138:
sorbents use amine groups which are charged when the pH is below about 9. Strong anion exchange sorbents are useful because any strongly acidic impurities in the sample will bind to the sorbent and usually will not be eluted with the analyte of interest; to recover a strong acid a weak anion exchange cartridge should be used. To elute the analyte from either the strong or weak sorbent, the stationary phase is washed with a solvent that neutralizes the charge of either the analyte, the stationary phase, or both. Once the charge is neutralized, the electrostatic interaction between the analyte and the stationary phase no longer exists and the analyte will elute from the cartridge.
171:. These can be mounted on its specific type of extraction manifold. The manifold allows multiple samples to be processed by holding several SPE media in place and allowing for an equal number of samples to pass through them simultaneously. In a standard cartridge SPE manifold up to 24 cartridges can be mounted in parallel, while a typical disk SPE manifold can accommodate 6 disks. Most SPE manifolds are equipped with a vacuum port, where vacuum can be applied to speed up the extraction process by pulling the liquid sample through the stationary phase. The analytes are collected in sample tubes inside or below the manifold after they pass through the stationary phase.
56:), to separate a mixture into desired and undesired components. The result is that either the desired analytes of interest or undesired impurities in the sample are retained on the stationary phase. The portion that passes through the stationary phase is collected or discarded, depending on whether it contains the desired analytes or undesired impurities. If the portion retained on the stationary phase includes the desired analytes, they can then be removed from the stationary phase for collection in an additional step, in which the stationary phase is rinsed with an appropriate
147:
aliphatic carboxylic acids, which are charged when the pH is above about 5. Strong cation exchange sorbents are useful because any strongly basic impurities in the sample will bind to the sorbent and usually will not be eluted with the analyte of interest; to recover a strong base a weak cation exchange cartridge should be used. To elute the analyte from either the strong or weak sorbent, the stationary phase is washed with a solvent that neutralizes ionic interaction between the analyte and the stationary phase.
45:) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
104:
wet the silica surface. The sample is then added to the cartridge. As the sample passes through the stationary phase, the polar analytes in the sample will interact and retain on the polar sorbent while the solvent, and other non-polar impurities pass through the cartridge. After the sample is loaded, the cartridge is washed with a non-polar solvent to remove further impurities. Then, the analyte is eluted with a polar solvent or a buffer of the appropriate pH.
802:
96:
31:
87:, for each component in the mixture. The chemical considerations for the selection of stationary and mobile phases are similar to those for liquid column chromatography and many of the adsorbents/materials used are the same. The theory, procedures, and aims are different, however, and as an extractive technique it has a unique niche in modern chemical science.
79:, in the sense of having a mobile phase, carrying mixtures through a stationary phase, packed inside a column. The chromatographic process is harnessed to create a solid-liquid extractive technique—allowing separation of a mixture of components by taking advantage of large differences between the solid and liquid phase K
174:
Solid phase extraction cartridges and disks can be purchased with several stationary phases, each of which separates analytes depending on different chemical properties. The basis of most stationary phases is silica that has been bonded to a specific functional group. Some of these functional groups
146:
Cation exchange sorbents are derivatized with functional groups that interact and retain positively charged cations, such as bases. Strong cation exchange sorbents contain aliphatic sulfonic acid groups that are always negatively charged in aqueous solution, and weak cation exchange sorbents contain
103:
A typical solid phase extraction involves five basic steps. First, the cartridge is equilibrated with a non-polar or slightly polar solvent, which wets the surface and penetrates the bonded phase. Then water, or buffer of the same composition as the sample, is typically washed through the column to
63:
It is possible to have an incomplete recovery of the analytes by SPE caused by incomplete extraction or elution. In the case of an incomplete extraction, the analytes do not have enough affinity for the stationary phase and part of them will remain in the permeate. In an incomplete elution, part of
137:
Anion exchange sorbents are derivatized with positively charged functional groups that interact and retain negatively charged anions, such as acids. Strong anion exchange sorbents contain quaternary ammonium groups that have a permanent positive charge in aqueous solutions, and weak anion exchange
116:
Reversed phase SPE separates analytes based on their polarity. The stationary phase of a reversed phase SPE cartridge is derivatized with hydrocarbon chains, which retain compounds of mid to low polarity due to the hydrophobic effect. The analyte can be eluted by washing the cartridge with a
128:
Ion exchange sorbents separate analytes based on electrostatic interactions between the analyte of interest and the positively or negatively charged groups on the stationary phase. For ion exchange to occur, both the stationary phase and sample must be at a pH where both are charged.
107:
A stationary phase of polar functionally bonded silicas with short carbons chains frequently makes up the solid phase. This stationary phase will adsorb polar molecules which can be collected with a more polar solvent.
175:
include hydrophobic alkyl or aryl chains chains of variable length (for reversed phase), quaternary ammonium or amino groups (for anion exchange), and aliphatic sulfonic acid or carboxyl groups (for cation exchange).
34:
A typical solid phase extraction manifold. The cartridges drip into the chamber below, where tubes collect the effluent. A vacuum port with gauge is used to control the vacuum applied to the chamber.
120:
A stationary phase of silicon with carbon chains is commonly used. Relying on mainly non-polar, hydrophobic interactions, only non-polar or very weakly polar compounds will adsorb to the surface.
67:
Many of the adsorbents/materials are the same as in chromatographic methods, but SPE is distinctive, with aims separate from chromatography, and so has a unique niche in modern chemical science.
202:
and non-volatile) from different kinds of media, that can be in liquid or gas phase. The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as
618:
309:
Augusto, Fabio; Hantao, Leandro W.; Mogollón, Noroska G.S.; Braga, Soraia C.G.N. (2013). "New materials and trends in sorbents for solid-phase extraction".
528:
M. Abdel-Rehim, AstraZeneca
Application “Syringe for solid phase microextraction”, Current Patents Gazette, week 0310, WO 03019149, p. 77, (2003).
366:
589:
579:
569:
611:
186:(SPME), is a solid phase extraction technique that involves the use of a fiber coated with an extracting phase, that can be a liquid (
441:"Selectivity of solid phase extraction of freshwater dissolved organic matter and its effect on ultrahigh resolution mass spectra"
971:
776:
266:
Hennion, Marie-Claire (1999). "Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography".
786:
966:
766:
604:
741:
53:
816:
801:
183:
836:
876:
761:
99:
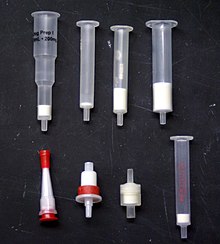
A selection of solid phase extraction cartridges, available in many sizes, shapes, and types of stationary phase.
646:
919:
686:
711:
199:
901:
896:
701:
661:
564:
E. M. Thurman, M. S. Mills, Solid-Phase
Extraction: Principles and Practice, Wiley-Interscience, 1998,
851:
736:
681:
375:
203:
84:
439:
Raeke, Julia; Lechtenfeld, Oliver J.; Wagner, Martin; Herzsprung, Peter; Reemtsma, Thorsten (2016).
64:
the analytes remain in the sorbent because the eluent used does not have a strong enough affinity.
671:
547:
393:
228:
731:
627:
574:
Nigel J.K. Simpson, Solid-Phase
Extraction: Principles, Techniques, and Applications, CRC, 2000,
421:
334:
871:
746:
706:
585:
575:
565:
511:
503:
468:
460:
413:
326:
291:
283:
248:
52:), to a solid packing inside a small column, through which the sample is passed (known as the
539:
495:
452:
405:
318:
275:
240:
117:
non-polar solvent, which disrupts the interaction of the analyte and the stationary phase.
929:
866:
791:
771:
751:
726:
666:
540:
945:
656:
76:
279:
17:
960:
886:
861:
486:
Abdel-Rehim, Mohamed (2011). "Microextraction by packed sorbent (MEPS): A tutorial".
156:
425:
338:
846:
831:
721:
691:
49:
48:
SPE uses the affinity of solutes, dissolved or suspended in a liquid (known as the
409:
244:
821:
676:
167:) device, a SPE method that uses a packed sorbent material in a liquid handling
322:
155:
The stationary phase comes in the form of a packed syringe-shaped cartridge, a
841:
826:
716:
651:
499:
207:
507:
464:
417:
330:
287:
252:
924:
95:
515:
472:
295:
30:
881:
856:
641:
756:
596:
456:
440:
195:
191:
187:
168:
696:
57:
584:
James S. Fritz, Analytical Solid-Phase
Extraction, Wiley-VCH, 1999,
206:
is reached or, in case of short time pre-equilibrium, with help of
891:
94:
29:
600:
394:"Past, Present, and Future of Solid Phase Extraction: A Review"
229:"Past, Present, and Future of Solid Phase Extraction: A Review"
392:
Buszewski, Boguslaw; Szultka, Malgorzata (July 2012).
542:
938:
910:
809:
634:
227:Buszewski, Boguslaw; Szultka, Malgorzata (2012).
445:Environmental Science: Processes & Impacts
360:
358:
356:
354:
352:
350:
348:
612:
8:
27:Process to separate compounds by properties
619:
605:
597:
398:Critical Reviews in Analytical Chemistry
233:Critical Reviews in Analytical Chemistry
219:
194:), which extracts different kinds of
7:
311:TrAC Trends in Analytical Chemistry
25:
161:microextraction by packed sorbent
159:, a 47- or 90-mm flat disk, or a
800:
368:Guide to Solid Phase Extraction
546:. Wiley-Interscience. p.
1:
538:Mitra, Somenath, ed. (2003).
280:10.1016/S0021-9673(99)00832-8
410:10.1080/07373937.2011.645413
245:10.1080/07373937.2011.645413
268:Journal of Chromatography A
184:Solid-phase microextraction
179:Solid-phase microextraction
75:SPE is in fact a method of
988:
837:Electrostatic precipitator
323:10.1016/j.trac.2012.08.012
91:Normal phase SPE procedure
877:Rotary vacuum-drum filter
798:
500:10.1016/j.aca.2011.05.037
920:Aqueous two-phase system
742:Liquid–liquid extraction
817:API oil–water separator
687:Dissolved air flotation
972:Extraction (chemistry)
782:Solid-phase extraction
488:Analytica Chimica Acta
100:
71:SPE and chromatography
39:Solid-phase extraction
35:
18:Solid phase extraction
902:Vacuum ceramic filter
897:Sublimation apparatus
702:Electrochromatography
662:Cross-flow filtration
98:
33:
967:Analytical chemistry
852:Fractionating column
647:Acid–base extraction
628:Separation processes
85:equilibrium constant
672:Cyclonic separation
732:Gravity separation
457:10.1039/C6EM00200E
381:on 13 January 2012
112:Reversed phase SPE
101:
36:
954:
953:
872:Rapid sand filter
767:Recrystallization
747:Electroextraction
707:Electrofiltration
590:978-0-471-24667-1
580:978-0-8247-0021-8
570:978-0-471-61422-7
16:(Redirected from
979:
804:
621:
614:
607:
598:
552:
551:
545:
535:
529:
526:
520:
519:
483:
477:
476:
436:
430:
429:
389:
383:
382:
380:
374:, archived from
373:
365:Supelco (1998),
362:
343:
342:
306:
300:
299:
263:
257:
256:
224:
198:(including both
124:Ion exchange SPE
54:stationary phase
21:
987:
986:
982:
981:
980:
978:
977:
976:
957:
956:
955:
950:
934:
912:
906:
867:Protein skimmer
805:
796:
792:Ultrafiltration
772:Reverse osmosis
752:Microfiltration
727:Froth flotation
667:Crystallization
630:
625:
561:
559:Further reading
556:
555:
537:
536:
532:
527:
523:
485:
484:
480:
438:
437:
433:
391:
390:
386:
378:
371:
364:
363:
346:
308:
307:
303:
265:
264:
260:
226:
225:
221:
216:
181:
153:
144:
142:Cation exchange
135:
126:
114:
93:
82:
73:
28:
23:
22:
15:
12:
11:
5:
985:
983:
975:
974:
969:
959:
958:
952:
951:
949:
948:
946:Unit operation
942:
940:
936:
935:
933:
932:
927:
922:
916:
914:
908:
907:
905:
904:
899:
894:
889:
884:
879:
874:
869:
864:
859:
854:
849:
844:
839:
834:
829:
824:
819:
813:
811:
807:
806:
799:
797:
795:
794:
789:
784:
779:
774:
769:
764:
759:
754:
749:
744:
739:
734:
729:
724:
719:
714:
709:
704:
699:
694:
689:
684:
679:
674:
669:
664:
659:
657:Chromatography
654:
649:
644:
638:
636:
632:
631:
626:
624:
623:
616:
609:
601:
595:
594:
592:
582:
572:
560:
557:
554:
553:
530:
521:
494:(2): 119–128.
478:
451:(7): 918–927.
431:
404:(3): 198–213.
384:
344:
301:
258:
239:(3): 198–213.
218:
217:
215:
212:
210:or agitation.
190:) or a solid (
180:
177:
152:
149:
143:
140:
134:
133:Anion exchange
131:
125:
122:
113:
110:
92:
89:
80:
77:chromatography
72:
69:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
984:
973:
970:
968:
965:
964:
962:
947:
944:
943:
941:
937:
931:
928:
926:
923:
921:
918:
917:
915:
909:
903:
900:
898:
895:
893:
890:
888:
887:Spinning cone
885:
883:
880:
878:
875:
873:
870:
868:
865:
863:
862:Mixer-settler
860:
858:
855:
853:
850:
848:
845:
843:
840:
838:
835:
833:
830:
828:
825:
823:
820:
818:
815:
814:
812:
808:
803:
793:
790:
788:
785:
783:
780:
778:
777:Sedimentation
775:
773:
770:
768:
765:
763:
762:Precipitation
760:
758:
755:
753:
750:
748:
745:
743:
740:
738:
735:
733:
730:
728:
725:
723:
720:
718:
715:
713:
710:
708:
705:
703:
700:
698:
695:
693:
690:
688:
685:
683:
680:
678:
675:
673:
670:
668:
665:
663:
660:
658:
655:
653:
650:
648:
645:
643:
640:
639:
637:
633:
629:
622:
617:
615:
610:
608:
603:
602:
599:
593:
591:
587:
583:
581:
577:
573:
571:
567:
563:
562:
558:
549:
544:
543:
534:
531:
525:
522:
517:
513:
509:
505:
501:
497:
493:
489:
482:
479:
474:
470:
466:
462:
458:
454:
450:
446:
442:
435:
432:
427:
423:
419:
415:
411:
407:
403:
399:
395:
388:
385:
377:
370:
369:
361:
359:
357:
355:
353:
351:
349:
345:
340:
336:
332:
328:
324:
320:
316:
312:
305:
302:
297:
293:
289:
285:
281:
277:
274:(1–2): 3–54.
273:
269:
262:
259:
254:
250:
246:
242:
238:
234:
230:
223:
220:
213:
211:
209:
205:
201:
197:
193:
189:
185:
178:
176:
172:
170:
166:
162:
158:
157:96 well plate
150:
148:
141:
139:
132:
130:
123:
121:
118:
111:
109:
105:
97:
90:
88:
86:
78:
70:
68:
65:
61:
59:
55:
51:
46:
44:
40:
32:
19:
847:Filter press
832:Depth filter
781:
722:Flocculation
692:Distillation
541:
533:
524:
491:
487:
481:
448:
444:
434:
401:
397:
387:
376:the original
367:
314:
310:
304:
271:
267:
261:
236:
232:
222:
182:
173:
164:
160:
154:
145:
136:
127:
119:
115:
106:
102:
74:
66:
62:
50:mobile phase
47:
42:
38:
37:
822:Belt filter
787:Sublimation
677:Decantation
204:equilibrium
961:Categories
911:Multiphase
842:Evaporator
827:Centrifuge
717:Filtration
712:Extraction
652:Adsorption
642:Absorption
214:References
208:convection
151:Cartridges
925:Azeotrope
635:Processes
508:0003-2670
465:2050-7887
418:1040-8347
331:0165-9936
317:: 14–23.
288:0021-9673
253:1040-8347
939:Concepts
930:Eutectic
882:Scrubber
857:Leachate
737:Leaching
682:Dialysis
516:21801877
473:27363664
426:98381163
339:96825406
296:10526783
200:volatile
196:analytes
913:systems
810:Devices
757:Osmosis
192:sorbent
188:polymer
169:syringe
697:Drying
588:
578:
568:
514:
506:
471:
463:
424:
416:
337:
329:
294:
286:
251:
58:eluent
892:Still
422:S2CID
379:(PDF)
372:(PDF)
335:S2CID
83:, or
586:ISBN
576:ISBN
566:ISBN
512:PMID
504:ISSN
469:PMID
461:ISSN
414:ISSN
327:ISSN
292:PMID
284:ISSN
249:ISSN
165:MEPS
548:113
496:doi
492:701
453:doi
406:doi
319:doi
276:doi
272:856
241:doi
60:.
43:SPE
963::
510:.
502:.
490:.
467:.
459:.
449:18
447:.
443:.
420:.
412:.
402:42
400:.
396:.
347:^
333:.
325:.
315:43
313:.
290:.
282:.
270:.
247:.
237:42
235:.
231:.
81:eq
620:e
613:t
606:v
550:.
518:.
498::
475:.
455::
428:.
408::
341:.
321::
298:.
278::
255:.
243::
163:(
41:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.