1603:(MISCAPs). In short, parylene N and AF-4 (those parylenes with no functional groups) are pin-hole free at ~14 Å. This results because the parylene repeat units possess a phenyl ring and due to the high electronic polarizability of the phenyl ring adjacent repeat units order themselves in the XY-plane. As a result of this interaction parylene MLs are surface independent, except for transition metals, which de-activate the triplet (benzoid) state and therefore the parylenes cannot be initiated. This finding of parylenes as molecular layers is very powerful for industrial applications because of the robustness of the process and that the MLs are deposited at room temperature. In this way parylenes can be used as diffusion barriers and for reducing the polarizability of surface (de-activation of oxide surfaces). Combining the properties of the reactive parylenes with the observation that they can form dense pin-hole-free molecular layers, parylene X has been utilized as a genome sequencing interface layer.
1440:
727:
barrier against water then the apolar chemistries such as parylene E are much more effective. For moisture barriers the three principal material parameters to be optimized are: coating density, coating polarity (olefin chemistry is best) and a glass-transition temperature above room temperature and ideally above the service limit of the printed-circuit board, device or part. In this regard parylene E is a best choice although it has a low density compared to, for example, parylene C.
1352:
1240:
600:
342:
20:
1587:(SAMs). SAMs are long-chain alkyl chains, which interact with surfaces based on sulfur-metal interaction (alkylthiolates) or a sol-gel type reaction with a hydroxylated oxide surface (trichlorosilyl alkyls or trialkoxy alkyls). However, unless the gold or oxide surface is carefully treated and the alkyl chain is long, these SAMs form disordered monolayers, which do not pack well. This lack of packing causes issues in, for example,
1459:
1135:, meaning that it is stronger at lower temperatures than higher temperatures. There is critical threshold temperature above which there is practically no physisorption, and hence no deposition. The closer the deposition temperature is to the threshold temperature the weaker the physisorption. Parylene C has a higher threshold temperature, 90 °C, and therefore has a much higher deposition rate, greater than 1
389:
mitigates the deposition of a parylene-like material on the downside of the pyrolysis tube. This material becomes carbonized and generates particles in situ to contaminate clean rooms and create defects on printed-circuit boards that are often called "stringers and nodules". Parylene N and E do not have this problem and therefore are preferred for manufacturing and clean room use.
1161:
1115:
28:
1080:-xylylene intermediate has two quantum mechanical states, the benzoid state (triplet state) and the quinoid state (singlet state). The triplet state is effectively the initiator and the singlet state is effectively the monomer. The triplet state can be de-activated when in contact with transition metals or metal oxides including Cu/CuO
1393:
491:
1146:
Another relevant property for the deposition process is polarizability, which determines how strongly the monomer interacts with the surface. Deposition of halogenated parylenes strongly correlates with molecular weight of the monomer. The fluorinated variants are an exception: the polarizability of
1057:
Parts to be coated need to be clean in order to ensure good adherence of the film. Since the monomer diffuses, areas that are not to be coated must be hermetically sealed, without gaps, crevices or other openings. The part must be maintained in a relatively narrow window of pressure and temperature.
713:
Parylenes are relatively flexible (0.5 GPa for parylene N), except for cross-linked parylene X (1.0 GPa), and have poor oxidative resistance (~60–100 °C, depending on failure criteria) and UV stability, except for parylene AF-4. However, parylene AF-4 is more expensive due to
704:
in its as-deposited condition and it does not appreciably become more crystalline until it undergoes a crystallographic phase transformation at ~220 °C to hexagonal, at which point it becomes highly crystalline like the fluorinated parylenes. It can reach 80% crystallinity at anneal temperatures
1602:
measurements, where MLs thicker than 10 Å had an equilibrium contact angle of 80 degrees (same as bulk parylene N) but those thinner had a reduced contact angle. This was also confirmed with electrical measurements (bias-temperature stress measurements) using metal-insulator-semiconductor capacitors
1294:
Selection of a leaving group may consider its toxicity (which excludes sulfur and amine-based reactions), how easily it leaves the precursor, and possible interference with the polymerization. The leaving group can either be trapped before the deposition chamber, or it can be highly volatile so that
311:
Union
Carbide went on to undertake research into the synthesis of numerous parylene precursors, including parylene AF-4, throughout the 1960s into the early 1970s. Union Carbide purchased NovaTran (a parylene coater) in 1984 and combined it with other electronic chemical coating businesses to
726:
As a moisture diffusion barrier, the efficacy of halogneated parylene coatings scales non-linearly with their density. Halogen atoms such as F, Cl and Br add much density to the coating and therefore allow the coating to be a better diffusion barrier; however, if parylenes are used as a diffusion
388:
that may harm vacuum pumps and other equipment. The chlorine atom leaves the phenyl ring in the pyrolysis tube at all temperatures; however, optimizing the pyrolysis temperature will minimize this problem. The free-radical (phenyl radical) generated in this process is not resonance-stabilized and
693:
Parylene thin films and coatings are transparent; however, they are not amorphous except for the alkylated parylenes. i.e. parylene E. As a result, of the coatings being semi-crystalline, they scatter light. Parylene N and C have a low degree of crystallinity; however, parylene VT-4 and AF-4 are
364:
Parylene C is the most used variety, due to its low cost of its precursor and to the balance of its properties as dielectric and moisture barrier properties and ease of deposition. A major disadvantage for many applications is its insolubility in any solvent at room temperature, which prevents
315:
There are parylene coating service companies located around the world, but there is limited commercial availability of parylene. The paracyclophane precursors can be purchased for parylene N, C, D, AF-4 and VT-4. Parylene services are provided for N, C, AF-4, VT-4 and E (copolymer of N and E).
479:
467:
1606:
One caveat with the molecular layer parylenes, namely they are deposited as oligomers and not high polymer. As a result, a vacuum anneal is needed to convert the oligomers to high polymer. For parylene N that temperature is 250 °C, whereas it is 300 °C for payrlene AF-4.
2598:
Laibinis, Paul E.; Whitesides, George M.; Allara, David L.; Tao, Yu Tai; Parikh, Atul N.; Nuzzo, Ralph G. (1991). "Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold".
717:
Nearly all the parylenes are insoluble at room temperature, except for the alkylated parylenes, one of which is parylene E, and the alkylated-ethynyl parylenes. This lack of solubility has made it difficult to re-work printed circuit boards coated with parylene.
1054:-xylylene or a derivative thereof. This method has one very strong benefit, namely it does not generate any byproducts besides the parylene polymer, which would need to be removed from the reaction chamber and could interfere with the polymerization.
2653:
Fadeev, Alexander Y.; McCarthy, Thomas J. (2000). "Self-Assembly is Not the Only
Reaction Possible between Alkyltrichlorosilanes and Surfaces: Monomolecular and Oligomeric Covalently Attached Layers of Dichloro- and Trichloroalkylsilanes on Silicon".
546:
While parylene coatings are mostly used to protect an object from water and other chemicals, some applications require a coating that can bind to adhesives or other coated parts, or immobilize various molecules such as dyes, catalysts, or enzymes.
501:
These substitutions increase the intermolecular (chain-to-chain) distance, which makes the polymer more soluble and permeable. For example, compared to parylene C, parylene M was shown to have a lower dielectric constant (2.48 vs. 3.2 at
432:
Another fluorinated variant is parylene VT-4 (also called parylene F), with fluorine substituted for the four hydrogens on the aryl ring. This variant is marketed by Kisco with the trademark
Parylene CF. Because of the aliphatic
369:
Parylene C is also the most commonly used because of its relatively low cost. It can be deposited at room temperature while still possessing a high degree of conformality and uniformity and a moderate deposition rate in a batch process.
594:
Parylene AM is more reactive than the A variant. The amine of the latter, being adjacent to the phenyl ring, is in resonance stabilization and therefore less basic. However, parylene A is much easier to synthesize and hence cheaper.
307:
at temperatures exceeding 550 °C and in vacuum below 1 Torr. This process did not require a solvent and resulted in chemically resistant films free from pinholes. Union
Carbide commercialized a parylene coating system in 1965.
1358:
The advantage to this process is the low cost of synthesis for the precursor. The precursor is also a liquid and can be delivered by standard methods developed in the semiconductor industry, such as with a vaporizer, vaporizer with a
1142:
An important property of the monomer is the so-called 'sticking coefficient', that expresses the degree to which it adsorbs on the polymer. A lower coefficient results more uniform deposition thickness and a more conformal coating.
672:/parylene C) of parylene have been deposited at near-room temperature previously. With strongly electron withdrawing comonomers, parylene can be used as an initiator to initiate polymerizations, such as with N-phenyl
1575:. There are numerous other applications as parylene is an excellent moisture barrier. It is the most bio-accepted coating for stents, defibrillators, pacemakers and other devices permanently implanted into the body.
2315:
P. K. Wu; G. -R. Yang; L. You; D. Mathur; A. Cocoziello; C. -I. Lang; J. A. Moore; T. -M. Lu; H. Bakru (1997). "Deposition of High Purity
Parylene- F Using Low Pressure Low Temperature Chemical Vapor Deposition".
1551:
Since the coating process takes place at ambient temperature in a mild vacuum, it can be applied even to temperature-sensitive objects such as dry biological specimens. The low temperature also results in low
1139:/s, while still yielding fairly uniform coatings. In contrast, the threshold temperature of parylene AF-4 is very close to room temperature (30–35 °C), as a result, its deposition efficiency is poor.
1251:-cyclophane precursor dimer can be sublimed below <100 °C and cracked at 700–750 °C, higher than the temperature (680 °C) used to crack the unsubstituted cyclophane since the −CF
2455:
D.M. Dobkin, S. Mokhtari, M. Schmidt, A. Pant, L. Robinson, Mechanisms of
Deposition of SiO2 from TEOS and Related Organosilicon Compounds and Ozone" J. Electrochem. Soc. 142(7), 2332-40 (1995).
381:
manufacture. Moreover, some of the dimer precursor is decomposed by breaking of the aryl-chlorine bond during pyrolysis, generating carbonaceous material that contaminates the coating, and
2626:
Wasserman, Stephen R.; Tao, Yu Tai; Whitesides, George M. (1989). "Structure and reactivity of alkylsiloxane monolayers formed by reaction of alkyltrichlorosilanes on silicon substrates".
714:
a three-step synthesis of its precursor with low yield and poor deposition efficiency. Their UV stability is so poor that parylene cannot be exposed to regular sunlight without yellowing.
2235:
P. Kramer; A. K. Sharma; E. E. Hennecke; H. Yasuda (2003). "Polymerization of para-xylylene derivatives (parylene polymerization). I. Deposition kinetics for parylene N and parylene C".
1559:
Parylene AF-4 and VT-4 are both fluorinated and as a result very expensive compared to parylene N and C, which has severely limited their commercial use, except for niche applications.
1267:− bond. This resonance-stabilized intermediate is transported to a room temperature deposition chamber where polymerization occurs under low pressure (1–100 mTorr) conditions.
1978:
J. J. Senkevich; B. W. Woods; J. J. McMahon; P.-I Wang (2007). "Thermomechanical
Properties of Parylene X, A Room-Temperature Chemical Vapor Depositable Crosslinkable Polymer".
2681:
Senkevich, Jay J.; Mitchell, Christopher J.; Yang, G.-R.; Lu, T.-M. (2002). "Surface
Chemistry of Mercaptan and Growth of Pyridine Short-Chain Alkoxy Silane Molecular Layers".
2208:
H. J. Reich; D. J. Cram (1969). "Macro rings. XXXVI. Ring expansion, racemization, and isomer interconversions in the paracyclophane system through a diradical intermediate".
1833:
J. J. Senkevich; C. J. Mitchell; A. Vijayaraghavan; E. V. Barnat; J. F. McDonald; T.-M. Lu (2002). "The Unique
Structure/Properties of Chemical Vapor Deposited Parylene E".
1428:− is the leaving group; while it condenses in the deposition chamber, it does not interfere with the deposition of the polymer. This precursor is much less expensive than
1382:
and removed from monomer flow. Special precautions are needed since bromine and HBr are toxic and corrosive towards most metals and metal alloys, and bromine can damage
168:. Some of these variants are designated in commerce by letter-number codes such as "parylene C" and "parylene AF-4". Some of these names are registered
1556:
in the thin film. Moreover, the only gas in the deposition chamber is the monomer, without any solvents, catalysts, or byproducts that could attack the object.
550:
These "reactive" parylene coatings can be obtained with chemically active substituents. Two commercially available products are parylene A, featuring one
2129:
J.J. Senkevich; C.J. Wiegand; G.-R. Yang; T.-M. Lu (2004). "Selective
Deposition of Ultra-thin Poly(p-xylylene) Films on Dielectrics versus Copper Surfaces".
1868:
J. F. Gaynor; J. J. Senkevich; S. B. Desu (1996). "A New Method for Fabricating High Performance Polymeric Thin Films by Chemical Vapor Polymerization".
694:
highly crystalline ~60% in their as-deposited condition (hexagonal crystal structure) and therefore are generally not suitable as optical materials.
518:
for a 1-mil coating) but better solubility. However, the copolymer of parylene N and E has equivalent barrier performance of parylene C.
357:
bridge by other functional groups. The most common of these variants is parylene C, which has one hydrogen atom in the aryl ring replaced by
303:
A more efficient route was found in 1965 by William F. Gorham at Union Carbide. He deposited parylene films by the thermal decomposition of
2582:
1734:
1700:
1061:
The process involves three steps: generation of the gaseous monomer, adsorption on the part's surface, and polymerization of adsorbed film.
1087:
Polymerization may proceed by a variety of routes that differ in the transient termination of the growing chains, such as a radical group −
2114:
2097:
1951:
1439:
1220:
The same method can be used to deposit substituted parylenes. For example, parylene C can be obtained from the dimeric precursor
2305:-xylylene) from Alkoxide Precursors I: Optical Properties and Thermal Stability". Chemical Vapor Deposition, volume 17, pages 235-240.
1084:. Many of the parylenes exhibit this selectivity based on quantum mechanical deactivation of the triplet state, including parylene X.
680:
nanocomposites, parylene C could be used as a sacrificial layer to make nanoporous silica thin films with a porosity of >90%.
1505:, chemically resistant, and mostly impermeable to gases (including water vapor) and inorganic and organic liquids (including strong
2753:"Bias-Temperature Stability of Ultra Thin Parylene Capped PETEOS Dielectrics: Influence of Surface Oxygen on Copper Ion Diffusion"
641:, allowing parylene-to-parylene bonding without any by-products during processing. Unlike most other variants, parylene X is
613:
attached to the phenyl ring in some of the units. This variant, which contains no elements other than hydrogen and carbon, can be
2492:
J. J. Senkevich; S. B. Desu (1999). "Compositional studies of near-room temperature thermal CVD of poly(chloro-p-xylylene)/SiO
1654:
1592:
1239:
2173:
J. B. Fortin & T.-M. Lu (2000). "Mass spectrometry study during the vapor deposition of poly-para-xylylene thin films".
2005:
J.B. Fortin & T.-M. Lu (2001). "Ultraviolet radiation induced degradation of poly-para-xylylene (parylene) thin films".
510:), a lower dielectric constant (2.34 vs. 3.05 at 10 kHz), slightly worse moisture barrier properties (4.1 vs. 0.6
1751:
1436:; whereas the generation and delivery of the gaseous monomer of the Gorham process are difficult to measure and control.
1371:
lower the pyrolysis temperature, resulting in less char residue and a better coating. By either method an atomic bromine
1073:-xylylene monomer requires a minimum threshold temperature. For parylene N, its threshold temperature is 40 °C.
490:
397:
Another common halogenated variant is parylene AF-4, with the four hydrogen atoms on the aliphatic chain replaced by
697:
Parylene C will become more crystalline if heated at elevated temperatures until its melting point at 270 °C.
221:
or other chemicals to terminate the chain; and the coatings can be applied at or near room temperature, without any
47:
425:(Teflon), consistent with its superior oxidative and UV stability. Parylene AF-4 has been used to protect outdoor
406:
1432:-cyclophane. Moreover, being a liquid just above room temperature, this precursor can delivered reliably using a
701:
200:
1771:
1722:
1584:
1451:
The same chemistry can generate parylene AM-2 can be generated from the precursor α,α'-dimethyl-α,α'-dimethoxy-
538:-substituted variant trademarked by Kisco). The solubility of parylene AM-2 is not as good as parylene E.
478:
466:
1470:
Another example of this approach is the synthesis of parylene AF-4 from α,α'-diphenoxy-α,α,α',α'-tetrafluoro-
1537:
422:
2359:
Pebalk, A. V.; Kardash, I. E.; Kozlova, N. V.; Zaitseva, E. L.; Kozlov, Yu. A.; Pravednikov, A. N. (1980).
1903:
J.J. Senkevich; S. B. Desu (1999). "Near-Room-Temperature Thermal Chemical Vapor Deposition of Poly(chloro-
294:
1364:
1198:. This method (Gorham process) yields 100% monomer with no by-products or decomposition of the monomer.
1194:
at a relatively low temperature, then decomposing the vapor at 450–700 °C and pressure 0.01–1.0
2084:
2372:
1568:
515:
378:
1795:
W. F. Gorham (1966). "A New, General Synthetic Method for the Preparation of Linear Poly-p-xylylenes".
1498:
Parylenes may confer several desirable qualities to the coated parts. Among other properties, they are
1392:
2067:
1553:
2764:
2717:
2505:
2325:
2244:
2182:
2014:
1877:
1842:
1804:
1433:
1405:
1050:
Parylene coatings are generally applied by chemical vapor deposition in an atmosphere of the monomer
426:
402:
312:
form the Specialty Coating Systems division. The division was sold to Cookson Electronics in 1994.
1521:
1372:
1214:
626:
304:
1458:
1351:
2733:
2521:
2341:
1132:
196:
176:
521:
Replacement of one hydrogen by methyl on each carbon of the ethyl bridge yields parylene AM-2,
2797:
2578:
2572:
2276:
1730:
1696:
1666:
1184:
382:
188:
165:
1628:
Microwave electronics (e.g., protection of PTFE dielectric substrates from oil contamination)
2772:
2725:
2690:
2663:
2635:
2608:
2553:
2513:
2474:
2438:
2333:
2252:
2217:
2190:
2138:
2109:
2049:
2022:
1987:
1947:
1920:
1885:
1850:
1812:
1690:
1510:
1376:
630:
373:
Also, the chlorine on the phenyl ring of the parylene C repeat unit is problematic for
1567:
Parylene C and to a lesser extent AF-4, SF, HT (all the same polymer) are used for coating
2385:
1755:
1726:
1692:
Chemical vapor deposition polymerization: the growth and properties of parylene thin films
1181:
642:
634:
567:
214:
2768:
2721:
2509:
2329:
2248:
2186:
2018:
1881:
1846:
1808:
1180:-xylylene monomer is normally generated during the coating process by evaporating the
2465:
Senkevich, Jay J. (2013). "Parylene AF-4 via the Trapping of a Phenoxy Leaving Group".
1572:
599:
361:. Another common variant is parylene D, with two such substitutions on the ring.
341:
234:
19:
2098:"Selective growth of poly(p-phenylene vinylene) prepared by chemical vapor deposition"
2026:
2791:
2737:
1599:
1413:
1128:
606:
2544:
J. J. Senkevich & P.-I. Wang (2009). "Molecular Layer Chemistry via Parylenes".
2525:
2345:
449:. Substitution may occur on either the phenyl ring or the ethylene bridge, or both.
2040:
Senkevich, Jay J. (2014). "Tert-Butylethynyl-parylene and Phenylethynyl-parylene".
1598:
The observation that parylenes could form ordered molecular layers (MLs) came with
1284:
453:
437:− units, it has poor oxidative and UV stability, but still better than N, C, or D.
1524:(average in-plane and out-of-plane: 2.67 parylene N and 2.5 parylene AF-4, SF, HT)
1737:
2271:
1748:
1502:
1206:
654:
457:
446:
2256:
1816:
1114:
2415:
2400:
2337:
1517:
1221:
1191:
614:
421:− unit that comprises the ethylene chain is the same as the repeating unit of
180:
160:
The name is also used for several polymers with the same backbone, where some
2429:
Senkevich, Jay J. (2011). "Non-Halogen Liquid Precursor Route to Parylene".
2115:
10.1002/(SICI)1521-4095(199907)11:10<814::AID-ADMA814>3.0.CO;2-Z
1247:
The standard Gorham process is shown above for parylene AF-4. The octafluoro
1210:
1136:
673:
665:
503:
354:
184:
169:
2557:
2478:
2442:
2142:
2053:
1991:
1160:
401:
atoms. This variant is also marketed under the trade names of parylene SF (
349:
Derivatives of parylene can be obtained by replacing hydrogen atoms on the
329:
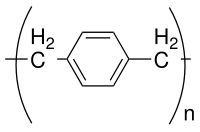
Parylene N is the un-substituted polymer obtained by polymerization of the
2517:
1952:
10.1002/(SICI)1521-3862(199912)5:6<257::AID-CVDE257>3.0.CO;2-J
1889:
1646:
Protection of plastic, rubber, etc., from harmful environmental conditions
657:
directly to the paracyclophane base molecule to impart color to parylene.
293:-xylylene as the precursor by observing that reaction with iodine yielded
1650:
1588:
1368:
1229:
638:
398:
358:
238:
218:
192:
161:
106:
2639:
2612:
2221:
2729:
1527:
Stable and accepted in biological tissues, having been approved by the
1475:
1360:
1343:
222:
204:
53:
43:
27:
2777:
2752:
2751:
Senkevich, Jay J.; Wang, Pei-I.; Wiegand, Chris J.; Lu, T.-M. (2004).
2694:
2667:
2194:
2159:
2085:
https://www.paryleneconformalcoating.com/#TheParyleneDepositionProcess
1924:
1854:
1528:
1386:
1228:, except that the temperature must be carefully controlled since the
622:
618:
350:
78:
1719:
1367:. Originally the precursor was just thermally cracked, but suitable
429:
displays and lighting from water, salt and pollutants successfully.
2160:"GlobalTop Technology | Taiwan | Aluminum Nitride Powder"
1622:
Hydrophobic coating (moisture barriers, e.g., for biomedical hoses)
1457:
1438:
1391:
1383:
1159:
1113:
551:
535:
452:
Specifically, replacement of one hydrogen on the phenyl ring by a
340:
26:
18:
1618:
Parylene films have been used in various applications, including
300:
as the only product. The reaction yield was only a few percent.
1634:
Sensors in rough environment (e.g., automotive fuel/air sensors)
1506:
1233:
1195:
374:
2708:
Z. Yapu (2003). "Stiction and anti-stiction in MEMS and NEMS".
2574:
Dekker encyclopédia of nanoscience and nanotechnology, Volume 1
2571:
James A. Schwarz; Cristian I. Contescu; Karol Putyera (2004).
2272:"Tricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene"
1375:
is given off from each methyl end, which can be converted to
1298:
For example, the precursor α,α'-dibromo-α,α,α',α'-tetrafluoro-
1127:
The monomer polymerizes only after it is physically adsorbed (
2083:
Horn, Sean "The Parylene Deposition Process: Pre-Deposition"
1238:
1147:
parylene AF-4 is low, resulting in inefficient deposition.
365:
removal of the coating when the part has to be re-worked.
817:
Linear coefficient of thermal expansion at 25 °C (ppm)
625:
salts to generate the corresponding metalorganic complexes
605:
Another reactive variant is parylene X, which features an
1490:−, which can be condensed before the deposition chamber.
1964:
C. Chiang, A. S. Mack, C. Pan, Y.-L. Ling, D. B. Fraser
1653:, e.g., for guiding catheters, acupuncture needles and
1279:
Another route to generation of the monomer is to use a
2398:
Lee, Chung J.; Wang, Hui; Foggiato, Giovanni Antonio,
1797:
Journal of Polymer Science Part A-1: Polymer Chemistry
1404:
A similar synthesis for parylene N uses the precursor
1283:-xylene precursor with a suitable substituent on each
2237:
Journal of Polymer Science: Polymer Chemistry Edition
1625:
Barrier layers (e.g., for filter, diaphragms, valves)
1938:
J. J. Senkevich (1999). "CVD of NanoPorous Silica".
514:), and equivalent dielectric breakdown 5–6 kV/
2293:J.J. Senkevich (2011): "CVD of Poly(α,α'-dimethyl-
1534:Dense and pinhole free, for thickness above 1.4 nm
1131:) on the part's surface. This process has inverse
705:up to 400 °C, after which point it degrades.
2068:"Specialty Coating Systems - Parylene Properties"
1772:"Parylene: The Truly Conformal Thin Film Coating"
506:). Parylene E had a lower tensile modulus (
237:as one of the thermal decomposition products of
195:and in medicine to prevent adverse reactions to
1543:Stable to oxidation up to 350 °C (AF-4, SF, HT)
566:in each unit, and parylene AM, with one
8:
2539:
2537:
2535:
1720:The foundations of vacuum coating technology
1583:The classic molecular layer chemistries are
1474:-xylene. In this case, the leaving group is
834:Thermal conductivity at 25 °C (W/(m·K))
617:by heat or with UV light and can react with
2289:
2287:
2154:
2152:
1828:
1826:
1758:. Scscoatings.com. Retrieved on 2012-06-04.
460:yields parylene M and E respectively.
445:The hydrogen atoms can be replaced also by
183:, moisture barriers, or protection against
105:−. It can be obtained by polymerization of
2175:Journal of Vacuum Science and Technology A
1835:Journal of Vacuum Science and Technology A
1790:
1788:
1766:
1764:
1714:
1712:
1640:Corrosion protection for metallic surfaces
1546:Low coefficient of friction (AF-4, HT, SF)
1540:and uniformly thick, even within cavities.
1205:-xylene involving several steps involving
199:. These coatings are typically applied by
175:Coatings of parylene are often applied to
2776:
2361:Vysokomolekulyarnye Soedineniya, Seriya A
2297:-xylylene and Poly(α,α,α',α'-tetramethyl-
2113:
1342:yields parylene AF-4 with elimination of
2601:Journal of the American Chemical Society
2270:H. E. Winberg and F. S. Fawcett (1973).
2210:Journal of the American Chemical Society
1637:Electronics for space travel and defense
745:
591:per unit. Both are trademarks of Kisco.
2096:K. M. Vaeth & K. F. Jensen (1999).
1689:Jeffrey B. Fortin; Toh-Ming Lu (2003).
1676:
1118:Possible parylene polymerization routes
462:
2381:
2370:
1684:
1682:
1680:
289:above 1000 °C. Szwarc identified
1462:α,α'-diphenoxy-α,α,α',α'-tetrafluoro-
7:
1295:it does not condense in the latter.
217:because its polymerization needs no
976:Water absorption (% after 24 hours)
800:Short-term service temperature (°C)
783:Continuous service temperature (°C)
233:Parylene was discovered in 1947 by
1201:The dimer can be synthesized from
14:
1643:Reinforcement of micro-structures
1531:for various medical applications.
345:Repeating unit of parylene C
1350:
1243:Gorham process for parylene AF-4
598:
489:
477:
465:
191:). They are also used to reduce
2404:, Issue date: October 31, 2000.
2318:Journal of Electronic Materials
1259:− bond is stronger than the −CH
1069:Polymerization of the adsorbed
1027:Dynamic coefficient of friction
851:Specific heat at 20°C (J/(g·K))
377:compliance, especially for the
1655:microelectromechanical systems
1494:Characteristics and advantages
1287:, whose elimination generates
1010:Static coefficient of friction
689:Transparency and crystallinity
1:
2027:10.1016/S0040-6090(01)01355-4
1443:α,α'-dimethyl-α,α'-dimethoxy-
2419:, Issue date: March 9, 2004.
1236:bond breaks at 680 °C.
702:monoclinic crystal structure
534:(not to be confused with an
164:atoms are replaced by other
1779:Plasma Ruggedized Solutions
1631:Implantable medical devices
653:It is possible to attach a
441:Alkyl-substituted parylenes
46:whose backbone consists of
2814:
2577:. CRC Press. p. 263.
2257:10.1002/pol.1984.170220218
1966:Mat. Res. Soc. Symp. Proc.
1817:10.1002/pol.1966.150041209
1695:. Springer. pp. 4–7.
1099:or a negative anion group
676:. Using the parylene C/SiO
23:Repeating unit of parylene
2546:Chemical Vapor Deposition
2467:Chemical Vapor Deposition
2431:Chemical Vapor Deposition
2338:10.1007/s11664-997-0280-8
2131:Chemical Vapor Deposition
2042:Chemical Vapor Deposition
1980:Chemical Vapor Deposition
1940:Chemical Vapor Deposition
1585:self-assembled monolayers
213:Parylene is considered a
201:chemical vapor deposition
661:Parylene-like copolymers
637:" and can be used as an
633:. It can also undergo "
333:-xylylene intermediate.
203:in an atmosphere of the
42:is the common name of a
2757:Applied Physics Letters
925:Elongation to break (%)
731:Industry specifications
709:Mechanical and chemical
668:and nanocomposites (SiO
179:and other equipment as
2558:10.1002/cvde.200804266
2498:Chemistry of Materials
2479:10.1002/cvde.201304321
2443:10.1002/cvde.201104304
2143:10.1002/cvde.200304179
2054:10.1002/cvde.201307071
1992:10.1002/cvde.200606541
1913:Chemistry of Materials
1569:printed circuit boards
1467:
1448:
1401:
1244:
1173:
1119:
512:g-mil/atom-100in2-24hr
346:
36:
24:
2710:Acta Mechanica Sinica
2518:10.1007/s003390051076
2473:(10–11–12): 327–331.
2416:U.S. patent 6,703,462
2401:U.S. patent 6,140,456
1968:vol. 381, 123 (1995).
1890:10.1557/JMR.1996.0233
1461:
1442:
1395:
1242:
1163:
1156:From the cyclic dimer
1117:
868:Young's modulus (psi)
393:Fluorinated parylenes
379:printed circuit board
344:
337:Chlorinated parylenes
187:and chemical attack (
181:electrical insulation
30:
22:
1749:SCS Coatings History
1614:Typical applications
1518:electrical insulator
1434:mass-flow controller
1365:mass-flow controller
942:Yield elongation (%)
508:175 kpi vs. 460 kpsi
405:) and HT parylene (
2769:2004ApPhL..84.2617S
2722:2003AcMSn..19....1Z
2640:10.1021/la00088a035
2613:10.1021/ja00019a011
2510:2000ApPhA..70..541S
2330:1997JEMat..26..949W
2301:-xylylene)-co-poly(
2249:1984JPoSA..22..475K
2222:10.1021/ja01041a016
2187:2000JVSTA..18.2459F
2019:2001TSF...397..223F
1882:1996JMatR..11.1842G
1847:2002JVSTA..20.1445S
1809:1966JPoSA...4.3027G
1522:dielectric constant
1215:Hofmann elimination
645:(non-crystalline).
298:-xylylene di-iodide
177:electronic circuits
172:in some countries.
2730:10.1007/BF02487448
2102:Advanced Materials
1754:2012-01-12 at the
1725:2009-10-07 at the
1468:
1449:
1402:
1245:
1174:
1151:Monomer generation
1133:Arrhenius kinetics
1120:
766:Melting point (°C)
542:Reactive parylenes
347:
37:
25:
2778:10.1063/1.1691488
2695:10.1021/la010970f
2668:10.1021/la000471z
2584:978-0-8247-5047-3
2496:nanocomposites".
2380:Missing or empty
2277:Organic Syntheses
2216:(13): 3517–3526.
2195:10.1116/1.1289773
1925:10.1021/cm990042q
1911:Nanocomposites".
1855:10.1116/1.1487870
1803:(12): 3027–3039.
1735:978-3-540-20410-7
1729:, Springer, 2003
1702:978-1-4020-7688-6
1667:Conformal coating
1271:From substituted
1043:
1042:
993:Rockwell hardness
885:Tensile strength
700:Parylene N has a
649:Colored parylenes
383:hydrogen chloride
197:implanted devices
189:conformal coating
166:functional groups
35:-xylylene monomer
16:Chemical compound
2805:
2783:
2782:
2780:
2748:
2742:
2741:
2705:
2699:
2698:
2678:
2672:
2671:
2650:
2644:
2643:
2623:
2617:
2616:
2595:
2589:
2588:
2568:
2562:
2561:
2541:
2530:
2529:
2489:
2483:
2482:
2462:
2456:
2453:
2447:
2446:
2426:
2420:
2418:
2411:
2405:
2403:
2396:
2390:
2389:
2383:
2378:
2376:
2368:
2356:
2350:
2349:
2312:
2306:
2291:
2282:
2280:
2267:
2261:
2260:
2232:
2226:
2225:
2205:
2199:
2198:
2170:
2164:
2163:
2156:
2147:
2146:
2126:
2120:
2119:
2117:
2093:
2087:
2081:
2075:
2074:
2072:
2064:
2058:
2057:
2048:(1–2–3): 39–43.
2037:
2031:
2030:
2013:(1–2): 223–228.
2007:Thin Solid Films
2002:
1996:
1995:
1975:
1969:
1962:
1956:
1955:
1935:
1929:
1928:
1900:
1894:
1893:
1865:
1859:
1858:
1830:
1821:
1820:
1792:
1783:
1782:
1776:
1768:
1759:
1746:
1740:
1716:
1707:
1706:
1686:
1579:Molecular layers
1554:intrinsic stress
1489:
1487:
1486:
1427:
1425:
1424:
1381:
1377:hydrogen bromide
1354:
1341:
1339:
1338:
1330:
1329:
1321:
1320:
1312:
1311:
1168:-xylylene dimer
1110:
1109:
1108:
1098:
1097:
1096:
908:
888:
761:Parylene HT/AF4
746:
743:
742:
738:
612:
602:
590:
589:
588:
580:
579:
565:
564:
563:
533:
532:
531:
513:
509:
493:
481:
469:
420:
419:
418:
387:
288:
287:
286:
276:
275:
274:
266:
265:
255:
253:
252:
156:
155:
154:
144:
143:
142:
134:
133:
123:
121:
120:
104:
103:
102:
92:
91:
90:
76:
75:
74:
66:
65:
2813:
2812:
2808:
2807:
2806:
2804:
2803:
2802:
2788:
2787:
2786:
2750:
2749:
2745:
2707:
2706:
2702:
2680:
2679:
2675:
2652:
2651:
2647:
2625:
2624:
2620:
2597:
2596:
2592:
2585:
2570:
2569:
2565:
2543:
2542:
2533:
2495:
2491:
2490:
2486:
2464:
2463:
2459:
2454:
2450:
2428:
2427:
2423:
2414:
2413:Lee, Chung J.,
2412:
2408:
2399:
2397:
2393:
2379:
2369:
2358:
2357:
2353:
2314:
2313:
2309:
2292:
2285:
2269:
2268:
2264:
2234:
2233:
2229:
2207:
2206:
2202:
2172:
2171:
2167:
2158:
2157:
2150:
2128:
2127:
2123:
2108:(10): 814–820.
2095:
2094:
2090:
2082:
2078:
2070:
2066:
2065:
2061:
2039:
2038:
2034:
2004:
2003:
1999:
1977:
1976:
1972:
1963:
1959:
1937:
1936:
1932:
1910:
1902:
1901:
1897:
1867:
1866:
1862:
1832:
1831:
1824:
1794:
1793:
1786:
1774:
1770:
1769:
1762:
1756:Wayback Machine
1747:
1743:
1727:Wayback Machine
1717:
1710:
1703:
1688:
1687:
1678:
1674:
1664:
1616:
1610:
1581:
1573:medical devices
1565:
1550:
1496:
1485:
1482:
1481:
1480:
1478:
1423:
1420:
1419:
1418:
1416:
1406:α,α'-dimethoxy-
1396:α,α'-dimethoxy-
1379:
1337:
1334:
1333:
1332:
1328:
1325:
1324:
1323:
1319:
1316:
1315:
1314:
1310:
1307:
1306:
1305:
1303:
1277:
1266:
1262:
1258:
1254:
1158:
1153:
1125:
1107:
1104:
1103:
1102:
1100:
1095:
1092:
1091:
1090:
1088:
1083:
1067:
1048:
1046:Coating process
906:
905:Yield strength
886:
744:
740:
736:
734:
733:
724:
711:
691:
686:
679:
671:
663:
651:
635:click chemistry
610:
587:
584:
583:
582:
578:
575:
574:
573:
571:
562:
559:
558:
557:
555:
544:
530:
525:
524:
523:
522:
511:
507:
497:
494:
485:
482:
473:
470:
443:
436:
417:
414:
413:
412:
410:
395:
385:
368:
339:
327:
322:
285:
282:
281:
280:
278:
273:
270:
269:
268:
264:
261:
260:
259:
257:
251:
248:
247:
246:
244:
231:
215:"green" polymer
153:
150:
149:
148:
146:
141:
138:
137:
136:
132:
129:
128:
127:
125:
119:
116:
115:
114:
112:
101:
98:
97:
96:
94:
89:
86:
85:
84:
82:
77:− connected by
73:
70:
69:
68:
64:
61:
60:
59:
57:
17:
12:
11:
5:
2811:
2809:
2801:
2800:
2790:
2789:
2785:
2784:
2743:
2700:
2673:
2645:
2618:
2590:
2583:
2563:
2552:(4–6): 91–94.
2531:
2493:
2484:
2457:
2448:
2437:(4–6): 76–79.
2421:
2406:
2391:
2351:
2324:(8): 949–953.
2307:
2283:
2262:
2243:(2): 475–491.
2227:
2200:
2165:
2148:
2121:
2088:
2076:
2059:
2032:
1997:
1970:
1957:
1930:
1919:(7): 1814–21.
1908:
1907:-xylylene)/SiO
1895:
1876:(7): 1842–50.
1860:
1822:
1784:
1760:
1741:
1718:Mattox, D. M.
1708:
1701:
1675:
1673:
1670:
1663:
1660:
1659:
1658:
1647:
1644:
1641:
1638:
1635:
1632:
1629:
1626:
1623:
1615:
1612:
1580:
1577:
1564:
1561:
1548:
1547:
1544:
1541:
1535:
1532:
1525:
1514:
1495:
1492:
1483:
1421:
1356:
1355:
1335:
1326:
1317:
1308:
1276:
1269:
1264:
1260:
1256:
1252:
1157:
1154:
1152:
1149:
1124:
1121:
1105:
1093:
1081:
1066:
1065:Polymerization
1063:
1047:
1044:
1041:
1040:
1037:
1034:
1031:
1028:
1024:
1023:
1020:
1017:
1014:
1011:
1007:
1006:
1003:
1000:
997:
994:
990:
989:
986:
983:
980:
977:
973:
972:
969:
966:
963:
960:
959:Density (g/cm)
956:
955:
952:
949:
946:
943:
939:
938:
935:
932:
929:
926:
922:
921:
918:
915:
912:
909:
902:
901:
898:
895:
892:
889:
882:
881:
878:
875:
872:
869:
865:
864:
861:
858:
855:
852:
848:
847:
844:
841:
838:
835:
831:
830:
827:
824:
821:
818:
814:
813:
810:
807:
804:
801:
797:
796:
793:
790:
787:
784:
780:
779:
776:
773:
770:
767:
763:
762:
759:
756:
753:
750:
732:
729:
723:
720:
710:
707:
690:
687:
685:
682:
677:
669:
662:
659:
650:
647:
585:
576:
560:
543:
540:
526:
499:
498:
495:
488:
486:
483:
476:
474:
471:
464:
442:
439:
434:
415:
394:
391:
338:
335:
326:
323:
321:
318:
305:paracyclophane
283:
271:
262:
249:
235:Michael Szwarc
230:
227:
151:
139:
130:
117:
99:
87:
79:1,2-ethanediyl
71:
62:
15:
13:
10:
9:
6:
4:
3:
2:
2810:
2799:
2796:
2795:
2793:
2779:
2774:
2770:
2766:
2762:
2758:
2754:
2747:
2744:
2739:
2735:
2731:
2727:
2723:
2719:
2715:
2711:
2704:
2701:
2696:
2692:
2688:
2684:
2677:
2674:
2669:
2665:
2661:
2657:
2649:
2646:
2641:
2637:
2633:
2629:
2622:
2619:
2614:
2610:
2606:
2602:
2594:
2591:
2586:
2580:
2576:
2575:
2567:
2564:
2559:
2555:
2551:
2547:
2540:
2538:
2536:
2532:
2527:
2523:
2519:
2515:
2511:
2507:
2503:
2499:
2488:
2485:
2480:
2476:
2472:
2468:
2461:
2458:
2452:
2449:
2444:
2440:
2436:
2432:
2425:
2422:
2417:
2410:
2407:
2402:
2395:
2392:
2387:
2374:
2366:
2362:
2355:
2352:
2347:
2343:
2339:
2335:
2331:
2327:
2323:
2319:
2311:
2308:
2304:
2300:
2296:
2290:
2288:
2284:
2279:
2278:
2273:
2266:
2263:
2258:
2254:
2250:
2246:
2242:
2238:
2231:
2228:
2223:
2219:
2215:
2211:
2204:
2201:
2196:
2192:
2188:
2184:
2180:
2176:
2169:
2166:
2161:
2155:
2153:
2149:
2144:
2140:
2136:
2132:
2125:
2122:
2116:
2111:
2107:
2103:
2099:
2092:
2089:
2086:
2080:
2077:
2069:
2063:
2060:
2055:
2051:
2047:
2043:
2036:
2033:
2028:
2024:
2020:
2016:
2012:
2008:
2001:
1998:
1993:
1989:
1985:
1981:
1974:
1971:
1967:
1961:
1958:
1953:
1949:
1946:(6): 257–60.
1945:
1941:
1934:
1931:
1926:
1922:
1918:
1914:
1906:
1899:
1896:
1891:
1887:
1883:
1879:
1875:
1871:
1870:J. Mater. Res
1864:
1861:
1856:
1852:
1848:
1844:
1841:(4): 1445–9.
1840:
1836:
1829:
1827:
1823:
1818:
1814:
1810:
1806:
1802:
1798:
1791:
1789:
1785:
1780:
1773:
1767:
1765:
1761:
1757:
1753:
1750:
1745:
1742:
1739:
1736:
1732:
1728:
1724:
1721:
1715:
1713:
1709:
1704:
1698:
1694:
1693:
1685:
1683:
1681:
1677:
1671:
1669:
1668:
1661:
1656:
1652:
1649:Reduction of
1648:
1645:
1642:
1639:
1636:
1633:
1630:
1627:
1624:
1621:
1620:
1619:
1613:
1611:
1608:
1604:
1601:
1600:contact angle
1596:
1594:
1590:
1586:
1578:
1576:
1574:
1570:
1562:
1560:
1557:
1555:
1545:
1542:
1539:
1536:
1533:
1530:
1526:
1523:
1519:
1515:
1512:
1508:
1504:
1501:
1500:
1499:
1493:
1491:
1477:
1473:
1465:
1460:
1456:
1454:
1446:
1441:
1437:
1435:
1431:
1415:
1414:methoxy group
1411:
1409:
1399:
1394:
1390:
1388:
1385:
1378:
1374:
1370:
1366:
1362:
1353:
1349:
1348:
1347:
1345:
1301:
1296:
1292:
1291:-xylylene.
1290:
1286:
1285:methyl groups
1282:
1274:
1270:
1268:
1250:
1241:
1237:
1235:
1231:
1227:
1225:
1218:
1216:
1212:
1208:
1204:
1199:
1197:
1193:
1189:
1186:
1183:
1179:
1171:
1167:
1162:
1155:
1150:
1148:
1144:
1140:
1138:
1134:
1130:
1123:Physisorption
1122:
1116:
1112:
1085:
1079:
1074:
1072:
1064:
1062:
1059:
1055:
1053:
1045:
1038:
1035:
1032:
1029:
1026:
1025:
1021:
1018:
1015:
1012:
1009:
1008:
1004:
1001:
998:
995:
992:
991:
987:
984:
981:
978:
975:
974:
970:
967:
964:
961:
958:
957:
953:
950:
947:
944:
941:
940:
936:
933:
930:
927:
924:
923:
919:
916:
913:
910:
904:
903:
899:
896:
893:
890:
884:
883:
879:
876:
873:
870:
867:
866:
862:
859:
856:
853:
850:
849:
845:
842:
839:
836:
833:
832:
828:
825:
822:
819:
816:
815:
811:
808:
805:
802:
799:
798:
794:
791:
788:
785:
782:
781:
777:
774:
771:
768:
765:
764:
760:
757:
754:
751:
748:
747:
739:
730:
728:
721:
719:
715:
708:
706:
703:
698:
695:
688:
683:
681:
675:
667:
660:
658:
656:
648:
646:
644:
640:
636:
632:
628:
624:
620:
616:
608:
607:ethinyl group
603:
601:
596:
592:
570:amine group −
569:
554:substituent −
553:
548:
541:
539:
537:
529:
519:
517:
505:
496:Parylene AM-2
492:
487:
480:
475:
468:
463:
461:
459:
455:
450:
448:
440:
438:
430:
428:
424:
408:
404:
400:
392:
390:
384:
380:
376:
371:
366:
362:
360:
356:
352:
343:
336:
334:
332:
324:
319:
317:
313:
309:
306:
301:
299:
297:
292:
243:
241:
236:
228:
226:
224:
220:
216:
211:
209:
206:
202:
198:
194:
190:
186:
182:
178:
173:
171:
167:
163:
158:
111:
109:
80:
55:
51:
50:
45:
41:
34:
29:
21:
2763:(14): 2617.
2760:
2756:
2746:
2713:
2709:
2703:
2686:
2682:
2676:
2662:(18): 7268.
2659:
2655:
2648:
2631:
2627:
2621:
2607:(19): 7152.
2604:
2600:
2593:
2573:
2566:
2549:
2545:
2501:
2497:
2487:
2470:
2466:
2460:
2451:
2434:
2430:
2424:
2409:
2394:
2382:|title=
2373:cite journal
2364:
2360:
2354:
2321:
2317:
2310:
2302:
2298:
2294:
2275:
2265:
2240:
2236:
2230:
2213:
2209:
2203:
2178:
2174:
2168:
2137:(5): 247–9.
2134:
2130:
2124:
2105:
2101:
2091:
2079:
2062:
2045:
2041:
2035:
2010:
2006:
2000:
1986:(1): 55–59.
1983:
1979:
1973:
1965:
1960:
1943:
1939:
1933:
1916:
1912:
1904:
1898:
1873:
1869:
1863:
1838:
1834:
1800:
1796:
1778:
1744:
1738:Google books
1691:
1665:
1617:
1609:
1605:
1597:
1582:
1566:
1563:Applications
1558:
1549:
1497:
1471:
1469:
1463:
1452:
1450:
1444:
1429:
1407:
1403:
1397:
1373:free-radical
1357:
1299:
1297:
1293:
1288:
1280:
1278:
1272:
1248:
1246:
1223:
1219:
1202:
1200:
1187:
1177:
1175:
1169:
1165:
1145:
1141:
1126:
1086:
1077:
1075:
1070:
1068:
1060:
1056:
1051:
1049:
725:
722:Permeability
716:
712:
699:
696:
692:
664:
652:
631:Ag-acetylide
627:Cu-acetylide
615:cross-linked
604:
597:
593:
549:
545:
527:
520:
500:
454:methyl group
451:
447:alkyl groups
444:
431:
396:
372:
367:
363:
353:ring or the
348:
330:
328:
314:
310:
302:
295:
290:
239:
232:
212:
210:-xylylene.
207:
174:
159:
107:
48:
39:
38:
32:
2689:(5): 1587.
2634:(4): 1074.
2504:(5): 1814.
2367:(5): 972–6.
2181:(5): 2459.
1571:(PCBs) and
1538:Homogeneous
1520:with a low
1503:Hydrophobic
1226:-cyclophane
1207:bromination
1164:The cyclic
1129:physisorbed
655:chromophore
458:ethyl group
54:benzenediyl
1672:References
1455:-xylene.
1192:cyclophane
1172:cyclophane
758:Parylene D
755:Parylene C
752:Parylene N
749:Properties
684:Properties
666:Copolymers
484:Parylene E
472:Parylene M
325:Parylene N
170:trademarks
2738:110540114
1595:devices.
1369:catalysts
1211:amination
988:<0.01
674:maleimide
643:amorphous
568:methylene
355:aliphatic
320:Varieties
219:initiator
185:corrosion
110:-xylylene
81:bridges −
2798:Polymers
2792:Category
2716:(1): 1.
2683:Langmuir
2656:Langmuir
2628:Langmuir
2526:96072554
2346:94987047
1752:Archived
1723:Archived
1662:See also
1651:friction
1589:stiction
1302:-xylene
1275:-xylenes
1230:chlorine
1222:dichloro
962:1.1–1.12
880:370,000
778:>500
639:adhesive
409:). The −
399:fluorine
359:chlorine
193:friction
162:hydrogen
40:Parylene
2765:Bibcode
2718:Bibcode
2506:Bibcode
2326:Bibcode
2245:Bibcode
2183:Bibcode
2015:Bibcode
1878:Bibcode
1843:Bibcode
1805:Bibcode
1476:phenoxy
1466:-xylene
1447:-xylene
1410:-xylene
1400:-xylene
1387:O-rings
1363:, or a
1361:bubbler
1344:bromine
985:<0.1
982:<0.1
979:<0.1
877:380,000
874:400,000
871:350,000
502:1
242:-xylene
229:History
223:solvent
205:monomer
56:rings −
44:polymer
2736:
2581:
2524:
2344:
1781:. PRS.
1733:
1699:
1529:US FDA
1412:. The
1182:cyclic
920:5,000
900:7,500
897:11,000
894:10,000
846:0.096
735:": -->
623:silver
619:copper
456:or an
351:phenyl
2734:S2CID
2522:S2CID
2342:S2CID
2071:(PDF)
1775:(PDF)
1516:Good
1511:bases
1507:acids
1384:viton
1185:dimer
1039:0.13
1022:0.15
1005:R122
971:1.32
968:1.418
965:1.289
917:9,000
914:8,000
911:6,100
907:(psi)
891:7,000
887:(psi)
863:1.04
857:0.712
854:0.837
840:0.084
837:0.126
552:amine
536:amine
403:Kisco
2579:ISBN
2386:help
1731:ISBN
1697:ISBN
1593:MEMS
1509:and
1472:para
1430:para
1300:para
1289:para
1281:para
1249:para
1234:aryl
1224:para
1213:and
1196:Torr
1188:para
1176:The
1170:para
1166:para
1076:The
1052:para
1036:0.31
1033:0.29
1030:0.25
1019:0.33
1016:0.29
1013:0.25
954:2.0
937:200
812:450
795:350
737:edit
611:C≡CH
423:PTFE
375:RoHS
331:para
296:para
291:para
240:para
208:para
157:.
108:para
49:para
33:para
31:The
2773:doi
2726:doi
2691:doi
2664:doi
2636:doi
2609:doi
2605:113
2554:doi
2514:doi
2475:doi
2439:doi
2334:doi
2253:doi
2218:doi
2191:doi
2139:doi
2110:doi
2050:doi
2023:doi
2011:397
1988:doi
1948:doi
1921:doi
1886:doi
1851:doi
1813:doi
1591:in
1380:HBr
1313:Br)
1304:(CF
1263:−CH
1255:−CF
1002:R80
999:R80
996:R85
951:3.0
948:2.9
945:2.5
934:200
931:200
928:250
829:36
809:120
806:100
792:100
775:380
772:290
769:420
629:or
621:or
516:mil
504:kHz
433:−CH
427:LED
407:SCS
386:HCl
2794::
2771:.
2761:84
2759:.
2755:.
2732:.
2724:.
2714:19
2712:.
2687:18
2685:.
2660:16
2658:.
2630:.
2603:.
2550:15
2548:.
2534:^
2520:.
2512:.
2502:11
2500:.
2471:19
2469:.
2435:17
2433:.
2377::
2375:}}
2371:{{
2365:22
2363:.
2340:.
2332:.
2322:26
2320:.
2286:^
2274:.
2251:.
2241:22
2239:.
2214:91
2212:.
2189:.
2179:18
2177:.
2151:^
2135:10
2133:.
2106:11
2104:.
2100:.
2046:20
2044:.
2021:.
2009:.
1984:13
1982:.
1942:.
1917:11
1915:.
1884:.
1874:11
1872:.
1849:.
1839:20
1837:.
1825:^
1811:.
1799:.
1787:^
1777:.
1763:^
1711:^
1679:^
1513:).
1479:CH
1426:CO
1389:.
1346:.
1322:(C
1217:.
1209:,
1137:nm
1111::
1101:CH
1089:CH
826:38
823:35
820:69
803:80
789:80
786:60
581:NH
572:CH
556:NH
411:CF
279:CH
225:.
147:CH
95:CH
83:CH
2781:.
2775::
2767::
2740:.
2728::
2720::
2697:.
2693::
2670:.
2666::
2642:.
2638::
2632:5
2615:.
2611::
2587:.
2560:.
2556::
2528:.
2516::
2508::
2494:2
2481:.
2477::
2445:.
2441::
2388:)
2384:(
2348:.
2336::
2328::
2303:p
2299:p
2295:p
2281:.
2259:.
2255::
2247::
2224:.
2220::
2197:.
2193::
2185::
2162:.
2145:.
2141::
2118:.
2112::
2073:.
2056:.
2052::
2029:.
2025::
2017::
1994:.
1990::
1954:.
1950::
1944:5
1927:.
1923::
1909:2
1905:p
1892:.
1888::
1880::
1857:.
1853::
1845::
1819:.
1815::
1807::
1801:4
1705:.
1657:.
1488:O
1484:5
1464:p
1453:p
1445:p
1422:3
1417:H
1408:p
1398:p
1340:)
1336:4
1331:H
1327:6
1318:2
1309:2
1273:p
1265:2
1261:2
1257:2
1253:2
1232:-
1203:p
1190:-
1178:p
1106:2
1094:2
1082:x
1078:p
1071:p
860:—
843:—
741:]
678:2
670:2
609:−
586:2
577:2
561:2
528:n
435:2
416:2
284:3
277:−
272:4
267:H
263:6
258:C
256:−
254:C
250:3
245:H
152:2
145:=
140:4
135:H
131:6
126:C
124:=
122:C
118:2
113:H
100:2
93:−
88:2
72:4
67:H
63:6
58:C
52:-
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.