121:
144:
339:
370:
295:
225:
31:
1704:
1555:
1623:
1460:
65:
116:
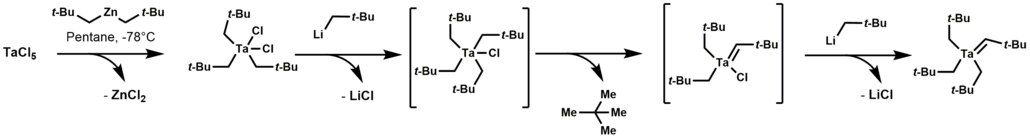
Tantalum alkylidene complexes arise by treating trialkyltantalum dichloride with alkyl lithium reagents. This reaction initially forms a thermally unstable tetraalkyl-monochloro-tantalum complex, which undergoes α-hydrogen elimination, followed by alkylation of the remaining chloride.
600:
Brennessel, William W.; Romanenkov, Alexander; Young, Victor G.; Ellis, John E. (2019). "Tantalum isocyanide complexes: TaI(CNDipp)6 (Dipp is 2,6-diisopropylphenyl) and ionic [Ta(CNDipp)7][Ta(CNDipp)6], a formal disproportionation product of the 17-electron Ta0 metalloradical
386:
of organotin compounds with tantalum(V) chloride. These organotantalum reagents promote the conjugate allylation of enones. Although the direct allylation of carbonyl groups is prevalent throughout the literature, little has been reported on the conjugate allylation of enones.
920:
Eisenberger, Patrick; Ayinla, Rashidat O.; Lauzon, Jean Michel P.; Schafer, Laurel L. (2009-10-19). "Tantalum–Amidate
Complexes for the Hydroaminoalkylation of Secondary Amines: Enhanced Substrate Scope and Enantioselective Chiral Amine Synthesis".
999:
Payne, Philippa R.; Garcia, Pierre; Eisenberger, Patrick; Yim, Jacky C.-H.; Schafer, Laurel L. (2013-05-03). "Tantalum
Catalyzed Hydroaminoalkylation for the Synthesis of α- and β-Substituted N-Heterocycles".
478:
McLain, S. J.; Wood, C. D.; Schrock, R. R. (1977-05-01). "Multiple metal-carbon bonds. 6. The reaction of niobium and tantalum neopentylidene complexes with simple olefins: a route to metallocyclopentanes".
776:
Takai, Kazuhiko; Kataoka, Y.; Utimoto, K. (1990-03-01). "Tantalum-alkyne complexes as synthetic intermediates. Stereoselective preparation of trisubstituted allylic alcohols from acetylenes and aldehydes".
741:
Bruck, M. A.; Copenhaver, A. S.; Wigley, D. E. (1987-10-01). "Alkyne cyclizations at reduced tantalum centers: synthesis and molecular structure of (.eta.6-C6Me6)Ta(O-2,6-i-Pr2C6H3)2Cl".
1043:
Shibata, Ikuya; Kano, Takeyoshi; Kanazawa, Nobuaki; Fukuoka, Shoji; Baba, Akio (2002-04-15). "Generation of
Organotantalum Reagents and Conjugate Addition to Enones".
964:
Dörfler, Jaika; Doye, Sven (2014-05-01). "A Commercially
Available Tantalum Catalyst for the Highly Regioselective Intermolecular Hydroaminoalkylation of Styrenes".
1138:
567:
J. E. Ellis; A. Davison (1976). "Tris[Bis(2-Methoxyethyl)Ether]Potassium and
Tetraphenylarsonium Hexacarbonylmetallates(1-) of Niobium and Tantalum".
644:
Pampaloni, G. (2010). "Aromatic hydrocarbons as ligands. Recent advances in the synthesis, the reactivity and the applications of bis(η6-arene) complexes".
331:. The chemistry developed by Maspero was later brought to fruition when Hartwig and Herzon reported the hydroaminoalkylation of olefins to form
56:. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
584:
531:
464:
1131:
671:
Labinger, Jay A.; Schwartz, Jeffrey; Townsend, John M. (1974-06-01). "Iodo- and hydridotantalum(III) complexes of dialkylacetylenes".
1841:
1056:
1124:
291:. Some tantalum-alkyne complexes are precursors to allylic alcohols. Tantalacyclopropenes are invoked as intermediates.
1836:
1693:
1688:
1683:
1678:
1673:
1668:
1663:
1658:
1653:
1648:
1643:
1638:
1628:
1571:
1465:
1386:
1381:
1238:
198:
1729:
1431:
1401:
1391:
1371:
1359:
1327:
1292:
1260:
1228:
1223:
1183:
514:
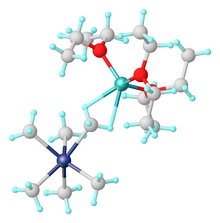
Endy Y.-J. Min; John E. Bercaw (2014). "Bis(η -Pentamethylcyclopentadienyl) Complexes of
Niobium and Tantalum".
351:
1198:
1162:
1116:
1774:
1769:
1764:
1759:
1754:
1749:
1744:
1739:
1734:
1719:
1709:
1560:
1535:
1530:
1515:
1500:
1480:
1426:
1354:
1337:
1287:
1282:
1277:
1272:
1248:
1208:
241:
under an atmosphere of CO gives the salts of . These same anions can be obtained by carbonylation of tantalum
179:
175:
143:
395:
Organotantalum compounds are of academic interest, but few or no commercial applications have been described.
338:
174:
Some of the first reported organotantalum complexes were cyclopentadienyl derivatives. These arise from the
1724:
1714:
1525:
1510:
1495:
1485:
1470:
1411:
1396:
1376:
1366:
1347:
1342:
1332:
1322:
1265:
1233:
1203:
1193:
369:
1633:
1548:
1490:
1453:
1448:
1436:
1416:
1406:
1312:
1307:
1302:
1243:
1218:
706:
Cotton, F. Albert; Hall, William T. (1979-08-01). "Reactions of tantalum(III) with alkynes and nitriles".
187:
183:
1441:
1253:
1188:
1178:
277:
120:
294:
1520:
1505:
1317:
1297:
1421:
77:
841:
626:
541:
359:
288:
42:
1103:
1068:
1060:
1025:
1017:
981:
946:
938:
902:
884:
833:
794:
758:
723:
688:
618:
580:
527:
496:
433:
261:
163:
1095:
1052:
1009:
973:
930:
892:
876:
825:
786:
750:
715:
680:
653:
610:
572:
519:
488:
460:
451:
Kleinhenz, S.; Pfennig, V.; Seppelt, K. (1998). "Preparation and
Structures of , , , and ".
425:
355:
1086:
Yamamoto, Yoshinori; Asao, Naoki (1993-09-01). "Selective reactions using allylic metals".
224:
553:
383:
347:
332:
316:
81:
569:
366:
of the tantalum-carbon bond, and β-hydrogen abstraction affords the alkylamine product.
897:
860:
92:
1830:
1793:
53:
845:
630:
363:
416:
Schrock, Richard R. (1979-03-01). "Alkylidene complexes of niobium and tantalum".
30:
129:
576:
523:
657:
614:
465:
10.1002/(SICI)1521-3765(19980904)4:9<1687::AID-CHEM1687>3.0.CO;2-R
312:
249:
137:
17:
1107:
1064:
1021:
985:
942:
888:
837:
798:
762:
727:
692:
500:
437:
273:
155:
1072:
1029:
977:
950:
934:
906:
622:
1057:
10.1002/1521-3773(20020415)41:8<1389::AID-ANIE1389>3.0.CO;2-D
829:
320:
151:
50:
1099:
790:
754:
719:
684:
492:
429:
159:
1013:
880:
1148:
133:
46:
861:"Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with
368:
337:
293:
242:
223:
142:
119:
64:
63:
186:. An example of this is the first transition metal trihydride,
1120:
812:
Clerici, Mario G.; Maspero, Federico (1980-01-01). "Catalytic
132:. They effect a number of reactions including: olefinations,
311:
Organotantalum compounds are invoked as intermediates in C-
136:
metathesis, hydroaminoalkylation of olefins, and conjugate
27:
Chemistry of compounds containing a carbon-to-tantalum bond
197:. More soluble and better developed are derivatives of
603:
571:. Inorganic Syntheses. Vol. 16. pp. 68–73.
1785:
162:react with tantalum alkylidene complexes to yield
859:Herzon, Seth B.; Hartwig, John F. (2007-05-01).
816:-Alkylation of Secondary Amines with Alkenes".
124:Synthesis of Tantalum Monoalkylidene Complexes
1132:
8:
342:Hartwig hydroaminoalkylation reaction scheme
87:Salts of are prepared by alkylation of TaF
1139:
1125:
1117:
373:Purposed mechanism of hydroaminoalkylation
1151:with other elements in the periodic table
896:
411:
409:
407:
260:Treatment of tantalum pentachloride with
869:Journal of the American Chemical Society
743:Journal of the American Chemical Society
708:Journal of the American Chemical Society
673:Journal of the American Chemical Society
481:Journal of the American Chemical Society
147:Tantalum Alkylidene Promoted Olefination
29:
923:Angewandte Chemie International Edition
403:
1168:
549:
539:
966:European Journal of Organic Chemistry
228:Tantalum Alkylidene Olefin Metathesis
7:
1810:Academic research, no widespread use
256:Tantalum arenes and alkyne complexes
287:Tantalum-alkyne complexes catalyze
60:Classes of organotantalum compounds
382:Organotantalum reagents arise via
233:Tantalum carbonyls and isocyanides
128:Tantalum alkylidene complexes are
25:
354:of the bisamide, which forms the
1702:
1621:
1553:
1458:
1158:
779:The Journal of Organic Chemistry
518:. Vol. 36. pp. 52–57.
646:Coordination Chemistry Reviews
516:Inorganic Syntheses: Volume 36
1:
418:Accounts of Chemical Research
1858:
577:10.1002/9780470132470.ch21
524:10.1002/9781118744994.ch11
252:complexes are also known.
199:pentamethylcyclopentadiene
170:Cyclopentadienyl complexes
1699:
1618:
1170:
1166:
1156:
658:10.1016/j.ccr.2009.05.014
615:10.1107/S2053229619000834
176:salt metathesis reactions
1842:Organometallic chemistry
307:Tantalum-amido complexes
298:Utimoto's Synthesis of (
180:sodium cyclopentadienide
73:Alkyl and aryl complexes
39:Organotantalum chemistry
1805:Many uses in chemistry
1800:Core organic chemistry
978:10.1002/ejoc.201400082
935:10.1002/anie.200903656
374:
352:β-hydrogen abstraction
343:
303:
229:
184:tantalum pentachloride
148:
125:
69:
35:
372:
341:
297:
278:aluminium trichloride
248:A number of tantalum
227:
146:
123:
67:
33:
830:10.1055/s-1980-29002
358:. Subsequent olefin
112:Alkylidene complexes
41:is the chemistry of
34:Tantalum-Carbon Bond
1100:10.1021/cr00022a010
791:10.1021/jo00293a008
755:10.1021/ja00255a056
720:10.1021/ja00511a064
685:10.1021/ja00819a047
493:10.1021/ja00452a064
430:10.1021/ar50135a004
289:cyclotrimerizations
78:Pentamethyltantalum
1837:Tantalum compounds
865:-Alkyl Arylamines"
375:
344:
304:
302:)-Allylic Alcohols
230:
149:
126:
70:
43:chemical compounds
36:
1824:
1823:
1780:
1779:
1045:Angewandte Chemie
1014:10.1021/ol400729v
972:(13): 2790–2797.
929:(44): 8361–8365.
881:10.1021/ja0718366
875:(21): 6690–6691.
749:(21): 6525–6527.
714:(17): 5094–5095.
679:(12): 4009–4011.
586:978-0-470-13247-0
533:978-1-118-74499-4
487:(10): 3519–3520.
262:hexamethylbenzene
237:Reduction of TaCl
164:olefin metathesis
16:(Redirected from
1849:
1816:
1811:
1806:
1801:
1706:
1705:
1625:
1624:
1557:
1556:
1462:
1461:
1159:
1141:
1134:
1127:
1118:
1112:
1111:
1094:(6): 2207–2293.
1088:Chemical Reviews
1083:
1077:
1076:
1051:(8): 1447–1450.
1040:
1034:
1033:
1008:(9): 2182–2185.
996:
990:
989:
961:
955:
954:
917:
911:
910:
900:
856:
850:
849:
809:
803:
802:
785:(6): 1707–1708.
773:
767:
766:
738:
732:
731:
703:
697:
696:
668:
662:
661:
652:(5–6): 402–419.
641:
635:
634:
597:
591:
590:
564:
558:
557:
551:
547:
545:
537:
511:
505:
504:
475:
469:
468:
448:
442:
441:
413:
356:metallaaziridine
317:secondary amines
80:was reported by
21:
1857:
1856:
1852:
1851:
1850:
1848:
1847:
1846:
1827:
1826:
1825:
1820:
1819:
1814:
1809:
1804:
1799:
1781:
1703:
1622:
1554:
1459:
1152:
1145:
1115:
1085:
1084:
1080:
1042:
1041:
1037:
1002:Organic Letters
998:
997:
993:
963:
962:
958:
919:
918:
914:
858:
857:
853:
811:
810:
806:
775:
774:
770:
740:
739:
735:
705:
704:
700:
670:
669:
665:
643:
642:
638:
599:
598:
594:
587:
566:
565:
561:
548:
538:
534:
513:
512:
508:
477:
476:
472:
450:
449:
445:
415:
414:
405:
401:
393:
384:transmetalation
380:
378:Transmetalation
350:may proceed by
348:catalytic cycle
330:
326:
309:
283:
271:
267:
258:
240:
235:
220:
216:
212:
208:
204:
201:such as Cp*TaCl
195:
191:
172:
114:
106:
102:
90:
82:Richard Schrock
75:
62:
28:
23:
22:
15:
12:
11:
5:
1855:
1853:
1845:
1844:
1839:
1829:
1828:
1822:
1821:
1818:
1817:
1812:
1807:
1802:
1797:
1794:Chemical bonds
1790:
1789:
1787:
1783:
1782:
1778:
1777:
1772:
1767:
1762:
1757:
1752:
1747:
1742:
1737:
1732:
1727:
1722:
1717:
1712:
1707:
1700:
1697:
1696:
1691:
1686:
1681:
1676:
1671:
1666:
1661:
1656:
1651:
1646:
1641:
1636:
1631:
1626:
1619:
1616:
1615:
1611:
1610:
1607:
1604:
1601:
1598:
1595:
1592:
1589:
1586:
1583:
1580:
1577:
1574:
1569:
1566:
1563:
1558:
1551:
1546:
1542:
1541:
1538:
1533:
1528:
1523:
1518:
1513:
1508:
1503:
1498:
1493:
1488:
1483:
1478:
1473:
1468:
1463:
1456:
1451:
1445:
1444:
1439:
1434:
1429:
1424:
1419:
1414:
1409:
1404:
1399:
1394:
1389:
1384:
1379:
1374:
1369:
1364:
1362:
1357:
1351:
1350:
1345:
1340:
1335:
1330:
1325:
1320:
1315:
1310:
1305:
1300:
1295:
1290:
1285:
1280:
1275:
1270:
1268:
1263:
1257:
1256:
1251:
1246:
1241:
1236:
1231:
1226:
1221:
1215:
1214:
1211:
1206:
1201:
1196:
1191:
1186:
1181:
1175:
1174:
1171:
1169:
1167:
1165:
1157:
1154:
1153:
1146:
1144:
1143:
1136:
1129:
1121:
1114:
1113:
1078:
1035:
991:
956:
912:
851:
824:(4): 305–306.
804:
768:
733:
698:
663:
636:
609:(2): 135–140.
601:Ta(CNDipp)6".
592:
585:
559:
550:|journal=
532:
506:
470:
443:
402:
400:
397:
392:
389:
379:
376:
328:
324:
308:
305:
281:
269:
265:
257:
254:
238:
234:
231:
218:
214:
210:
206:
202:
193:
189:
171:
168:
113:
110:
109:
108:
107:→ Li + 5 LiF
104:
100:
93:methyl lithium
88:
74:
71:
68:Structure of .
61:
58:
26:
24:
18:Organotantalum
14:
13:
10:
9:
6:
4:
3:
2:
1854:
1843:
1840:
1838:
1835:
1834:
1832:
1813:
1808:
1803:
1798:
1795:
1792:
1791:
1788:
1784:
1776:
1773:
1771:
1768:
1766:
1763:
1761:
1758:
1756:
1753:
1751:
1748:
1746:
1743:
1741:
1738:
1736:
1733:
1731:
1728:
1726:
1723:
1721:
1718:
1716:
1713:
1711:
1708:
1701:
1698:
1695:
1692:
1690:
1687:
1685:
1682:
1680:
1677:
1675:
1672:
1670:
1667:
1665:
1662:
1660:
1657:
1655:
1652:
1650:
1647:
1645:
1642:
1640:
1637:
1635:
1632:
1630:
1627:
1620:
1617:
1613:
1612:
1608:
1605:
1602:
1599:
1596:
1593:
1590:
1587:
1584:
1581:
1578:
1575:
1573:
1570:
1567:
1564:
1562:
1559:
1552:
1550:
1547:
1544:
1543:
1539:
1537:
1534:
1532:
1529:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1507:
1504:
1502:
1499:
1497:
1494:
1492:
1489:
1487:
1484:
1482:
1479:
1477:
1474:
1472:
1469:
1467:
1464:
1457:
1455:
1452:
1450:
1447:
1446:
1443:
1440:
1438:
1435:
1433:
1430:
1428:
1425:
1423:
1420:
1418:
1415:
1413:
1410:
1408:
1405:
1403:
1400:
1398:
1395:
1393:
1390:
1388:
1385:
1383:
1380:
1378:
1375:
1373:
1370:
1368:
1365:
1363:
1361:
1358:
1356:
1353:
1352:
1349:
1346:
1344:
1341:
1339:
1336:
1334:
1331:
1329:
1326:
1324:
1321:
1319:
1316:
1314:
1311:
1309:
1306:
1304:
1301:
1299:
1296:
1294:
1291:
1289:
1286:
1284:
1281:
1279:
1276:
1274:
1271:
1269:
1267:
1264:
1262:
1259:
1258:
1255:
1252:
1250:
1247:
1245:
1242:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1220:
1217:
1216:
1212:
1210:
1207:
1205:
1202:
1200:
1197:
1195:
1192:
1190:
1187:
1185:
1182:
1180:
1177:
1176:
1172:
1164:
1161:
1160:
1155:
1150:
1147:Compounds of
1142:
1137:
1135:
1130:
1128:
1123:
1122:
1119:
1109:
1105:
1101:
1097:
1093:
1089:
1082:
1079:
1074:
1070:
1066:
1062:
1058:
1054:
1050:
1046:
1039:
1036:
1031:
1027:
1023:
1019:
1015:
1011:
1007:
1003:
995:
992:
987:
983:
979:
975:
971:
967:
960:
957:
952:
948:
944:
940:
936:
932:
928:
924:
916:
913:
908:
904:
899:
894:
890:
886:
882:
878:
874:
870:
866:
864:
855:
852:
847:
843:
839:
835:
831:
827:
823:
819:
815:
808:
805:
800:
796:
792:
788:
784:
780:
772:
769:
764:
760:
756:
752:
748:
744:
737:
734:
729:
725:
721:
717:
713:
709:
702:
699:
694:
690:
686:
682:
678:
674:
667:
664:
659:
655:
651:
647:
640:
637:
632:
628:
624:
620:
616:
612:
608:
604:
596:
593:
588:
582:
578:
574:
570:
563:
560:
555:
543:
535:
529:
525:
521:
517:
510:
507:
502:
498:
494:
490:
486:
482:
474:
471:
466:
462:
458:
454:
447:
444:
439:
435:
431:
427:
424:(3): 98–104.
423:
419:
412:
410:
408:
404:
398:
396:
390:
388:
385:
377:
371:
367:
365:
361:
357:
353:
349:
340:
336:
334:
322:
318:
314:
306:
301:
296:
292:
290:
285:
279:
275:
263:
255:
253:
251:
246:
244:
232:
226:
222:
200:
196:
185:
181:
177:
169:
167:
165:
161:
157:
153:
145:
141:
140:of enones.
139:
135:
131:
122:
118:
111:
98:
97:
96:
94:
85:
83:
79:
72:
66:
59:
57:
55:
54:chemical bond
52:
48:
45:containing a
44:
40:
32:
19:
1815:Bond unknown
1475:
1091:
1087:
1081:
1048:
1044:
1038:
1005:
1001:
994:
969:
965:
959:
926:
922:
915:
872:
868:
862:
854:
821:
817:
813:
807:
782:
778:
771:
746:
742:
736:
711:
707:
701:
676:
672:
666:
649:
645:
639:
606:
602:
595:
568:
562:
515:
509:
484:
480:
473:
456:
453:Chem. Eur. J
452:
446:
421:
417:
394:
391:Applications
381:
364:protonolysis
345:
323:using Ta(NMe
310:
299:
286:
259:
247:
236:
173:
150:
130:nucleophilic
127:
115:
86:
76:
38:
37:
459:(9): 1687.
333:alkylamines
245:complexes.
1831:Categories
399:References
313:alkylation
250:isocyanide
166:products.
138:allylation
1796:to carbon
1108:0009-2665
1065:1521-3757
1022:1523-7060
986:1099-0690
943:1521-3773
889:0002-7863
838:0039-7881
818:Synthesis
799:0022-3263
763:0002-7863
728:0002-7863
693:0002-7863
552:ignored (
542:cite book
501:0002-7863
438:0001-4842
360:insertion
321:1-alkenes
274:aluminium
213:, and Cp*
156:propylene
84:in 1974.
1073:19750774
1030:23600625
951:19787670
907:17474747
846:94579838
631:73450348
623:30720451
152:Ethylene
103:+ 6 LiCH
51:tantalum
1614:
898:2590937
160:styrene
1786:Legend
1149:carbon
1106:
1071:
1063:
1028:
1020:
984:
949:
941:
905:
895:
887:
844:
836:
797:
761:
726:
691:
629:
621:
583:
530:
499:
436:
280:gives
276:, and
158:, and
134:olefin
91:using
47:carbon
842:S2CID
627:S2CID
319:with
243:arene
205:, Cp*
1104:ISSN
1069:PMID
1061:ISSN
1026:PMID
1018:ISSN
982:ISSN
970:2014
947:PMID
939:ISSN
903:PMID
885:ISSN
834:ISSN
822:1980
795:ISSN
759:ISSN
724:ISSN
689:ISSN
619:PMID
581:ISBN
554:help
528:ISBN
497:ISSN
434:ISSN
346:The
209:TaCl
182:and
49:-to-
1760:CEs
1755:CCf
1750:CBk
1745:CCm
1740:CAm
1735:CPu
1730:CNp
1720:CPa
1715:CTh
1694:CYb
1689:CTm
1684:CEr
1679:CHo
1674:CDy
1669:CTb
1664:CGd
1659:CEu
1654:CSm
1649:CPm
1644:CNd
1639:CPr
1634:CCe
1629:CLa
1609:Og
1606:Ts
1603:Lv
1600:Mc
1597:Fl
1594:Nh
1591:Cn
1588:Rg
1585:Ds
1582:Mt
1579:Hs
1576:Bh
1572:CSg
1568:Db
1565:Rf
1549:CRa
1545:Fr
1540:Rn
1536:CAt
1531:CPo
1526:CBi
1521:CPb
1516:CTl
1511:CHg
1506:CAu
1501:CPt
1496:CIr
1491:COs
1486:CRe
1476:CTa
1471:CHf
1466:CLu
1454:CBa
1449:CCs
1442:CXe
1432:CTe
1427:CSb
1422:CSn
1417:CIn
1412:CCd
1407:CAg
1402:CPd
1397:CRh
1392:CRu
1387:CTc
1382:CMo
1377:CNb
1372:CZr
1360:CSr
1355:CRb
1348:CKr
1343:CBr
1338:CSe
1333:CAs
1328:CGe
1323:CGa
1318:CZn
1313:CCu
1308:CNi
1303:CCo
1298:CFe
1293:CMn
1288:CCr
1278:CTi
1273:CSc
1266:CCa
1254:CAr
1249:CCl
1234:CSi
1229:CAl
1224:CMg
1219:CNa
1213:Ne
1184:CBe
1179:CLi
1173:He
1096:doi
1053:doi
1049:114
1010:doi
974:doi
931:doi
893:PMC
877:doi
873:129
826:doi
787:doi
751:doi
747:109
716:doi
712:101
681:doi
654:doi
650:254
611:doi
573:doi
520:doi
489:doi
461:doi
426:doi
315:of
272:),
217:TaH
192:TaH
178:of
99:TaF
1833::
1775:No
1770:Md
1765:Fm
1725:CU
1710:Ac
1561:Lr
1481:CW
1437:CI
1367:CY
1283:CV
1261:CK
1244:CS
1239:CP
1209:CF
1204:CO
1199:CN
1194:CC
1189:CB
1163:CH
1102:.
1092:93
1090:.
1067:.
1059:.
1047:.
1024:.
1016:.
1006:15
1004:.
980:.
968:.
945:.
937:.
927:48
925:.
901:.
891:.
883:.
871:.
867:.
840:.
832:.
820:.
793:.
783:55
781:.
757:.
745:.
722:.
710:.
687:.
677:96
675:.
648:.
625:.
617:.
607:75
605:.
579:.
546::
544:}}
540:{{
526:.
495:.
485:99
483:.
455:.
432:.
422:12
420:.
406:^
362:,
335::
284:.
268:Me
264:(C
221:.
188:Cp
154:,
95::
1140:e
1133:t
1126:v
1110:.
1098::
1075:.
1055::
1032:.
1012::
988:.
976::
953:.
933::
909:.
879::
863:N
848:.
828::
814:C
801:.
789::
765:.
753::
730:.
718::
695:.
683::
660:.
656::
633:.
613::
589:.
575::
556:)
536:.
522::
503:.
491::
467:.
463::
457:4
440:.
428::
329:5
327:)
325:2
300:E
282:2
270:6
266:6
239:5
219:3
215:2
211:2
207:2
203:4
194:3
190:2
105:3
101:5
89:5
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.