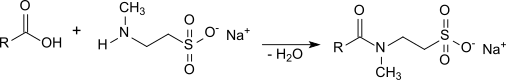149:
120:
90:) and produced under the trade name Igepon at the Hoechst plant. Taurates rapidly spread due to their lime resistance and their oil-removing effect in textile treatment, as detergent raw material and in cosmetics applications. They had a breakthrough in particular because they do not felt wool during washing (as opposed to soap). The production of taurates decreased after the outbreak of the
17:
316:
294:
126:
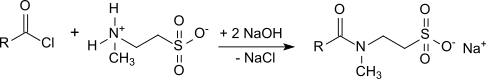
The formation of (at least) equimolar amounts of sodium chloride is problematic, as they worsen the properties of surfactant mixtures with such taurates. The high salt content also makes the resulting taurates hygroscopic and corrosive. Another disadvantage of the
Schotten-Baumann method is the
378:
356:
159:-methyltaurine already begins At temperatures above 200 °C and the resulting taurates darken and develop an unpleasant smell. Therefore, more recent variants of the direct amidation aim at gentler process conditions using suitable catalysts, such as
139:. This synthesis pathway for taurates is therefore complicated and expensive. An advantage of the Schotten-Baumann method, however, is the very low content of free fatty acids in the end product. Taurates are also accessible by direct amidation of
203:, as for example isethionates. They are very mild surfactants with good foaming ability and high foam stability, even in the presence of fats and oils. Taurates retain their good washing properties even in
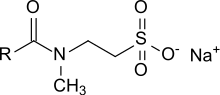
50:(2-methylaminoethanesulfonic acid) and a lipophilic residue, consisting of a long-chain carboxylic acid (fatty acid), both linked via an amide bond. The fatty acids used could be lauric (C
223:, liquid soaps and cleansers, face lotions, skin creams, bubble baths, syndet soaps), textile processing (wetting agents and detergents, dye dispersants), in
155:
The excess fatty acid (added for a favorable equilibrium) usually remain in the product, which can interfere with some applications. The decomposition of
289:, Guenther, Fritz; Münz, Ferdinand & Haussmann, Hans, "Sulphuric acid derivatives of amides", published 1933-10-24, assigned to
148:
354:, Walele, Ismail I. & Syed, Samad A., "Process for making N-acyl taurides", published 1995-07-18, assigned to Finetex, Inc.
211:. Taurates are suitable in concentrations of about 2% as co-surfactants because of their good compatibility with all nonionic and anionic surfactants.
119:
78:) are used. Besides sodium, no other counterions play a relevant role (these could be e. g. ammonium or other alkali or alkaline earth metals).
273:
245:
311:, Burnette, Llewellyn W. & Chiddix, Max E., "Production of N-acyl taurides", published 1959-05-31, assigned to
179:
At room temperature, taurates are usually pasty masses, which dissolve well in water and react then neutral to slightly alkaline (
103:
143:-methyltaurine or its sodium salt with the corresponding fatty acid for 10 hours at 220 °C under nitrogen.
411:
128:
187:, rat, oral is 7800 mg·kg for cocoyl tauride). They are easily biodegradable, they are not prone to
406:
373:, Day, James F., "Preparation of N-acyl taurates", published 1996-03-05, assigned to
370:
351:
308:
286:
416:
219:
Taurates are used as mild, well-foaming surfactants in body cleansing and personal care products (
160:
269:
241:
334:
136:
86:
The surfactant group of the taurates was developed by I.G. Farben in
Germany (just like the
224:
188:
44:
107:
400:
132:
240:, Wiley-VCH Verlag GmbH & Co. KGaA, 3. vollst. überarb. u. erw. Auflage (2012),
199:, taurates are stable in a much wider pH range (about 2–10) than the corresponding
91:
87:
312:
94:, since only poor quality fatty acids were available due to the fat management.
40:
204:
196:
192:
168:
164:
290:
16:
374:
208:
338:
220:
135:) and the accumulation of large amounts of waste materials, such as
200:
20:
Generic structure of a taurate. R is an odd numbered alkyl group C
15:
43:. They are composed of a hydrophilic head group, consisting of
268:, München: C.H.Beck 2005, XVIII + 460 S., 29 Abb., 20 Tab.,
180:
391:
Sicherheitsdatenblatt für
Geropon® TC 42 der Rhodia S.A.
191:, but they are harmful to aquatic organisms (like all
333:, J. Amer. Oil Chem. Soc., 39(11), 1962, 477–478,
106:which is the reaction of long-chain carboxylic
266:Hoechst. Ein I.G. Farben Werk im Dritten Reich
331:Reaction of Fatty Acids with N-Methyl Taurine
110:with aqueous solutions of the sodium salt of
8:
127:hazardousness of the raw materials (such as
257:
66:), but mainly mixtures of oleic acid (C
227:and in other industrial applications.
238:Kosmetik und Hygiene von Kopf bis Fuß
7:
102:Taurates were first obtained by the
183:7–8). Their toxicity is low (the LD
14:
313:General Aniline & Film Corp.
147:
118:
39:) are a group of mild anionic
1:
329:L.W. Burnette, M.E. Chiddix,
131:) and the intermediates (the
28:with n = 7 – 17 carbon atoms.
225:crop protection formulations
70:) and coconut fatty acid (C
433:
236:Wilfried Umbach (Hrsg.),
291:I.G. Farbenindustrie AG
104:Schotten-Baumann method
375:Hoechst Celanese Corp.
129:phosphorus trichloride
29:
19:
62:) or stearic acid (C
264:Stefan H. Lindner:
339:10.1007/BF02637229
161:sodium borohydride
30:
274:978-3-406-52959-7
246:978-3-527-30996-2
424:
392:
389:
383:
382:
381:
377:
367:
361:
360:
359:
355:
348:
342:
327:
321:
320:
319:
315:
305:
299:
298:
297:
293:
283:
277:
262:
195:). Due to their
151:
137:phosphonic acids
122:
114:-methyltaurine.
432:
431:
427:
426:
425:
423:
422:
421:
397:
396:
395:
390:
386:
379:
369:
368:
364:
357:
350:
349:
345:
328:
324:
317:
307:
306:
302:
295:
285:
284:
280:
263:
259:
255:
233:
217:
189:bioaccumulation
186:
177:
100:
84:
77:
73:
69:
65:
61:
57:
53:
27:
23:
12:
11:
5:
430:
428:
420:
419:
414:
412:Sulfonic acids
409:
399:
398:
394:
393:
384:
362:
343:
322:
300:
278:
256:
254:
251:
250:
249:
232:
229:
216:
213:
184:
176:
173:
153:
152:
133:acyl chlorides
124:
123:
108:acid chlorides
99:
96:
83:
80:
75:
71:
67:
63:
59:
58:), palmitic (C
55:
54:), myristic (C
51:
48:-methyltaurine
25:
21:
13:
10:
9:
6:
4:
3:
2:
429:
418:
415:
413:
410:
408:
405:
404:
402:
388:
385:
376:
372:
366:
363:
353:
347:
344:
340:
336:
332:
326:
323:
314:
310:
304:
301:
292:
288:
282:
279:
275:
271:
267:
261:
258:
252:
247:
243:
239:
235:
234:
230:
228:
226:
222:
214:
212:
210:
206:
202:
198:
194:
190:
182:
174:
172:
170:
166:
162:
158:
150:
146:
145:
144:
142:
138:
134:
130:
121:
117:
116:
115:
113:
109:
105:
97:
95:
93:
89:
81:
79:
49:
47:
42:
38:
34:
18:
407:Carboxamides
387:
365:
346:
330:
325:
303:
281:
265:
260:
237:
218:
178:
156:
154:
140:
125:
111:
101:
92:World War II
88:isethionates
85:
45:
36:
32:
31:
417:Surfactants
193:surfactants
41:surfactants
401:Categories
371:US 5496959
352:US 5434276
309:US 2880219
287:US 1932180
253:References
231:Literature
205:hard water
197:amide bond
175:Properties
169:zinc oxide
165:boric acid
98:Production
221:shampoos
209:seawater
37:taurides
33:Taurates
82:History
380:
358:
318:
296:
272:
244:
201:esters
270:ISBN
242:ISBN
68:18:1
35:(or
26:2n+1
335:doi
215:Use
207:or
167:or
74:– C
403::
185:50
181:pH
171:.
163:,
76:18
64:18
60:16
56:14
52:12
341:.
337::
276:.
248:.
157:N
141:N
112:N
72:8
46:N
24:H
22:n
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.