148:
38:
130:
330:. For example, canines may not be good models for solid oral dosage forms because the characteristic carnivore intestine is underdeveloped compared to the omnivore's, and gastric emptying rates are increased. Also, rodents can not act as models for antibiotic drugs because the resulting alteration to their intestinal flora causes significant
251:
337:
Medical device studies also use this basic premise. Most studies are performed in larger species such as dogs, pigs and sheep which allow for testing in a similar sized model as that of a human. In addition, some species are used for similarity in specific organs or organ system physiology (swine
368:
in the research-based pharmaceutical industry has been reduced in recent years both for ethical and cost reasons. However, most research will still involve animal based testing for the need of similarity in anatomy and physiology that is required for diverse product development.
250:(GLP) testing for safety of the device and its components. Some medical devices will also undergo biocompatibility testing which helps to show whether a component of the device or all components are sustainable in a living model. Most preclinical studies must adhere to GLPs in
733:
678:
634:
476:
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H (December 2015).
723:
439:
On average, only one in every 5,000 compounds that drug companies discover and put through preclinical testing becomes an approved drug. Of the drugs started in clinical trials on humans, only 10 percent secure F.D.A. approval.
285:
The information collected from these studies is vital so that safe human testing can begin. Typically, in drug development studies animal testing involves two species. The most commonly used models are
778:
773:
703:
788:
357:, and other similar international and regional authorities usually require safety testing in at least two mammalian species, including one non-rodent species, prior to human trials authorization.
708:
793:
738:
624:
802:
753:
658:
668:
614:
783:
718:
693:
48:
743:
698:
673:
385:
per mass patient basis. Generally a 1/100 uncertainty factor or "safety margin" is included to account for interspecies (1/10) and inter-individual (1/10) differences.
663:
768:
763:
807:
334:. Depending on a drug's functional groups, it may be metabolized in similar or different ways between species, which will affect both efficacy and toxicology.
728:
713:
648:
639:
619:
206:
Companies use stylized statistics to illustrate the risks in preclinical research, such as that on average, only one in every 5,000 compounds that enters
629:
180:(testing in humans) and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.
688:
683:
758:
544:
748:
273:
tests will be performed. Studies of drug toxicity include which organs are targeted by that drug, as well as if there are any long-term
109:
81:
378:
88:
147:
255:
246:
in humans. Medical devices that do not have drug attached will not undergo these additional tests and may go directly to
95:
350:
56:
592:
404:
381:(NOAELs) on drugs are established, which are used to determine initial phase 1 clinical trial dosage levels on a mass
184:
77:
428:
67:
52:
828:
537:
354:
331:
247:
833:
310:
The choice of species is based on which will give the best correlation to human trials. Differences in the
222:
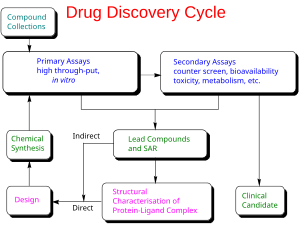
Each class of product may undergo different types of preclinical research. For instance, drugs may undergo
311:
530:
102:
399:
452:
424:
319:
235:
508:
382:
196:
479:"Discovery and resupply of pharmacologically active plant-derived natural products: A review"
653:
577:
498:
490:
394:
227:
223:
161:
338:
for dermatological and coronary stent studies; goats for mammary implant studies; dogs for
315:
587:
567:
503:
478:
433:
365:
243:
207:
192:
177:
822:
494:
211:
274:
17:
183:
The main goals of preclinical studies are to determine a starting, safe dose for
572:
553:
327:
323:
254:
Guidelines to be acceptable for submission to regulatory agencies such as the
322:, or other considerations make certain models more appropriate based on the
239:
200:
512:
263:
188:
339:
295:
287:
269:
66:
if you can. Unsourced or poorly sourced material may be challenged and
343:
231:
526:
522:
299:
291:
31:
734:
Nucleoside and nucleotide reverse-transcriptase inhibitors
453:"Drug Approvals - From Invention to Market...12 Years!"
63:
679:
Dual serotonin and norepinephrine reuptake inhibitors
138:
605:
560:
210:to the stage of preclinical development becomes an
228:pharmacokinetics (what the body does to the drug)
224:pharmacodynamics (what the drug does to the body)
724:Non-nucleoside reverse-transcriptase inhibitors
242:estimate a safe starting dose of the drug for
62:Please review the contents of the article and
538:
8:
191:of the product, which typically include new
176:) is a stage of research that begins before
545:
531:
523:
349:Importantly, the regulatory guidelines of
277:effects or toxic effects causing illness.
502:
684:Selective serotonin reuptake inhibitors
416:
7:
238:. This data allows researchers to
774:Bcr-Abl tyrosine-kinase inhibitors
25:
789:Neurokinin 1 receptor antagonists
664:Dipeptidyl peptidase-4 inhibitors
379:no-observed-adverse-effect levels
779:Cannabinoid receptor antagonists
495:10.1016/j.biotechadv.2015.08.001
146:
128:
36:
608:and development of drug classes
326:, site of activity, or noxious
256:Food & Drug Administration
64:add the appropriate references
1:
625:Angiotensin receptor blockers
429:"The Solution to Drug Prices"
377:Based on preclinical trials,
218:Types of preclinical research
803:Melatonin receptor agonists
754:Thalidomide and its analogs
709:Memantine and related drugs
659:Cyclooxygenase 2 inhibitors
405:Phases of clinical research
373:No observable effect levels
49:reliable medical references
850:
669:Direct thrombin inhibitors
606:Case studies of discovery
784:CCR5 receptor antagonists
248:good laboratory practices
78:"Preclinical development"
55:or relies too heavily on
27:Stage of drug development
719:Neuraminidase inhibitors
694:HIV-protease inhibitors
615:5α-Reductase inhibitors
166:preclinical development
744:Proton pump inhibitors
483:Biotechnology Advances
258:in the United States.
187:and assess potential
699:Integrase inhibitors
674:Direct Xa inhibitors
427:(9 September 2015).
185:first-in-human study
400:Preclinical imaging
174:nonclinical studies
170:preclinical studies
18:Preclinical studies
769:Tubulin inhibitors
320:circulatory system
236:toxicology testing
197:prescription drugs
816:
815:
764:TRPV1 antagonists
704:Lipase inhibitors
306:Choice of species
158:
157:
137:
136:
113:
16:(Redirected from
841:
829:Drug development
808:Renin inhibitors
654:c-Met inhibitors
578:Drug development
547:
540:
533:
524:
517:
516:
506:
489:(8): 1582–1614.
473:
467:
466:
464:
463:
449:
443:
442:
421:
395:Drug development
346:studies; etc.).
261:Typically, both
162:drug development
150:
139:
132:
131:
123:
120:
114:
112:
71:
40:
39:
32:
21:
849:
848:
844:
843:
842:
840:
839:
838:
819:
818:
817:
812:
797:
739:PDE5 inhibitors
729:NS5A inhibitors
714:mTOR inhibitors
643:
607:
601:
561:Steps in design
556:
551:
521:
520:
475:
474:
470:
461:
459:
451:
450:
446:
423:
422:
418:
413:
391:
375:
363:
332:adverse effects
316:enzyme activity
308:
302:are also used.
283:
244:clinical trials
220:
193:medical devices
178:clinical trials
154:
153:
152:
151:
133:
129:
124:
118:
115:
72:
61:
57:primary sources
41:
37:
28:
23:
22:
15:
12:
11:
5:
847:
845:
837:
836:
834:Drug discovery
831:
821:
820:
814:
813:
811:
810:
805:
800:
795:
791:
786:
781:
776:
771:
766:
761:
756:
751:
746:
741:
736:
731:
726:
721:
716:
711:
706:
701:
696:
691:
686:
681:
676:
671:
666:
661:
656:
651:
649:Cephalosporins
646:
641:
637:
632:
627:
622:
620:ACE inhibitors
617:
611:
609:
603:
602:
600:
599:
598:
597:
596:
595:
585:
575:
570:
568:Drug discovery
564:
562:
558:
557:
552:
550:
549:
542:
535:
527:
519:
518:
468:
444:
434:New York Times
415:
414:
412:
409:
408:
407:
402:
397:
390:
387:
374:
371:
366:Animal testing
362:
361:Ethical issues
359:
307:
304:
282:
281:Animal testing
279:
240:allometrically
219:
216:
208:drug discovery
156:
155:
145:
144:
143:
142:
135:
134:
127:
125:
44:
42:
35:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
846:
835:
832:
830:
827:
826:
824:
809:
806:
804:
801:
799:
792:
790:
787:
785:
782:
780:
777:
775:
772:
770:
767:
765:
762:
760:
757:
755:
752:
750:
747:
745:
742:
740:
737:
735:
732:
730:
727:
725:
722:
720:
717:
715:
712:
710:
707:
705:
702:
700:
697:
695:
692:
690:
687:
685:
682:
680:
677:
675:
672:
670:
667:
665:
662:
660:
657:
655:
652:
650:
647:
645:
638:
636:
635:Beta-blockers
633:
631:
630:Antiandrogens
628:
626:
623:
621:
618:
616:
613:
612:
610:
604:
594:
591:
590:
589:
586:
584:
581:
580:
579:
576:
574:
571:
569:
566:
565:
563:
559:
555:
548:
543:
541:
536:
534:
529:
528:
525:
514:
510:
505:
500:
496:
492:
488:
484:
480:
472:
469:
458:
454:
448:
445:
441:
436:
435:
430:
426:
420:
417:
410:
406:
403:
401:
398:
396:
393:
392:
388:
386:
384:
380:
372:
370:
367:
360:
358:
356:
352:
347:
345:
341:
335:
333:
329:
325:
321:
317:
313:
305:
303:
301:
297:
293:
289:
280:
278:
276:
272:
271:
266:
265:
259:
257:
253:
249:
245:
241:
237:
233:
229:
225:
217:
215:
213:
212:approved drug
209:
204:
202:
198:
194:
190:
186:
181:
179:
175:
171:
168:(also termed
167:
163:
149:
141:
140:
126:
122:
111:
108:
104:
101:
97:
94:
90:
87:
83:
80: –
79:
75:
74:Find sources:
69:
65:
59:
58:
54:
50:
45:This article
43:
34:
33:
30:
19:
582:
486:
482:
471:
460:. Retrieved
456:
447:
438:
432:
419:
376:
364:
348:
336:
309:
284:
275:carcinogenic
268:
262:
260:
221:
205:
182:
173:
169:
165:
159:
116:
106:
99:
92:
85:
73:
53:verification
46:
29:
798:antagonists
583:Preclinical
573:Hit to lead
554:Drug design
457:MedicineNet
328:metabolites
324:dosage form
294:, although
201:diagnostics
47:needs more
823:Categories
689:Gliflozins
462:2021-04-21
425:Emanuel EJ
411:References
89:newspapers
119:June 2020
759:Triptans
644:agonists
588:Clinical
513:26281720
389:See also
264:in vitro
189:toxicity
749:Statins
504:4748402
340:gastric
300:porcine
296:primate
270:in vivo
103:scholar
68:removed
593:Phases
511:
501:
344:cancer
292:canine
288:murine
234:, and
230:(PK),
226:(PD),
199:, and
105:
98:
91:
84:
76:
110:JSTOR
96:books
794:5-HT
640:Beta
509:PMID
342:and
298:and
290:and
267:and
232:ADME
214:.
82:news
51:for
499:PMC
491:doi
440:...
383:API
355:EMA
351:FDA
312:gut
252:ICH
172:or
164:,
160:In
825::
507:.
497:.
487:33
485:.
481:.
455:.
437:.
431:.
353:,
318:,
314:,
203:.
195:,
70:.
796:3
642:2
546:e
539:t
532:v
515:.
493::
465:.
121:)
117:(
107:·
100:·
93:·
86:·
60:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.