
1675:PMC
1667:doi
1607:PMC
1599:doi
1558:PMC
1548:doi
1536:111
1382:PMC
1374:doi
1280:doi
1209:PMC
1199:doi
1138:doi
990:doi
809:sec
674:of
574:is
572:WHO
270:EPA
223:CID
97:sec
1768::
1683:.
1673:.
1665:.
1655:29
1653:.
1649:.
1637:^
1623:.
1615:.
1605:.
1595:74
1593:.
1589:.
1566:.
1556:.
1546:.
1534:.
1530:.
1482:.
1412:.
1390:.
1380:.
1370:14
1368:.
1364:.
1323:.
1288:.
1276:41
1274:.
1270:.
1239:.
1217:.
1207:.
1193:.
1189:.
1154:.
1146:.
1134:25
1132:.
1128:.
1081:.
1065:,
1034:.
1012:.
1004:.
996:.
986:27
984:.
980:.
891:.
880:^
722:,
692:.
471:)
374:23
368:12
1716:.
1691:.
1669::
1661::
1631:.
1601::
1574:.
1550::
1542::
1515:.
1493:.
1468:.
1446:.
1424:.
1398:.
1376::
1349:.
1327:.
1296:.
1282::
1225:.
1201::
1195:8
1162:.
1140::
1113:.
1092:.
1038:.
1020:.
992::
965:.
927:.
901:.
710:p
511:N
487:)
485:D
482:n
480:(
383:3
380:O
377:N
371:H
365:C
272:)
268:(
140:)
31:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.