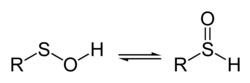279:
31:
471:
98:
SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy
434:
226:
were used to identify 2-propenesulfenic formed when garlic is cut or crushed and to demonstrate that this sulfenic acid has a lifetime of less than one second. The pharmacological activity of certain drugs, such as
556:
Goto K, Holler M, Okazaki R (1997). "Synthesis, Structure, and
Reactions of a Sulfenic Acid Bearing a Novel Bowl-Type Substituent: The First Synthesis of a Stable Sulfenic Acid by Direct Oxidation of a Thiol".
282:
Dioctadecyl 3,3'-thiodipropanoate: Oxidation to the sulfoxide and subsequent Ei elimination generates a sulfenic acid. This material is used as a polymer stabilizer where it protects against long term heat
181:
that detoxify peroxides. They function by the conversion of a cysteine residue to a sulfenic acid. The sulfenic acid then converts to a disulfide by reaction with another residue of cysteine.
584:
Ishii A, Komiya K, Nakayama J (1996). "Synthesis of a Stable
Sulfenic Acid by Oxidation of a Sterically Hindered Thiol (Thiophenetriptycene-8-thiol)1 and Its Characterization".
1083:
Harrop, Todd C.; Mascharak, Pradip K. (2004). "Fe(III) and Co(III) Centers with
Carboxamido Nitrogen and Modified Sulfur Coordination: Lessons Learned from Nitrile Hydratase".
123:, the structure of such stabilized sulfenic acids were shown to be R–S–O–H. The stable, sterically hindered sulfenic acid 1-triptycenesulfenic acid has been found to have a
1056:
Armstrong, C.; Plant, M.A.; Scott, G. (February 1975). "Mechanisms of antioxidant action: the nature of the redox behaviour of thiodipropionate esters in polypropylene".
1117:
1009:
874:
266:
Sulfenic acid forms part of the series of chemical reactions that occur when cutting onions. The lachrymal glands are irritated by the end product of the reactions,
1198:
2117:
2122:
965:
Braverman, S., "Rearrangements involving sulfenic acids and their derivatives," in
Sulfenic Acids and Derivatives, 1990, John Wiley & Sons.
1040:
692:
207:. 1-Propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, the lachrymatory factor synthase, giving
982:
Michael
Carrasco, Robert J. Jones, Scott Kamel, H. Rapoport, Thien Truong (1992). "N-(Benzyloxycarbonyl)-L-Vinylglycine Methyl Ester".
107:–S–O–H. Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form
308:
38:, spectroscopic measurements as well as theoretical studies indicate that the structure on the left predominates almost exclusively.
1149:"Phenylsulfenylation of Nonactivated Carbon Atom by Photolysiis of Alkyl Benzenesulfenated: Preparation of 2-Phenylthio-5-heptanol"
504:
have the formula RSOR′. They arise by the reaction of sulfenyl chlorides on alcohols. Sulfenate esters are intermediates in the
2145:
1191:
2021:
743:
Block E, Dane AJ, Thomas S, Cody RB (2010). "Applications of Direct
Analysis in Real Time–Mass Spectrometry (DART-MS) in
1594:
1184:
505:
474:
1631:
267:
208:
134:
278:
2104:
443:
where they protects against long term heat ageing, structures based on thiodipropionate esters are popular.
100:
2004:
2111:
1999:
1003:
868:
120:
83:
51:
708:
Vaidya V, Ingold KU, Pratt DA (2009). "Garlic: Source of the
Ultimate Antioxidants – Sulfenic Acids".
2080:
1525:
646:
Rhee, Sue Goo; Kil, In Sup (2017). "Multiple
Functions and Regulation of Mammalian Peroxiredoxins".
1386:
440:
260:
94:
In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH
222:, is thought to be responsible for garlic’s potent antioxidant activity. Mass spectrometry with a
611:
McGrath AJ, Garrett GE, Valgimigli L, Pratt DA (2010). "The redox chemistry of sulfenic acids".
30:
2070:
2040:
1798:
1420:
1100:
1036:
919:
856:
807:
768:
725:
688:
663:
628:
490:
470:
455:
1775:
1269:
1207:
1160:
1131:
1092:
1065:
1028:
991:
966:
909:
901:
846:
838:
799:
760:
717:
655:
620:
593:
566:
538:
1994:
1753:
1748:
1731:
1714:
1515:
1264:
659:
223:
2065:
2060:
1936:
1931:
1926:
1719:
1686:
1470:
1452:
1442:
1032:
914:
889:
851:
826:
174:
937:
489:
in organic nomenclature denotes the RS group (R ≠ H). One example is methane
2139:
2085:
2033:
1964:
1850:
1840:
1835:
1825:
1770:
1765:
1681:
1676:
1666:
1520:
1475:
1437:
1425:
1396:
1274:
1069:
145:
108:
71:
63:
1127:
62:. It is the first member of the family of organosulfur oxoacids, which also include
2016:
1903:
1898:
1875:
1626:
1465:
1391:
1328:
1323:
1301:
1257:
1242:
1232:
751:-Oxide and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums".
291:
232:
905:
682:
2075:
2028:
1989:
1870:
1758:
1743:
1738:
1726:
1291:
1286:
1252:
1247:
1237:
1215:
1122:
478:
451:
240:
236:
149:
35:
1984:
1975:
1855:
1810:
1706:
1671:
1661:
1601:
1537:
1460:
1408:
970:
803:
228:
1165:
1148:
1126:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
995:
747:
Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl
Trisulfane
1951:
1865:
1830:
1815:
1803:
1646:
1621:
1430:
1135:
287:
255:
to the corresponding protein sulfenic acids is suggested to be important in
244:
43:
1104:
923:
860:
811:
772:
729:
721:
667:
632:
82:), respectively. The base member of the sulfenic acid series with R = H is
1959:
1913:
1880:
1576:
1482:
1356:
1311:
1296:
248:
158:
137:(bde) of 71.9 ± 0.3 kcal/mol, which can be compared to a p
542:
144:
of ≥14 and O–H BDE of ~88 kcal/mol for the (valence) isoelectronic
17:
1921:
1845:
1696:
1691:
1656:
1641:
1636:
1606:
1589:
1413:
1340:
1306:
252:
219:
112:
55:
1096:
842:
764:
624:
597:
570:
2009:
1941:
1785:
1494:
1487:
1381:
1362:
1351:
1335:
1281:
447:
299:
203:
194:
190:
178:
155:
152:
116:
1176:
788:"Sulfenic acids as reactive intermediates in xenobiotic metabolism"
787:
1890:
1860:
1793:
1651:
1616:
1611:
1584:
1532:
1499:
1403:
1227:
277:
256:
198:
29:
247:
is proposed to involve sulfenic acid intermediates. Oxidation of
1318:
827:"Formation, Reactivity, and Detection of Protein Sulfenic Acids"
1180:
1025:
Reference Module in
Materials Science and Materials Engineering
462:
group is proposed as the nucleophile that attacks the nitrile.
429:{\displaystyle {\ce {R-S(O)CH2CH2-R' -> R-SOH + CH2=CH-R'}}}
1147:
Petrovic, Goran; Saicic, Radomir N.; Cekovic, Zivorad (2005).
189:
Sulfenic acids are produced by the enzymatic decomposition of
124:
529:
Penn RE, Block E, Revelle LK (1978). "Methanesulfenic Acid".
890:"Sulfenic acid chemistry, detection and cellular lifetime"
401:
358:
345:
508:
of allyl sulfoxides. Sulfenamides have the formula RSNR′
894:
Biochimica et Biophysica Acta (BBA) - General Subjects
34:
While sulfenic acids have the potential of exhibiting
311:
2053:
1973:
1950:
1912:
1889:
1784:
1705:
1575:
1552:
1508:
1451:
1374:
1349:
1214:
684:Garlic and Other Alliums: The Lore and the Science
428:
888:Gupta, Vinayak; Kate S. Carroll (February 2014).
439:Compounds which react in this manner are used as
193:and related compounds following tissue damage to
1192:
1023:Kröhnke, C. (2016). "Polymer Stabilization".
8:
1008:: CS1 maint: multiple names: authors list (
873:: CS1 maint: multiple names: authors list (
1572:
1371:
1199:
1185:
1177:
938:"Why does chopping an onion make you cry?"
753:Journal of Agricultural and Food Chemistry
481:, yet another derivative of sulfenic acid.
1164:
913:
850:
412:
404:
400:
395:
380:
361:
357:
352:
344:
339:
324:
316:
312:
310:
961:
959:
613:Journal of the American Chemical Society
586:Journal of the American Chemical Society
559:Journal of the American Chemical Society
531:Journal of the American Chemical Society
469:
77:
792:Archives of Biochemistry and Biophysics
710:Angewandte Chemie International Edition
521:
290:can undergo thermal elimination via an
27:Organosulfur compound of the form R–SOH
1001:
866:
218:. 2-Propenesulfenic acid, formed from
660:10.1146/annurev-biochem-060815-014431
7:
1123:Compendium of Chemical Terminology
1033:10.1016/B978-0-12-803581-8.01487-9
825:Kettenhofen, NJ, Wood, MJ (2010).
25:
201:, and other plants of the genus
274:Organic and inorganic chemistry
786:Mansuy D, Dansette PM (2011).
687:. Royal Society of Chemistry.
374:
331:
325:
1:
1085:Accounts of Chemical Research
648:Annual Review of Biochemistry
1070:10.1016/0014-3057(75)90141-X
906:10.1016/j.bbagen.2013.05.040
177:are ubiquitous and abundant
2162:
506:Mislow-Evans rearrangement
2094:
971:10.1002/9780470772287.ch8
944:. The Library of Congress
804:10.1016/j.abb.2010.09.015
475:Cyclohexylthiophthalimide
58:with the general formula
1166:10.15227/orgsyn.081.0244
1058:European Polymer Journal
996:10.15227/orgsyn.070.0029
466:Other sulfenyl compounds
268:syn-Propanethial-S-oxide
165:Formation and occurrence
135:bond-dissociation energy
2105:chemical classification
1136:10.1351/goldbook.S06098
101:rotational spectroscopy
2146:Organosulfur compounds
722:10.1002/anie.200804560
482:
430:
284:
39:
2112:chemical nomenclature
473:
431:
281:
121:X-ray crystallography
119:. Through the use of
84:hydrogen thioperoxide
52:organosulfur compound
33:
309:
302:and sulfenic acids:
1568:not C, H or O)
619:(47): 16759–16761.
592:(50): 12836–12837.
543:10.1021/ja00479a068
477:is an example of a
441:polymer stabilizers
403:
360:
347:
261:signal transduction
133:of 12.5 and an O–H
111:, RS(O)SR, such as
2010:Hypervalent iodine
942:Everyday Mysteries
831:Chem. Res. Toxicol
681:Block, E. (2010).
483:
456:nitrile hydratases
426:
391:
348:
335:
285:
40:
2133:
2132:
2071:Sulfenyl chloride
2049:
2048:
1548:
1547:
1367:(only C, H and O)
1208:Functional groups
1153:Organic Syntheses
1097:10.1021/ar0301532
1042:978-0-12-803581-8
984:Organic Syntheses
843:10.1021/tx100237w
837:(11): 1633–1646.
765:10.1021/jf1000106
694:978-0-85404-190-9
625:10.1021/ja1083046
598:10.1021/ja962995k
571:10.1021/ja962994s
537:(11): 3622–3624.
491:sulfenyl chloride
450:are found at the
420:
411:
394:
387:
379:
369:
351:
338:
330:
323:
315:
270:, causing tears.
185:Garlic and onions
16:(Redirected from
2153:
2100:
2005:Trifluoromethoxy
1573:
1569:
1372:
1368:
1221:
1201:
1194:
1187:
1178:
1171:
1170:
1168:
1144:
1138:
1115:
1109:
1108:
1080:
1074:
1073:
1053:
1047:
1046:
1020:
1014:
1013:
1007:
999:
979:
973:
963:
954:
953:
951:
949:
934:
928:
927:
917:
885:
879:
878:
872:
864:
854:
822:
816:
815:
783:
777:
776:
759:(8): 4617–4625.
740:
734:
733:
705:
699:
698:
678:
672:
671:
643:
637:
636:
608:
602:
601:
581:
575:
574:
565:(6): 1460–1461.
553:
547:
546:
526:
502:Sulfenate esters
461:
446:Sulfenate-based
435:
433:
432:
427:
425:
424:
418:
416:
409:
408:
402:
399:
392:
385:
384:
377:
373:
367:
365:
359:
356:
349:
346:
343:
336:
334:
328:
321:
320:
313:
81:
69:
61:
21:
2161:
2160:
2156:
2155:
2154:
2152:
2151:
2150:
2136:
2135:
2134:
2129:
2098:
2090:
2045:
2000:Trichloromethyl
1995:Trifluoromethyl
1969:
1946:
1908:
1885:
1780:
1749:Phosphine oxide
1701:
1567:
1565:
1564:
1562:
1560:
1558:
1556:
1554:
1544:
1504:
1447:
1366:
1365:
1360:
1355:
1345:
1219:
1218:
1210:
1205:
1175:
1174:
1146:
1145:
1141:
1128:sulfenyl groups
1116:
1112:
1082:
1081:
1077:
1055:
1054:
1050:
1043:
1022:
1021:
1017:
1000:
981:
980:
976:
964:
957:
947:
945:
936:
935:
931:
887:
886:
882:
865:
824:
823:
819:
785:
784:
780:
742:
741:
737:
707:
706:
702:
695:
680:
679:
675:
645:
644:
640:
610:
609:
605:
583:
582:
578:
555:
554:
550:
528:
527:
523:
518:
511:
500:
496:
468:
459:
417:
366:
307:
306:
298:to yield vinyl
295:
276:
224:DART ion source
187:
172:
167:
143:
131:
106:
97:
92:
79:
75:
67:
59:
28:
23:
22:
15:
12:
11:
5:
2159:
2157:
2149:
2148:
2138:
2137:
2131:
2130:
2128:
2127:
2126:
2125:
2120:
2108:
2101:
2095:
2092:
2091:
2089:
2088:
2086:Sulfinylamines
2083:
2078:
2073:
2068:
2066:Phosphoramides
2063:
2061:Isothiocyanate
2057:
2055:
2051:
2050:
2047:
2046:
2044:
2043:
2038:
2037:
2036:
2026:
2025:
2024:
2014:
2013:
2012:
2007:
2002:
1997:
1992:
1981:
1979:
1971:
1970:
1968:
1967:
1962:
1956:
1954:
1948:
1947:
1945:
1944:
1939:
1937:Selenenic acid
1934:
1932:Seleninic acid
1929:
1927:Selenonic acid
1924:
1918:
1916:
1910:
1909:
1907:
1906:
1901:
1895:
1893:
1887:
1886:
1884:
1883:
1878:
1873:
1868:
1863:
1858:
1853:
1848:
1843:
1838:
1833:
1828:
1823:
1818:
1813:
1808:
1807:
1806:
1796:
1790:
1788:
1782:
1781:
1779:
1778:
1773:
1768:
1763:
1762:
1761:
1751:
1746:
1741:
1736:
1735:
1734:
1724:
1723:
1722:
1720:Phosphodiester
1711:
1709:
1703:
1702:
1700:
1699:
1694:
1689:
1684:
1679:
1674:
1669:
1664:
1659:
1654:
1649:
1644:
1639:
1634:
1629:
1624:
1619:
1614:
1609:
1604:
1599:
1598:
1597:
1592:
1581:
1579:
1570:
1566:(one element,
1550:
1549:
1546:
1545:
1543:
1542:
1541:
1540:
1530:
1529:
1528:
1523:
1512:
1510:
1506:
1505:
1503:
1502:
1497:
1492:
1491:
1490:
1480:
1479:
1478:
1473:
1468:
1457:
1455:
1449:
1448:
1446:
1445:
1443:Methylenedioxy
1440:
1435:
1434:
1433:
1428:
1418:
1417:
1416:
1411:
1401:
1400:
1399:
1389:
1384:
1378:
1376:
1369:
1347:
1346:
1344:
1343:
1338:
1333:
1332:
1331:
1326:
1316:
1315:
1314:
1309:
1304:
1299:
1294:
1289:
1279:
1278:
1277:
1272:
1262:
1261:
1260:
1255:
1250:
1245:
1240:
1235:
1224:
1222:
1220:(only C and H)
1212:
1211:
1206:
1204:
1203:
1196:
1189:
1181:
1173:
1172:
1139:
1110:
1091:(4): 253–260.
1075:
1064:(2): 161–167.
1048:
1041:
1015:
974:
955:
929:
900:(2): 847–875.
880:
817:
798:(1): 174–185.
778:
735:
700:
693:
673:
638:
603:
576:
548:
520:
519:
517:
514:
509:
494:
467:
464:
437:
436:
423:
415:
407:
398:
390:
383:
376:
372:
364:
355:
342:
333:
327:
319:
293:
275:
272:
212:-propanethial-
186:
183:
175:Peroxiredoxins
171:
170:Peroxiredoxins
168:
166:
163:
146:hydroperoxides
141:
129:
109:thiosulfinates
104:
95:
91:
88:
72:sulfonic acids
64:sulfinic acids
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2158:
2147:
2144:
2143:
2141:
2124:
2121:
2119:
2116:
2115:
2114:
2113:
2109:
2107:
2106:
2102:
2097:
2096:
2093:
2087:
2084:
2082:
2079:
2077:
2074:
2072:
2069:
2067:
2064:
2062:
2059:
2058:
2056:
2052:
2042:
2039:
2035:
2032:
2031:
2030:
2027:
2023:
2020:
2019:
2018:
2015:
2011:
2008:
2006:
2003:
2001:
1998:
1996:
1993:
1991:
1988:
1987:
1986:
1983:
1982:
1980:
1978:
1977:
1972:
1966:
1965:Telluroketone
1963:
1961:
1958:
1957:
1955:
1953:
1949:
1943:
1940:
1938:
1935:
1933:
1930:
1928:
1925:
1923:
1920:
1919:
1917:
1915:
1911:
1905:
1902:
1900:
1897:
1896:
1894:
1892:
1888:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1862:
1859:
1857:
1854:
1852:
1851:Sulfonic acid
1849:
1847:
1844:
1842:
1841:Sulfinic acid
1839:
1837:
1836:Thiosulfonate
1834:
1832:
1829:
1827:
1826:Thiosulfinate
1824:
1822:
1821:Sulfenic acid
1819:
1817:
1814:
1812:
1809:
1805:
1802:
1801:
1800:
1797:
1795:
1792:
1791:
1789:
1787:
1783:
1777:
1776:Phosphaallene
1774:
1772:
1771:Phosphaalkyne
1769:
1767:
1766:Phosphaalkene
1764:
1760:
1757:
1756:
1755:
1752:
1750:
1747:
1745:
1742:
1740:
1737:
1733:
1730:
1729:
1728:
1725:
1721:
1718:
1717:
1716:
1713:
1712:
1710:
1708:
1704:
1698:
1695:
1693:
1690:
1688:
1685:
1683:
1680:
1678:
1675:
1673:
1670:
1668:
1665:
1663:
1660:
1658:
1655:
1653:
1650:
1648:
1645:
1643:
1640:
1638:
1635:
1633:
1630:
1628:
1625:
1623:
1620:
1618:
1615:
1613:
1610:
1608:
1605:
1603:
1600:
1596:
1593:
1591:
1588:
1587:
1586:
1583:
1582:
1580:
1578:
1574:
1571:
1551:
1539:
1536:
1535:
1534:
1531:
1527:
1524:
1522:
1519:
1518:
1517:
1514:
1513:
1511:
1507:
1501:
1498:
1496:
1493:
1489:
1486:
1485:
1484:
1481:
1477:
1474:
1472:
1469:
1467:
1464:
1463:
1462:
1459:
1458:
1456:
1454:
1450:
1444:
1441:
1439:
1438:Ethylenedioxy
1436:
1432:
1429:
1427:
1424:
1423:
1422:
1419:
1415:
1412:
1410:
1407:
1406:
1405:
1402:
1398:
1395:
1394:
1393:
1390:
1388:
1385:
1383:
1380:
1379:
1377:
1373:
1370:
1364:
1358:
1353:
1348:
1342:
1339:
1337:
1334:
1330:
1327:
1325:
1322:
1321:
1320:
1317:
1313:
1310:
1308:
1305:
1303:
1300:
1298:
1295:
1293:
1290:
1288:
1285:
1284:
1283:
1280:
1276:
1273:
1271:
1268:
1267:
1266:
1263:
1259:
1256:
1254:
1251:
1249:
1246:
1244:
1241:
1239:
1236:
1234:
1231:
1230:
1229:
1226:
1225:
1223:
1217:
1213:
1209:
1202:
1197:
1195:
1190:
1188:
1183:
1182:
1179:
1167:
1162:
1158:
1154:
1150:
1143:
1140:
1137:
1133:
1129:
1125:
1124:
1119:
1114:
1111:
1106:
1102:
1098:
1094:
1090:
1086:
1079:
1076:
1071:
1067:
1063:
1059:
1052:
1049:
1044:
1038:
1034:
1030:
1026:
1019:
1016:
1011:
1005:
997:
993:
989:
985:
978:
975:
972:
968:
962:
960:
956:
943:
939:
933:
930:
925:
921:
916:
911:
907:
903:
899:
895:
891:
884:
881:
876:
870:
862:
858:
853:
848:
844:
840:
836:
832:
828:
821:
818:
813:
809:
805:
801:
797:
793:
789:
782:
779:
774:
770:
766:
762:
758:
754:
750:
746:
739:
736:
731:
727:
723:
719:
716:(1): 157–60.
715:
711:
704:
701:
696:
690:
686:
685:
677:
674:
669:
665:
661:
657:
653:
649:
642:
639:
634:
630:
626:
622:
618:
614:
607:
604:
599:
595:
591:
587:
580:
577:
572:
568:
564:
560:
552:
549:
544:
540:
536:
532:
525:
522:
515:
513:
507:
503:
498:
492:
488:
480:
476:
472:
465:
463:
457:
453:
449:
444:
442:
421:
413:
405:
396:
388:
381:
370:
362:
353:
340:
317:
305:
304:
303:
301:
297:
289:
280:
273:
271:
269:
264:
262:
258:
254:
250:
246:
242:
238:
234:
230:
225:
221:
217:
215:
211:
206:
205:
200:
196:
192:
184:
182:
180:
176:
169:
164:
162:
160:
157:
154:
151:
147:
140:
136:
132:
128:
122:
118:
114:
110:
102:
89:
87:
85:
73:
65:
57:
53:
49:
48:sulfenic acid
45:
37:
32:
19:
2110:
2103:
2017:Vinyl halide
1974:
1904:Borinic acid
1899:Boronic acid
1876:Thioxanthate
1820:
1216:Hydrocarbons
1156:
1152:
1142:
1121:
1113:
1088:
1084:
1078:
1061:
1057:
1051:
1024:
1018:
1004:cite journal
987:
983:
977:
946:. Retrieved
941:
932:
897:
893:
883:
869:cite journal
834:
830:
820:
795:
791:
781:
756:
752:
748:
744:
738:
713:
709:
703:
683:
676:
651:
647:
641:
616:
612:
606:
589:
585:
579:
562:
558:
551:
534:
530:
524:
501:
499:
486:
484:
445:
438:
286:
265:
251:residues in
233:esomeprazole
213:
209:
202:
188:
173:
138:
126:
93:
47:
41:
2081:Thiocyanate
2076:Sulfonamide
2041:Perchlorate
2029:Acyl halide
1990:Fluoroethyl
1871:Thionoester
1759:Phosphonium
1744:Phosphinate
1739:Phosphonous
1727:Phosphonate
1426:Hydroperoxy
1248:Cyclopropyl
654:: 749–775.
485:The prefix
479:sulfenamide
452:active site
241:clopidogrel
237:ticlopidine
36:tautomerism
1985:Haloalkane
1856:Thioketone
1811:Persulfide
1707:Phosphorus
1672:Isocyanate
1662:Isonitrile
1563:or oxygen
1561:hydrogen,
1557:not being
1538:Orthoester
1431:Dioxiranes
1409:Enol ether
1297:1-Propenyl
516:References
288:Sulfoxides
259:-mediated
229:omeprazole
103:) to be CH
90:Properties
2118:inorganic
1952:Tellurium
1866:Thioester
1831:Sulfoxide
1816:Disulfide
1804:Sulfonium
1754:Phosphine
1732:Phosphite
1715:Phosphate
1647:Carbamate
1622:Hydrazone
1555:element,
1553:Only one
1526:Anhydride
1265:Methylene
414:−
382:−
375:⟶
363:−
318:−
296:mechanism
245:prasugrel
68:R−S(=O)OH
44:chemistry
2140:Category
2099:See also
2034:Chloride
1960:Tellurol
1914:Selenium
1881:Xanthate
1595:Ammonium
1577:Nitrogen
1559:carbon,
1516:Carboxyl
1483:Aldehyde
1471:Acryloyl
1453:carbonyl
1357:hydrogen
1312:Cumulene
1105:15096062
924:23748139
861:20845928
812:20869346
773:20225897
730:19040240
668:28226215
633:21049943
487:sulfenyl
422:′
371:′
249:cysteine
18:Sulfenyl
2123:organic
1922:Selenol
1846:Sulfone
1799:Sulfide
1697:NONOate
1692:Nitroso
1682:Nitrite
1677:Nitrate
1667:Cyanate
1657:Nitrile
1642:Amidine
1637:Imidate
1607:Nitrene
1602:Hydrazo
1590:Enamine
1521:Acetoxy
1509:carboxy
1476:Benzoyl
1414:Epoxide
1397:Methoxy
1387:Alcohol
1341:Carbene
1275:Methine
1159:: 244.
948:1 April
915:4184475
852:2990351
497:SCl.
458:. The
454:of the
448:ligands
300:alkenes
253:protein
220:allicin
179:enzymes
113:allicin
76:R−S(=O)
56:oxoacid
2022:Iodide
1942:Selone
1786:Sulfur
1495:Ketone
1488:Ketene
1466:Acetyl
1421:Peroxy
1392:Alkoxy
1382:Acetal
1363:oxygen
1352:carbon
1336:Alkyne
1329:Benzyl
1324:Phenyl
1307:Allene
1302:Crotyl
1282:Alkene
1270:Bridge
1258:Pentyl
1243:Propyl
1233:Methyl
1103:
1039:
990:: 29.
922:
912:
859:
849:
810:
771:
745:Allium
728:
691:
666:
631:
283:ageing
243:, and
216:-oxide
204:Allium
199:onions
195:garlic
191:alliin
117:garlic
70:) and
60:R−S−OH
50:is an
2054:Other
1891:Boron
1861:Thial
1794:Thiol
1687:Nitro
1652:Imide
1632:Amide
1617:Oxime
1612:Imine
1585:Amine
1533:Ester
1500:Ynone
1404:Ether
1375:R-O-R
1350:Only
1292:Allyl
1287:Vinyl
1253:Butyl
1238:Ethyl
1228:Alkyl
1118:IUPAC
257:redox
115:from
1976:Halo
1461:Acyl
1361:and
1319:Aryl
1101:PMID
1037:ISBN
1010:link
950:2019
920:PMID
898:1840
875:link
857:PMID
808:PMID
769:PMID
726:PMID
689:ISBN
664:PMID
629:PMID
493:, CH
54:and
46:, a
1627:Azo
1161:doi
1132:doi
1130:".
1093:doi
1066:doi
1029:doi
992:doi
967:doi
910:PMC
902:doi
847:PMC
839:doi
800:doi
796:507
761:doi
718:doi
656:doi
621:doi
617:132
594:doi
590:118
567:doi
563:119
539:doi
535:100
460:S=O
386:SOH
210:syn
42:In
2142::
1359:,
1354:,
1157:81
1155:.
1151:.
1120:,
1099:.
1089:37
1087:.
1062:11
1060:.
1035:.
1027:.
1006:}}
1002:{{
988:70
986:.
958:^
940:.
918:.
908:.
896:.
892:.
871:}}
867:{{
855:.
845:.
835:23
833:.
829:.
806:.
794:.
790:.
767:.
757:58
755:.
724:.
714:48
712:.
662:.
652:86
650:.
627:.
615:.
588:.
561:.
533:.
512:.
410:CH
393:CH
350:CH
337:CH
263:.
239:,
235:,
231:,
197:,
161:.
148:,
86:.
80:OH
1200:e
1193:t
1186:v
1169:.
1163::
1134::
1107:.
1095::
1072:.
1068::
1045:.
1031::
1012:)
998:.
994::
969::
952:.
926:.
904::
877:)
863:.
841::
814:.
802::
775:.
763::
749:S
732:.
720::
697:.
670:.
658::
635:.
623::
600:.
596::
573:.
569::
545:.
541::
510:2
495:3
419:R
406:=
397:2
389:+
378:R
368:R
354:2
341:2
332:)
329:O
326:(
322:S
314:R
294:i
292:E
214:S
159:H
156:O
153:O
150:R
142:a
139:K
130:a
127:K
125:p
105:3
99:(
96:3
78:2
74:(
66:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.