453:
the mitochondrion creates a lower concentration of positively charged protons inside it, resulting in excess negative charge on the inside of the membrane. The electrical potential gradient is about -170 mV , negative inside (N). These gradients - charge difference and the proton concentration difference both create a combined electrochemical gradient across the membrane, often expressed as the proton-motive force (PMF). In mitochondria, the PMF is almost entirely made up of the electrical component but in chloroplasts the PMF is made up mostly of the pH gradient because the charge of protons H is neutralized by the movement of Cl and other anions. In either case, the PMF needs to be greater than about 460 mV (45 kJ/mol) for the ATP synthase to be able to make ATP.
2342:
211:
2238:
341:
1667:
chloroplast. Azzone et al. stressed that the inside phase (N side of the membrane) is the bacterial cytoplasm, mitochondrial matrix, or chloroplast stroma; the outside (P) side is the bacterial periplasmic space, mitochondrial intermembrane space, or chloroplast lumen. Furthermore, 3D tomography of the mitochondrial inner membrane shows its extensive invaginations to be stacked, similar to thylakoid disks; hence the mitochondrial intermembrane space is topologically quite similar to the chloroplast lumen.:
2229:. The electrons lost from Photosystem II get replaced by the oxidation of water, which is "split" into protons and oxygen by the oxygen-evolving complex (OEC, also known as WOC, or the water-oxidizing complex). To generate one molecule of diatomic oxygen, 10 photons must be absorbed by Photosystems I and II, four electrons must move through the two photosystems, and 2 NADPH are generated (later used for carbon dioxide fixation in the Calvin Cycle).
2067:
2428:, emitting hot acidic or alkaline water, would have created external proton gradients. These provided energy that primordial organisms could have exploited. To keep the flows separate, such an organism could have wedged itself in the rock of the hydrothermal vent, exposed to the hydrothermal flow on one side and the more alkaline water on the other. As long as the organism's membrane (or passive
69:
1171:
129:(ADP) into ATP. The ATP synthase contains two parts: CF0 (present in thylakoid membrane) and CF1 (protrudes on the outer surface of thylakoid membrane). The breakdown of the proton gradient leads to conformational change in CF1—providing enough energy in the process to convert ADP to ATP. The generation of ATP by chemiosmosis occurs in
2054:/ (Δp / 10.4 kJ·mol/mV) = 40.2 kJ·mol / (173.5 mV / 10.4 kJ·mol/mV) = 40.2 / 16.7 = 2.4. The actual ratio of the proton-binding c-subunit to the ATP-synthesizing beta-subunit copy numbers is 8/3 = 2.67, showing that under these conditions, the mitochondrion functions at 90% (2.4/2.67) efficiency.
300:
This was a radical proposal at the time, and was not well accepted. The prevailing view was that the energy of electron transfer was stored as a stable high potential intermediate, a chemically more conservative concept. The problem with the older paradigm is that no high energy intermediate was ever
452:
reactions to pump protons (hydrogen ions) out across the membrane, separating the charge across the membrane. In mitochondria, energy released by the electron transport chain is used to move protons from the mitochondrial matrix (N side) to the intermembrane space (P side). Moving the protons out of
1662:
is chosen to represent the change in potential energy per unit charge flowing into the cell as above. Furthermore, due to redox-driven proton pumping by coupling sites, the proton gradient is always inside-alkaline. For both of these reasons, protons flow in spontaneously, from the P side to the N
2057:
In fact, the thermodynamic efficiency is mostly lower in eukaryotic cells because ATP must be exported from the matrix to the cytoplasm, and ADP and phosphate must be imported from the cytoplasm. This "costs" one "extra" proton import per ATP, hence the actual efficiency is only 65% (= 2.4/3.67).
2440:
A proposed alternative source to chemiosmotic energy developing across membranous structures is if an electron acceptor, ferricyanide, is within a vesicle and the electron donor is outside, quinones transported by carbonaceous meteorites pick up electrons and protons from the donor. They would
1666:
The spontaneity of proton import (from the P to the N side) is universal in all bioenergetic membranes. This fact was not recognized before the 1990s, because the chloroplast thylakoid lumen was interpreted as an interior phase, but in fact it is topologically equivalent to the exterior of the
1165:
1928:
1663:
side; the available free energy is used to synthesize ATP (see below). For this reason, PMF is defined for proton import, which is spontaneous. PMF for proton export, i.e., proton pumping as catalyzed by the coupling sites, is simply the negative of PMF(import).
1543:
1248:
1402:
585:
2542:
996:
1321:
2441:
release electrons across the lipid membrane by diffusion to ferricyanide within the vesicles and release protons which produces gradients above pH 2, the process is conducive to the development of proton gradients.
433:) as a combination of proton and voltage (electrical potential) gradients across a membrane. The electrical gradient is a consequence of the charge separation across the membrane (when the protons H move without a
2345:
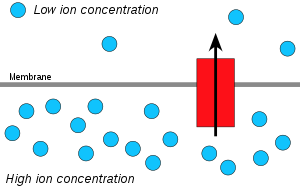
Early cell powered by external proton gradient near a deep-sea hydrothermal vent. As long as the membrane (or passive ion channels within it) is permeable to protons, the mechanism can function without ion
1764:; nevertheless, the concentrations on either side of the membrane need not be equal. Spontaneous movement across the potential membrane is determined by both concentration and electric potential gradients.
956:
1806:
1586:
1441:
2045:
1798:
2432:
within it) is permeable to protons, the mechanism can function without ion pumps. Such a proto-organism could then have evolved further mechanisms such as ion pumps and ATP synthase.
1701:
2718:
Azzone G, Benz R, Bertl A, Colombini M, Crofts A, Dilley R, Dimroth P, Dutton PL, Felle H, Harold F, Junge W (1993). "Transmembrane
Measurements Across Bioenergetic Membranes".
1467:
680:
2323:. The origin of the mitochondrion triggered the origin of eukaryotes, and the origin of the plastid the origin of the Archaeplastida, one of the major eukaryotic supergroups.
1762:
2750:
Silverstein TP (June 2014). "An exploration of how the thermodynamic efficiency of bioenergetic membrane systems varies with c-subunit stoichiometry of F₁F₀ ATP synthases".
1728:
1660:
705:
797:
752:
2012:
1610:
903:
615:
1633:
1474:
1670:
The energy expressed here as Gibbs free energy, electrochemical proton gradient, or proton-motive force (PMF), is a combination of two gradients across the membrane:
1184:
410:, however, are barriers for ions. This is why energy can be stored as a combination of these two gradients across the membrane. Only special membrane proteins like
988:
1988:
871:
845:
819:
641:
1330:
2918:"Chemiosmotic energy for primitive cellular life: Proton gradients are generated across lipid membranes by redox reactions coupled to meteoritic quinones"
1638:
It is worth noting that, as with any transmembrane transport process, the PMF is directional. The sign of the transmembrane electric potential difference
471:
1160:{\displaystyle \Delta \!\mu _{\mathrm {H} ^{+}}=F\Delta \!\psi +RT\ln {\frac {_{\text{N}}}{_{\text{P}}}}=F\Delta \!\psi -(\ln 10)RT\Delta \mathrm {pH} }
1548:
Note that for spontaneous proton import from the P side (relatively more positive and acidic) to the N side (relatively more negative and alkaline),
2365:
on earth, proposes that primordial organisms used thermal cycling as an energy source (thermosynthesis), functioning essentially as a heat engine:
1264:
3003:"The relation between the internal phosphorylation potential and the proton motive force in mitochondria during ATP synthesis and hydrolysis"
2843:
429:(PMF), derived from the electrochemical gradient mentioned earlier. It can be described as the measure of the potential energy stored (
2810:
2702:
2670:
2627:
2598:
2554:
254:
2341:
2213:. These protons then flow down their electrochemical potential gradient through an enzyme called ATP-synthase, creating ATP by the
388:
like protons H tend to diffuse down the electrical potential, from the positive (P) side of the membrane to the negative (N) side.
908:
2490:
Mitchell P (July 1961). "Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism".
1923:{\displaystyle \mathrm {ADP} ^{4-}+\mathrm {H} ^{+}+\mathrm {HOPO} _{3}^{2-}\rightarrow \mathrm {ATP} ^{4-}+\mathrm {H_{2}O} }
210:
3057:
3067:
444:
In most cases the proton-motive force is generated by an electron transport chain which acts as a proton pump, using the
2225:, then are raised to a higher energy level by light energy and then received by an electron acceptor and reduce NADP to
305:
grew too great to be ignored. Eventually the weight of evidence began to favor the chemiosmotic hypothesis, and in 1978
270:
1551:
258:
1407:
2237:
2470:
2146:
353:
214:
119:
273:, which in turn pass them to other proteins in the ETC. The energy at every redox transfer step is used to pump
2986:
Jeremy M. Berg; John L. Tymoczko; Lubert Stryer (eds.). "18.4. A Proton
Gradient Powers the Synthesis of ATP".
2460:
2302:
2218:
2202:
2174:
2134:
2017:
1770:
397:
310:
282:
266:
218:
177:
97:
42:
2319:
are the product of endosymbiosis and trace back to incorporated prokaryotes. This process is described in the
2259:
1677:
422:
is central to convert energy of spontaneous flow of protons through them into chemical energy of ATP bonds.
374:
force caused by a concentration gradient - all particles tend to diffuse from higher concentration to lower.
302:
2395:(generated ATP by change in electrical polarization of membrane during thermal cycling: thermosynthesis) →
2162:
2138:
2095:
2079:
430:
169:
54:
46:
38:
3062:
2830:. Cellular Origin, Life in Extreme Habitats and Astrobiology. Vol. 22. Springer. pp. 321–344.
2287:
2158:
340:
126:
1446:
653:
2385:(generated ATP by thermal cycling of subunit during suspension in convection cell: thermosynthesis) →
1737:
2987:
2929:
2872:
2499:
2450:
2320:
2295:
2107:
2083:
381:
278:
246:
173:
154:
58:
2584:
1709:
1641:
2615:
407:
377:
687:
2801:
2775:
2523:
2206:
1538:{\displaystyle \Delta \!p=-\Delta \!\psi +\left(59.1\,\mathrm {mV} \right)\Delta \!\mathrm {pH} }
757:
712:
415:
1993:
1591:
1243:{\displaystyle \Delta \!\mathrm {pH} =\mathrm {pH} _{\mathrm {N} }-\mathrm {pH} _{\mathrm {P} }}
884:
596:
2066:
3024:
2957:
2898:
2839:
2806:
2767:
2698:
2666:
2656:
2623:
2594:
2550:
2515:
2455:
2425:
2242:
2122:
1615:
462:
445:
349:
306:
242:
165:
77:
96:
from a region of high proton concentration to a region of lower proton concentration, and an
3014:
2947:
2937:
2888:
2880:
2831:
2759:
2727:
2570:
2507:
822:
344:
Energy conversion by the inner mitochondrial membrane and chemiosmotic coupling between the
118:
that makes ATP by chemiosmosis. It allows protons to pass through the membrane and uses the
73:
2291:
2246:
2214:
2210:
345:
967:
2933:
2876:
2503:
2980:
2952:
2917:
2893:
2860:
2299:
2250:
2190:
2178:
2087:
1936:
856:
830:
804:
626:
150:
62:
3019:
3002:
1397:{\displaystyle \Delta \!\mu _{\mathrm {H} ^{+}}=1\,\mathrm {kJ} \,\mathrm {mol} ^{-1}}
168:
proposed the chemiosmotic hypothesis in 1961. In brief, the hypothesis was that most
100:
of protons across a membrane can be harnessed to make ATP. This process is related to
3051:
2731:
2588:
2283:
2222:
2071:
644:
403:
222:
153:
through the thylakoid membrane to the thylakoid spaces. The stored energy is used to
123:
580:{\displaystyle \Delta \!G=zF\Delta \!\psi +RT\ln {\frac {_{\text{N}}}{_{\text{P}}}}}
2779:
2660:
2527:
2380:
2312:
2198:
2142:
2137:, which releases the energy of oxygen to create a proton gradient across the inner
848:
419:
360:
317:
290:
286:
193:
181:
130:
111:
17:
367:
The movement of ions across the membrane depends on a combination of two factors:
2835:
2145:
then uses the energy stored in this gradient to make ATP. This process is called
149:
during photosynthesis, an electron transport chain pumps H ions (protons) in the
3042:
2429:
2362:
2351:
2316:
2075:
874:
411:
321:
230:
146:
134:
81:
2942:
2763:
2465:
2370:
2254:
2126:
2110:. The last steps of this process occur in mitochondria. The reduced molecules
1933:
is also called phosphorylation potential. The equilibrium concentration ratio
434:
234:
2298:. These bacteria use the energy of light to create a proton gradient using a
2796:
2330:
2268:
2194:
2150:
2091:
371:
294:
281:
into the intermembrane space, storing energy in the form of a transmembrane
238:
93:
2961:
2902:
2771:
2519:
1170:
3028:
2997:
A set of experiments aiming to test some tenets of the chemiosmotic theory
2884:
2585:"Figure 10.22: Electron transport and ATP synthesis during photosynthesis"
68:
2826:
Muller AW (2012). "Life
Explained by Heat Engines". In Seckbach J (ed.).
2361:
A stepwise model for the emergence of chemiosmosis, a key element in the
2275:
2186:
2130:
1316:{\displaystyle \Delta \!p=-{\frac {\Delta \!\mu _{\mathrm {H^{+}} }}{F}}}
438:
325:
262:
200:
138:
50:
2333:
and an ADP molecule. This process is part of oxidative phosphorylation.
2307:
2279:
2103:
385:
329:
204:
142:
108:
across a selective membrane, which is why it is called "chemiosmosis".
101:
2697:(fourth ed.). New York - Basingstoke: W. H. Freeman and Company.
2511:
2182:
648:
618:
389:
356:
285:. The protons move back across the inner membrane through the enzyme
274:
185:
115:
89:
2407:(today's bacterial photosynthesis, which makes use of chemiosmosis).
465:. Let N denote the inside of a cell, and P denote the outside. Then
289:. The flow of protons back into the matrix of the mitochondrion via
2614:
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002).
2541:
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002).
2404:
added quinones and membrane-spanning light-induced electric dipoles
905:
is frequently interpreted as a molar electrochemical ion potential
301:
found, and the evidence for proton pumping by the complexes of the
2340:
2236:
2226:
2115:
1169:
449:
250:
209:
105:
67:
2616:"Figure 14-32: The importance of H-driven transport in bacteria"
2111:
414:
can sometimes allow ions to move across the membrane (see also:
189:
34:
2398:
added metastable, light-induced electric dipoles in membrane
951:{\displaystyle \Delta \!\mu _{\mathrm {X} ^{z+}}=\Delta \!G}
1612:) whereas PMF is positive (similar to redox cell potential
27:
Electrochemical principle that enables cellular respiration
396:
These two gradients taken together can be expressed as an
316:
Chemiosmotic coupling is important for ATP production in
293:
provides enough energy for ADP to combine with inorganic
799:
are the cation concentrations at P and N, respectively;
157:
ADP, making ATP, as protons move through ATP synthase.
2329:
is the third pathway that produces ATP from inorganic
2241:
Chemiosmotic coupling between the energy of sunlight,
2047:, for example in case of the mammalian mitochondrion:
2020:
1996:
1939:
1809:
1773:
1740:
1712:
1680:
1644:
1618:
1594:
1554:
1477:
1449:
1410:
1333:
1267:
1187:
999:
970:
911:
887:
859:
833:
807:
760:
715:
690:
656:
629:
599:
474:
199:
formed during the oxidative breakdown of energy-rich
2720:
Biochimica et
Biophysica Acta (BBA) - Bioenergetics
2205:, causing protons to be actively pumped across the
617:is the Gibbs free energy change per unit amount of
2070:Directions of chemiosmotic proton transfer in the
2039:
2006:
1982:
1922:
1792:
1756:
1722:
1695:
1654:
1627:
1604:
1580:
1537:
1461:
1435:
1396:
1315:
1242:
1159:
982:
950:
897:
865:
839:
813:
791:
746:
699:
674:
635:
609:
579:
418:). In the chemiosmotic hypothesis a transmembrane
2861:"The Hot Spring Hypothesis for an Origin of Life"
2543:"Proton Gradients Produce Most of the Cell's ATP"
2133:processing. These molecules pass electrons to an
2024:
2000:
1777:
1744:
1716:
1684:
1648:
1598:
1558:
1526:
1494:
1481:
1414:
1337:
1287:
1271:
1191:
1118:
1032:
1003:
944:
915:
891:
603:
494:
478:
2181:generate ATP by the action of chemiosmosis. The
1581:{\displaystyle \Delta \!\mu _{\mathrm {H} ^{+}}}
392:diffuse spontaneously in the opposite direction.
2916:Milshteyn D, Cooper G, Deamer D (August 2019).
2802:The Vital Question: Why Is Life The Way It Is?
2217:of ADP to ATP. The electrons from the initial
1436:{\displaystyle \Delta \!p=10.4\,\mathrm {mV} }
2745:
2743:
2741:
707:is the electric potential of N relative to P;
8:
2791:
2789:
2651:
2649:
2647:
2645:
2643:
2641:
2639:
2373:in natural waters causing thermal cycling →
461:The proton-motive force is derived from the
2665:(2nd ed.). San Diego: Academic Press.
2282:also can use chemiosmosis to generate ATP.
45:. An important example is the formation of
2979:Biochemistry textbook reference, from the
2688:
2686:
2684:
2682:
237:as a fairly energy-rich intermediate. The
3018:
2951:
2941:
2892:
2752:Journal of Bioenergetics and Biomembranes
2593:(2nd ed.). Sinauer Associates, Inc.
2040:{\displaystyle \Delta \!G_{\mathrm {p} }}
2030:
2029:
2019:
1995:
1966:
1958:
1949:
1944:
1938:
1910:
1905:
1893:
1882:
1869:
1864:
1850:
1840:
1835:
1822:
1811:
1808:
1793:{\displaystyle \Delta \!G_{\mathrm {p} }}
1783:
1782:
1772:
1739:
1711:
1685:
1679:
1643:
1617:
1593:
1570:
1565:
1563:
1553:
1527:
1510:
1509:
1476:
1454:
1453:
1448:
1425:
1424:
1409:
1385:
1374:
1372:
1364:
1363:
1349:
1344:
1342:
1332:
1298:
1293:
1292:
1281:
1266:
1233:
1232:
1224:
1213:
1212:
1204:
1192:
1186:
1174:A diagram of chemiosmotic phosphorylation
1149:
1100:
1090:
1085:
1073:
1063:
1058:
1051:
1015:
1010:
1008:
998:
969:
927:
922:
920:
910:
886:
858:
832:
806:
783:
770:
765:
759:
738:
725:
720:
714:
689:
663:
658:
655:
628:
598:
568:
555:
550:
538:
525:
520:
513:
473:
2149:because it uses energy released by the
2065:
339:
3043:Chemiosmosis (University of Wisconsin)
2482:
2189:are received by the antenna complex of
2305:. Non-photosynthetic bacteria such as
1696:{\displaystyle \Delta \!\mathrm {pH} }
98:electrochemical concentration gradient
7:
3007:The Journal of Biological Chemistry
2805:. Profile Books. pp. 129–140.
2294:synthesize ATP by a process called
1734:When a system reaches equilibrium,
881:The molar Gibbs free energy change
425:Hence researchers created the term
2031:
2021:
1997:
1973:
1970:
1967:
1945:
1916:
1907:
1889:
1886:
1883:
1860:
1857:
1854:
1851:
1836:
1818:
1815:
1812:
1784:
1774:
1741:
1713:
1689:
1686:
1681:
1645:
1619:
1595:
1566:
1555:
1531:
1528:
1523:
1514:
1511:
1491:
1478:
1455:
1429:
1426:
1411:
1381:
1378:
1375:
1368:
1365:
1345:
1334:
1295:
1284:
1268:
1234:
1228:
1225:
1214:
1208:
1205:
1196:
1193:
1188:
1153:
1150:
1146:
1115:
1086:
1059:
1029:
1011:
1000:
941:
923:
912:
888:
766:
721:
691:
659:
600:
551:
521:
491:
475:
25:
2201:. These electrons travel down an
2098:cells do not have outer membrane.
1462:{\displaystyle 298\,\mathrm {K} }
675:{\displaystyle \mathrm {X} ^{z+}}
255:nicotinamide adenine dinucleotide
221:and chemiosmosis — and occurs in
2859:Damer B, Deamer D (April 2020).
1757:{\displaystyle \Delta \!\rho =0}
1674:the concentration gradient (via
3001:Ogawa S, Lee TM (August 1984).
2106:releasing its energy is called
1990:can be calculated by comparing
963:electrochemical proton gradient
2992:(5th ed.). W. H. Freeman.
2590:The Cell: A Molecular Approach
2420:External proton gradient model
1977:
1963:
1955:
1940:
1878:
1723:{\displaystyle \Delta \!\psi }
1655:{\displaystyle \Delta \!\psi }
1469:this equation takes the form:
1137:
1125:
1097:
1081:
1070:
1054:
780:
761:
735:
716:
565:
546:
535:
516:
253:of a carrier molecule such as
229:Molecules such as glucose are
180:across the inner membranes of
1:
3020:10.1016/S0021-9258(18)90918-X
2620:Molecular Biology of the Cell
2547:Molecular Biology of the Cell
2401:(primitive photosynthesis) →
217:involves two processes — the
80:when the ions pass through a
2836:10.1007/978-94-007-2941-4_19
2732:10.1016/0005-2728(93)90002-W
2327:Chemiosmotic phosphorylation
2315:. In fact, mitochondria and
1767:The molar Gibbs free energy
1706:electric potential gradient
700:{\displaystyle \Delta \psi }
271:inner mitochondrial membrane
47:adenosine triphosphate (ATP)
41:bound structure, down their
792:{\displaystyle _{\text{N}}}
747:{\displaystyle _{\text{P}}}
259:flavin adenine dinucleotide
161:The chemiosmotic hypothesis
3084:
2943:10.1038/s41598-019-48328-5
2828:Genesis — in the Beginning
2349:
2102:The complete breakdown of
2007:{\displaystyle \Delta \!p}
1605:{\displaystyle \Delta \!G}
898:{\displaystyle \Delta \!G}
610:{\displaystyle \Delta \!G}
348:of redox reactions in the
2764:10.1007/s10863-014-9547-y
2659:; Ferguson S. J. (1992).
2471:Oxidative phosphorylation
2337:Emergence of chemiosmosis
2147:oxidative phosphorylation
354:oxidative phosphorylation
261:(FAD). The carriers pass
215:Oxidative phosphorylation
76:and can be used to power
2461:Electrochemical gradient
2303:electron transport chain
2203:electron transport chain
2135:electron transport chain
1628:{\displaystyle \Delta E}
1588:is negative (similar to
621:transferred from P to N;
398:electrochemical gradient
311:Nobel Prize in Chemistry
283:electrochemical gradient
267:electron transport chain
219:electron transport chain
178:electrochemical gradient
43:electrochemical gradient
2260:Halobacterium salinarum
2096:gram-positive bacterial
2080:gram-negative bacterial
303:electron transfer chain
2347:
2272:
2139:mitochondrial membrane
2099:
2041:
2008:
1984:
1924:
1794:
1758:
1724:
1697:
1656:
1629:
1606:
1582:
1539:
1463:
1437:
1398:
1317:
1244:
1175:
1161:
990:and as a consequence:
984:
952:
899:
867:
841:
815:
793:
748:
701:
676:
637:
611:
581:
431:chemiosmotic potential
364:
226:
170:adenosine triphosphate
122:difference to convert
85:
39:semipermeable membrane
3058:Biochemical reactions
2885:10.1089/ast.2019.2045
2357:Thermal cycling model
2350:Further information:
2344:
2288:green sulfur bacteria
2245:and phosphorylation (
2240:
2121:are generated by the
2069:
2042:
2009:
1985:
1925:
1795:
1759:
1725:
1698:
1657:
1630:
1607:
1583:
1540:
1464:
1438:
1399:
1318:
1254:Mitchell defined the
1245:
1173:
1162:
985:
953:
900:
868:
842:
816:
794:
749:
702:
677:
638:
612:
582:
343:
213:
176:cells comes from the
137:, as well as in most
127:adenosine diphosphate
71:
3068:Cellular respiration
2451:Cellular respiration
2388:added membrane and F
2376:added β-subunit of F
2321:endosymbiotic theory
2296:photophosphorylation
2108:cellular respiration
2084:cellular respiration
2018:
1994:
1937:
1807:
1771:
1738:
1710:
1678:
1642:
1616:
1592:
1552:
1475:
1447:
1408:
1331:
1265:
1185:
997:
968:
909:
885:
857:
831:
805:
758:
713:
688:
654:
627:
597:
472:
408:biological membranes
382:electrical potential
247:mitochondrial matrix
245:(acetyl-CoA) in the
72:An ion gradient has
59:cellular respiration
3013:(16): 10004–10011.
2934:2019NatSR...912447M
2877:2020AsBio..20..429D
2504:1961Natur.191..144M
2436:Meteoritic quinones
2392:ATP Synthase moiety
1877:
1256:proton-motive force
983:{\displaystyle z=1}
427:proton-motive force
378:Electrostatic force
336:Proton-motive force
172:(ATP) synthesis in
145:. For instance, in
49:by the movement of
33:is the movement of
18:Proton motive force
2922:Scientific Reports
2583:Cooper GM (2000).
2573:in Chemistry 1978.
2426:hydrothermal vents
2348:
2273:
2257:archaeal organism
2207:thylakoid membrane
2100:
2037:
2004:
1980:
1920:
1849:
1790:
1754:
1720:
1693:
1652:
1625:
1602:
1578:
1535:
1459:
1433:
1394:
1313:
1240:
1176:
1157:
980:
948:
895:
863:
837:
811:
789:
744:
697:
672:
633:
607:
577:
416:Membrane transport
365:
249:is coupled to the
227:
155:photophosphorylate
104:, the movement of
88:Hydrogen ions, or
86:
78:chemical reactions
53:ions (H) across a
2845:978-94-007-2940-7
2693:Stryer L (1995).
2498:(4784): 144–148.
2456:Citric acid cycle
2243:bacteriorhodopsin
2157:to phosphorylate
2090:). The bacterial
2050:H / ATP = ΔG
1983:{\displaystyle /}
1800:of ATP synthesis
1311:
1107:
1103:
1076:
866:{\displaystyle T}
840:{\displaystyle R}
814:{\displaystyle F}
786:
741:
636:{\displaystyle z}
575:
571:
541:
463:Gibbs free energy
446:Gibbs free energy
350:respiratory chain
307:Peter D. Mitchell
243:acetyl coenzyme A
166:Peter D. Mitchell
16:(Redirected from
3075:
3032:
3022:
2993:
2966:
2965:
2955:
2945:
2913:
2907:
2906:
2896:
2856:
2850:
2849:
2823:
2817:
2816:
2793:
2784:
2783:
2747:
2736:
2735:
2715:
2709:
2708:
2690:
2677:
2676:
2653:
2634:
2633:
2611:
2605:
2604:
2580:
2574:
2567:
2561:
2560:
2538:
2532:
2531:
2512:10.1038/191144a0
2487:
2267:). The archaeal
2193:, which excites
2153:of NADH and FADH
2046:
2044:
2043:
2038:
2036:
2035:
2034:
2013:
2011:
2010:
2005:
1989:
1987:
1986:
1981:
1976:
1962:
1954:
1953:
1948:
1929:
1927:
1926:
1921:
1919:
1915:
1914:
1901:
1900:
1892:
1876:
1868:
1863:
1845:
1844:
1839:
1830:
1829:
1821:
1799:
1797:
1796:
1791:
1789:
1788:
1787:
1763:
1761:
1760:
1755:
1729:
1727:
1726:
1721:
1702:
1700:
1699:
1694:
1692:
1661:
1659:
1658:
1653:
1634:
1632:
1631:
1626:
1611:
1609:
1608:
1603:
1587:
1585:
1584:
1579:
1577:
1576:
1575:
1574:
1569:
1544:
1542:
1541:
1536:
1534:
1522:
1518:
1517:
1468:
1466:
1465:
1460:
1458:
1442:
1440:
1439:
1434:
1432:
1403:
1401:
1400:
1395:
1393:
1392:
1384:
1371:
1356:
1355:
1354:
1353:
1348:
1322:
1320:
1319:
1314:
1312:
1307:
1306:
1305:
1304:
1303:
1302:
1282:
1249:
1247:
1246:
1241:
1239:
1238:
1237:
1231:
1219:
1218:
1217:
1211:
1199:
1166:
1164:
1163:
1158:
1156:
1108:
1106:
1105:
1104:
1101:
1095:
1094:
1089:
1079:
1078:
1077:
1074:
1068:
1067:
1062:
1052:
1022:
1021:
1020:
1019:
1014:
989:
987:
986:
981:
957:
955:
954:
949:
937:
936:
935:
934:
926:
904:
902:
901:
896:
872:
870:
869:
864:
846:
844:
843:
838:
823:Faraday constant
820:
818:
817:
812:
798:
796:
795:
790:
788:
787:
784:
778:
777:
769:
753:
751:
750:
745:
743:
742:
739:
733:
732:
724:
706:
704:
703:
698:
681:
679:
678:
673:
671:
670:
662:
642:
640:
639:
634:
616:
614:
613:
608:
586:
584:
583:
578:
576:
574:
573:
572:
569:
563:
562:
554:
544:
543:
542:
539:
533:
532:
524:
514:
309:was awarded the
74:potential energy
21:
3083:
3082:
3078:
3077:
3076:
3074:
3073:
3072:
3048:
3047:
3039:
3000:
2985:
2975:
2973:Further reading
2970:
2969:
2915:
2914:
2910:
2858:
2857:
2853:
2846:
2825:
2824:
2820:
2813:
2795:
2794:
2787:
2749:
2748:
2739:
2717:
2716:
2712:
2705:
2692:
2691:
2680:
2673:
2662:Bioenergetics 2
2655:
2654:
2637:
2630:
2613:
2612:
2608:
2601:
2582:
2581:
2577:
2568:
2564:
2557:
2540:
2539:
2535:
2489:
2488:
2484:
2479:
2447:
2438:
2422:
2391:
2379:
2369:self-organized
2359:
2354:
2339:
2292:purple bacteria
2247:chemical energy
2235:
2215:phosphorylation
2211:thylakoid lumen
2175:light reactions
2171:
2156:
2119:
2064:
2062:In mitochondria
2053:
2025:
2016:
2015:
1992:
1991:
1943:
1935:
1934:
1906:
1881:
1834:
1810:
1805:
1804:
1778:
1769:
1768:
1736:
1735:
1708:
1707:
1676:
1675:
1640:
1639:
1614:
1613:
1590:
1589:
1564:
1559:
1550:
1549:
1505:
1501:
1473:
1472:
1445:
1444:
1406:
1405:
1373:
1343:
1338:
1329:
1328:
1294:
1288:
1283:
1263:
1262:
1223:
1203:
1183:
1182:
1096:
1084:
1080:
1069:
1057:
1053:
1009:
1004:
995:
994:
966:
965:
921:
916:
907:
906:
883:
882:
855:
854:
829:
828:
803:
802:
779:
764:
756:
755:
734:
719:
711:
710:
686:
685:
657:
652:
651:
625:
624:
595:
594:
564:
549:
545:
534:
519:
515:
470:
469:
459:
346:chemical energy
338:
197:
163:
28:
23:
22:
15:
12:
11:
5:
3081:
3079:
3071:
3070:
3065:
3060:
3050:
3049:
3046:
3045:
3038:
3037:External links
3035:
3034:
3033:
2994:
2981:NCBI bookshelf
2974:
2971:
2968:
2967:
2908:
2871:(4): 429–452.
2851:
2844:
2818:
2812:978-1781250365
2811:
2785:
2758:(3): 229–241.
2737:
2710:
2704:978-0716720096
2703:
2678:
2671:
2657:Nicholls D. G.
2635:
2628:
2606:
2599:
2575:
2562:
2555:
2533:
2481:
2480:
2478:
2475:
2474:
2473:
2468:
2463:
2458:
2453:
2446:
2443:
2437:
2434:
2421:
2418:
2417:
2416:
2415:
2414:
2413:
2412:
2411:
2410:
2409:
2408:
2405:
2399:
2393:
2389:
2383:
2377:
2363:origin of life
2358:
2355:
2338:
2335:
2300:photosynthetic
2251:photosynthesis
2234:
2233:In prokaryotes
2231:
2219:light reaction
2191:Photosystem II
2179:photosynthesis
2170:
2167:
2154:
2117:
2088:photosynthesis
2063:
2060:
2051:
2033:
2028:
2023:
2003:
1999:
1979:
1975:
1972:
1969:
1965:
1961:
1957:
1952:
1947:
1942:
1931:
1930:
1918:
1913:
1909:
1904:
1899:
1896:
1891:
1888:
1885:
1880:
1875:
1872:
1867:
1862:
1859:
1856:
1853:
1848:
1843:
1838:
1833:
1828:
1825:
1820:
1817:
1814:
1786:
1781:
1776:
1753:
1750:
1747:
1743:
1732:
1731:
1719:
1715:
1704:
1691:
1688:
1683:
1651:
1647:
1624:
1621:
1601:
1597:
1573:
1568:
1562:
1557:
1533:
1530:
1525:
1521:
1516:
1513:
1508:
1504:
1500:
1497:
1493:
1490:
1487:
1484:
1480:
1457:
1452:
1431:
1428:
1423:
1420:
1417:
1413:
1391:
1388:
1383:
1380:
1377:
1370:
1367:
1362:
1359:
1352:
1347:
1341:
1336:
1325:
1324:
1310:
1301:
1297:
1291:
1286:
1280:
1277:
1274:
1270:
1252:
1251:
1236:
1230:
1227:
1222:
1216:
1210:
1207:
1202:
1198:
1195:
1190:
1168:
1167:
1155:
1152:
1148:
1145:
1142:
1139:
1136:
1133:
1130:
1127:
1124:
1121:
1117:
1114:
1111:
1099:
1093:
1088:
1083:
1072:
1066:
1061:
1056:
1050:
1047:
1044:
1041:
1038:
1035:
1031:
1028:
1025:
1018:
1013:
1007:
1002:
979:
976:
973:
947:
943:
940:
933:
930:
925:
919:
914:
894:
890:
879:
878:
862:
852:
836:
826:
810:
800:
782:
776:
773:
768:
763:
737:
731:
728:
723:
718:
708:
696:
693:
683:
669:
666:
661:
632:
622:
606:
602:
588:
587:
567:
561:
558:
553:
548:
537:
531:
528:
523:
518:
512:
509:
506:
503:
500:
497:
493:
490:
487:
484:
481:
477:
458:
455:
404:Lipid bilayers
394:
393:
375:
337:
334:
195:
162:
159:
151:stroma (fluid)
63:photosynthesis
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3080:
3069:
3066:
3064:
3061:
3059:
3056:
3055:
3053:
3044:
3041:
3040:
3036:
3030:
3026:
3021:
3016:
3012:
3008:
3004:
2998:
2995:
2991:
2990:
2983:
2982:
2977:
2976:
2972:
2963:
2959:
2954:
2949:
2944:
2939:
2935:
2931:
2927:
2923:
2919:
2912:
2909:
2904:
2900:
2895:
2890:
2886:
2882:
2878:
2874:
2870:
2866:
2862:
2855:
2852:
2847:
2841:
2837:
2833:
2829:
2822:
2819:
2814:
2808:
2804:
2803:
2798:
2792:
2790:
2786:
2781:
2777:
2773:
2769:
2765:
2761:
2757:
2753:
2746:
2744:
2742:
2738:
2733:
2729:
2725:
2721:
2714:
2711:
2706:
2700:
2696:
2689:
2687:
2685:
2683:
2679:
2674:
2672:9780125181242
2668:
2664:
2663:
2658:
2652:
2650:
2648:
2646:
2644:
2642:
2640:
2636:
2631:
2629:0-8153-4072-9
2625:
2621:
2617:
2610:
2607:
2602:
2600:0-87893-119-8
2596:
2592:
2591:
2586:
2579:
2576:
2572:
2566:
2563:
2558:
2556:0-8153-4072-9
2552:
2548:
2544:
2537:
2534:
2529:
2525:
2521:
2517:
2513:
2509:
2505:
2501:
2497:
2493:
2486:
2483:
2476:
2472:
2469:
2467:
2464:
2462:
2459:
2457:
2454:
2452:
2449:
2448:
2444:
2442:
2435:
2433:
2431:
2427:
2419:
2406:
2403:
2402:
2400:
2397:
2396:
2394:
2387:
2386:
2384:
2382:
2375:
2374:
2372:
2368:
2367:
2366:
2364:
2356:
2353:
2343:
2336:
2334:
2332:
2328:
2324:
2322:
2318:
2314:
2311:also contain
2310:
2309:
2304:
2301:
2297:
2293:
2289:
2285:
2284:Cyanobacteria
2281:
2277:
2270:
2266:
2262:
2261:
2256:
2252:
2248:
2244:
2239:
2232:
2230:
2228:
2224:
2223:Photosystem I
2220:
2216:
2212:
2208:
2204:
2200:
2196:
2192:
2188:
2184:
2180:
2176:
2168:
2166:
2164:
2160:
2152:
2148:
2144:
2140:
2136:
2132:
2128:
2124:
2120:
2113:
2109:
2105:
2097:
2093:
2089:
2085:
2081:
2077:
2073:
2072:mitochondrion
2068:
2061:
2059:
2055:
2048:
2026:
2001:
1959:
1950:
1911:
1902:
1897:
1894:
1873:
1870:
1865:
1846:
1841:
1831:
1826:
1823:
1803:
1802:
1801:
1779:
1765:
1751:
1748:
1745:
1717:
1705:
1673:
1672:
1671:
1668:
1664:
1649:
1636:
1622:
1599:
1571:
1560:
1546:
1519:
1506:
1502:
1498:
1495:
1488:
1485:
1482:
1470:
1450:
1421:
1418:
1415:
1389:
1386:
1360:
1357:
1350:
1339:
1327:For example,
1308:
1299:
1289:
1278:
1275:
1272:
1261:
1260:
1259:
1257:
1220:
1200:
1181:
1180:
1179:
1172:
1143:
1140:
1134:
1131:
1128:
1122:
1119:
1112:
1109:
1091:
1064:
1048:
1045:
1042:
1039:
1036:
1033:
1026:
1023:
1016:
1005:
993:
992:
991:
977:
974:
971:
964:
959:
945:
938:
931:
928:
917:
892:
876:
860:
853:
850:
834:
827:
824:
808:
801:
774:
771:
729:
726:
709:
694:
684:
667:
664:
650:
646:
645:charge number
630:
623:
620:
604:
593:
592:
591:
559:
556:
529:
526:
510:
507:
504:
501:
498:
495:
488:
485:
482:
479:
468:
467:
466:
464:
456:
454:
451:
447:
442:
440:
436:
432:
428:
423:
421:
417:
413:
409:
405:
401:
399:
391:
387:
383:
379:
376:
373:
370:
369:
368:
362:
358:
355:
351:
347:
342:
335:
333:
331:
327:
323:
319:
314:
312:
308:
304:
298:
297:to form ATP.
296:
292:
288:
284:
280:
276:
272:
269:(ETC) in the
268:
264:
260:
256:
252:
248:
244:
240:
236:
232:
224:
220:
216:
212:
208:
206:
202:
198:
191:
187:
184:by using the
183:
179:
175:
171:
167:
160:
158:
156:
152:
148:
144:
140:
136:
132:
128:
125:
124:phosphorylate
121:
117:
113:
109:
107:
103:
99:
95:
91:
83:
79:
75:
70:
66:
64:
60:
56:
52:
48:
44:
40:
36:
32:
19:
3063:Cell biology
3010:
3006:
2996:
2989:Biochemistry
2988:
2978:
2928:(1): 12447.
2925:
2921:
2911:
2868:
2865:Astrobiology
2864:
2854:
2827:
2821:
2800:
2755:
2751:
2723:
2719:
2713:
2695:Biochemistry
2694:
2661:
2619:
2609:
2589:
2578:
2565:
2546:
2536:
2495:
2491:
2485:
2439:
2430:ion channels
2423:
2381:ATP Synthase
2360:
2326:
2325:
2317:chloroplasts
2313:ATP synthase
2306:
2274:
2271:is omitted.
2264:
2258:
2199:energy level
2197:to a higher
2172:
2143:ATP synthase
2101:
2094:is omitted,
2056:
2049:
1932:
1766:
1733:
1669:
1665:
1637:
1547:
1471:
1326:
1255:
1253:
1177:
962:
960:
880:
849:gas constant
589:
460:
443:
426:
424:
420:ATP synthase
412:ion channels
402:
395:
366:
361:ATP synthase
322:chloroplasts
318:mitochondria
315:
299:
291:ATP synthase
287:ATP synthase
228:
223:mitochondria
182:mitochondria
164:
147:chloroplasts
135:chloroplasts
131:mitochondria
112:ATP synthase
110:
87:
31:Chemiosmosis
30:
29:
2622:. Garland.
2571:Nobel Prize
2549:. Garland.
2352:Abiogenesis
2265:H. halobium
2123:Krebs cycle
2076:chloroplast
875:temperature
384:gradient -
233:to produce
231:metabolized
120:free energy
3052:Categories
2726:(1): 1–3.
2477:References
2466:Glycolysis
2371:convection
2255:halophilic
2127:glycolysis
437:, such as
435:counterion
380:caused by
257:(NAD) and
235:acetyl CoA
2424:Deep-sea
2331:phosphate
2269:cell wall
2249:) during
2209:into the
2195:electrons
2169:In plants
2151:oxidation
2092:cell wall
2022:Δ
1998:Δ
1898:−
1879:→
1874:−
1827:−
1775:Δ
1746:ρ
1742:Δ
1718:ψ
1714:Δ
1682:Δ
1650:ψ
1646:Δ
1620:Δ
1596:Δ
1561:μ
1556:Δ
1524:Δ
1496:ψ
1492:Δ
1489:−
1479:Δ
1412:Δ
1387:−
1340:μ
1335:Δ
1290:μ
1285:Δ
1279:−
1269:Δ
1258:(PMF) as
1221:−
1189:Δ
1147:Δ
1132:
1123:−
1120:ψ
1116:Δ
1049:
1034:ψ
1030:Δ
1006:μ
1001:Δ
942:Δ
918:μ
913:Δ
889:Δ
695:ψ
692:Δ
601:Δ
511:
496:ψ
492:Δ
476:Δ
457:Equations
372:Diffusion
357:catalysed
324:and many
295:phosphate
277:from the
263:electrons
251:reduction
239:oxidation
201:molecules
174:respiring
37:across a
2962:31462644
2903:31841362
2799:(2015).
2772:24706236
2520:13771349
2445:See also
2276:Bacteria
2187:sunlight
2131:pyruvate
1404:implies
439:chloride
352:and the
326:bacteria
203:such as
139:bacteria
55:membrane
51:hydrogen
3029:6469951
2953:6713726
2930:Bibcode
2894:7133448
2873:Bibcode
2780:1840860
2528:1784050
2500:Bibcode
2308:E. coli
2280:archaea
2253:in the
2183:photons
2104:glucose
2082:cells (
2078:and in
961:For an
873:is the
847:is the
821:is the
647:of the
643:is the
619:cations
386:cations
359:by the
330:archaea
275:protons
265:to the
205:glucose
143:archaea
114:is the
102:osmosis
94:diffuse
92:, will
90:protons
82:channel
57:during
3027:
2960:
2950:
2901:
2891:
2842:
2809:
2797:Lane N
2778:
2770:
2701:
2669:
2626:
2597:
2553:
2526:
2518:
2492:Nature
2346:pumps.
2290:, and
2263:(syn.
2221:reach
2129:, and
1178:where
649:cation
590:where
390:Anions
279:matrix
186:energy
116:enzyme
84:(red).
2776:S2CID
2524:S2CID
2227:NADPH
2161:into
1703:) and
1443:. At
851:; and
450:redox
441:Cl).
106:water
3025:PMID
2958:PMID
2899:PMID
2840:ISBN
2807:ISBN
2768:PMID
2724:1183
2699:ISBN
2667:ISBN
2624:ISBN
2595:ISBN
2569:The
2551:ISBN
2516:PMID
2278:and
2173:The
2116:FADH
2114:and
2112:NADH
2086:and
2014:and
1507:59.1
1422:10.4
754:and
328:and
194:FADH
192:and
190:NADH
141:and
133:and
35:ions
3015:doi
3011:259
2948:PMC
2938:doi
2889:PMC
2881:doi
2832:doi
2760:doi
2728:doi
2508:doi
2496:191
2185:in
2177:of
2163:ATP
2159:ADP
1635:).
1451:298
448:of
406:of
241:of
188:of
61:or
3054::
3023:.
3009:.
3005:.
2999:–
2984:–
2956:.
2946:.
2936:.
2924:.
2920:.
2897:.
2887:.
2879:.
2869:20
2867:.
2863:.
2838:.
2788:^
2774:.
2766:.
2756:46
2754:.
2740:^
2722:.
2681:^
2638:^
2618:.
2587:.
2545:.
2522:.
2514:.
2506:.
2494:.
2286:,
2165:.
2141:.
2125:,
2074:,
1545:.
1135:10
1129:ln
1046:ln
958:.
508:ln
400:.
332:.
320:,
313:.
207:.
65:.
3031:.
3017::
2964:.
2940::
2932::
2926:9
2905:.
2883::
2875::
2848:.
2834::
2815:.
2782:.
2762::
2734:.
2730::
2707:.
2675:.
2632:.
2603:.
2559:.
2530:.
2510::
2502::
2390:o
2378:1
2155:2
2118:2
2052:p
2032:p
2027:G
2002:p
1978:]
1974:P
1971:T
1968:A
1964:[
1960:/
1956:]
1951:+
1946:H
1941:[
1917:O
1912:2
1908:H
1903:+
1895:4
1890:P
1887:T
1884:A
1871:2
1866:3
1861:O
1858:P
1855:O
1852:H
1847:+
1842:+
1837:H
1832:+
1824:4
1819:P
1816:D
1813:A
1785:p
1780:G
1752:0
1749:=
1730:.
1690:H
1687:p
1623:E
1600:G
1572:+
1567:H
1532:H
1529:p
1520:)
1515:V
1512:m
1503:(
1499:+
1486:=
1483:p
1456:K
1430:V
1427:m
1419:=
1416:p
1390:1
1382:l
1379:o
1376:m
1369:J
1366:k
1361:1
1358:=
1351:+
1346:H
1323:.
1309:F
1300:+
1296:H
1276:=
1273:p
1250:.
1235:P
1229:H
1226:p
1215:N
1209:H
1206:p
1201:=
1197:H
1194:p
1154:H
1151:p
1144:T
1141:R
1138:)
1126:(
1113:F
1110:=
1102:P
1098:]
1092:+
1087:H
1082:[
1075:N
1071:]
1065:+
1060:H
1055:[
1043:T
1040:R
1037:+
1027:F
1024:=
1017:+
1012:H
978:1
975:=
972:z
946:G
939:=
932:+
929:z
924:X
893:G
877:.
861:T
835:R
825:;
809:F
785:N
781:]
775:+
772:z
767:X
762:[
740:P
736:]
730:+
727:z
722:X
717:[
682:;
668:+
665:z
660:X
631:z
605:G
570:P
566:]
560:+
557:z
552:X
547:[
540:N
536:]
530:+
527:z
522:X
517:[
505:T
502:R
499:+
489:F
486:z
483:=
480:G
363:.
225:.
196:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.