1490:
1124:
122:
173:
249:
1597:
1837:
1118:
31:
1659:
1363:
239:
profiles were evaluated and tested in an attempt to determine the optimal way to deliver the drug, which was especially important given the puzzling failure of an existing extended-release formulation of methylphenidate (Ritalin SR) to act as expected. The zero-order (flat) release profile that the
244:
that an ascending pattern of drug delivery was necessary to maintain clinical effect. Trials designed to test this hypothesis were successful, and ALZA subsequently developed a modified PPOP design that utilized an overcoat of methylphenidate designed to release immediately and rapidly raise serum
245:
levels, followed by 10 hours of first-order (ascending) drug delivery from the modified PPOP design. This design was called the Push-Stick
Osmotic Pump (PSOP), and utilized two separate drug layers with different concentrations of methylphenidate in addition to the (now quite robust) push layer.
151:
later developed the
Controlled-Porosity Osmotic Pump (CPOP) with the intention of addressing some of the issues that led to Osmosin's withdrawal via a new approach to the final stage of the release mechanism. Unlike the EOP, the CPOP had no pre-formed hole in the outer shell for the drug to be
34:
A 54 mg tablet of
Concerta, which uses OROS technology. 22% of the drug is contained in the red overcoat, while the remaining 78% is split between two drug layers of differing concentration. The tablet uses an additional push layer that expands as water enters the tablet via the osmotic
104:, and differing intestinal environments. Using an osmotic pump to deliver drugs has additional inherent advantages regarding control over drug delivery rates. This allows for much more precise drug delivery over an extended period of time, which results in much more predictable
240:
PPOP was optimal at delivering failed to maintain its efficacy over time, which suggested that acute tolerance to methylphenidate formed over the course of the day. This explained why
Ritalin SR was inferior to twice-daily Ritalin IR, and led to the
152:
expelled out of. Instead, the CPOP's semipermeable membrane was designed to form numerous small pores upon contact with water through which the drug would be expelled via osmotic pressure. The pores were formed via the use of a pH insensitive
108:. However, osmotic release systems are relatively complicated, somewhat difficult to manufacture, and may cause irritation or even blockage of the GI tract due to prolonged release of irritating drugs from the non-deformable tablet.
669:
van den Berg, G; van
Steveninck, F; Gubbens-Stibbe, JM; Schoemaker, HC; de Boer, AG; Cohen, AF (1990). "Influence of food on the bioavailability of metoprolol from an OROS system; a study in healthy volunteers".
1035:
802:
Auiler, JF; Liu, K; Lynch, JM; Gelotte, CK (2002). "Effect of food on early drug exposure from extended-release stimulants: results from the
Concerta, Adderall XR Food Evaluation (CAFE) Study".
1028:
978:"Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies"
133:
in 1974, and was the first practical example of an osmotic pump based drug release system for oral use. It was introduced to the market in the early 1980s in
Osmosin (
759:
Modi, NB; Wang, B; Hu, WT; Gupta, SK (January 2000). "Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects".
1021:
532:
Conley, R; Gupta, SK; Sathyan, G (October 2006). "Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form".
235:
required multiple doses to be administered each day to attain long-lasting coverage, which made it an ideal candidate for the OROS technology. Multiple candidate
96:
Osmotic release systems have a number of major advantages over other controlled-release mechanisms. They are significantly less affected by factors such as
716:
Bass, DM; Prevo, M; Waxman, DS (2002). "Gastrointestinal safety of an extended-release, nondeformable, oral dosage form (OROS: a retrospective study".
228:
216:
438:
Malaterre, V; Ogorka, J; Loggia, N; Gurny, R (November 2009). "Oral osmotically driven systems: 30 years of development and clinical use".
1392:
1975:
208:. The initial design developed in 1982 by ALZA researchers was designated the Push-Pull Osmotic Pump (PPOP), and Procardia XL (
1980:
1473:
772:
1489:
1570:
1413:
1944:
1906:
1249:
1065:
142:
1858:
101:
1886:
1455:
192:
polymer for suspension of poorly soluble drugs) out of the exit hole at a controlled rate. Osmotic agents such as
2000:
1934:
1863:
1450:
1397:
1231:
1853:
1227:
478:
Theeuwes, F; Yum, SI; Haak, R; Wong, P (1991). "Systems for triggered, pulsed, and programmed drug delivery".
1881:
1924:
1891:
1690:
1522:
1254:
1175:
1102:
1044:
184:
drugs. This led to the development of an additional internal "push layer" composed of material (a swellable
180:
Both the EOP and CPOP were relatively simple designs, and were limited by their inability to deliver poorly
157:
56:
44:
1919:
1873:
1815:
1721:
1588:
1195:
1069:
880:
Eckenhoff, B; Yum, SI (April 1981). "The osmotic pump: novel research tool for optimizing drug regimens".
76:
60:
976:
Swanson, J; Gupta, S; Lam, A; Shoulson, I; Lerner, M; Modi, N; Lindemulder, E; Wigal, S (February 2003).
219:
An animation illustrating the exterior/interior compositions of a tablet of
Concerta, a PSOP OROS design.
1995:
1985:
1929:
1810:
1223:
1990:
1774:
1619:
1537:
1482:
1402:
1387:
1190:
487:
153:
1827:
1564:
1445:
1440:
1160:
1056:
267:
138:
121:
1123:
172:
1901:
1695:
1650:
1347:
1092:
827:
784:
741:
695:
651:
557:
511:
197:
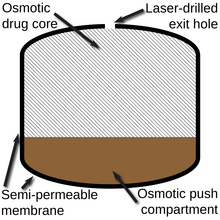
188:) that would expand as it absorbed water, which then pushed the drug layer (which incorporates a
35:
membrane. The drug is expelled via the laser-drilled hole visible on the left side of the tablet.
1751:
1746:
1584:
1325:
1087:
999:
928:
897:
862:
819:
776:
733:
687:
643:
605:
549:
503:
455:
252:
An illustration of the different inner components of a tablet of
Concerta, a PSOP OROS design.
48:
1685:
989:
920:
889:
854:
811:
768:
725:
679:
635:
595:
541:
495:
447:
335:
236:
205:
105:
80:
68:
1575:
381:
339:
331:
327:
323:
295:
224:
223:
In the early 1990s, an ALZA-funded research program began to develop a new dosage form of
193:
1013:
626:
Verma, RK; Mishra, B; Garg, S (July 2000). "Osmotically controlled oral drug delivery".
491:
1636:
1596:
1269:
499:
215:
148:
52:
248:
1969:
1845:
1779:
1701:
1670:
1435:
1264:
893:
729:
388:
346:
1836:
831:
788:
745:
655:
561:
515:
1820:
1756:
1741:
1716:
1554:
1274:
1117:
699:
360:
134:
30:
1914:
1802:
1675:
1631:
1609:
1527:
1497:
1431:
1280:
1219:
1048:
815:
451:
1798:
1769:
1329:
1303:
1165:
994:
977:
947:
924:
395:
374:
316:
309:
274:
241:
209:
181:
545:
1408:
1298:
1150:
1140:
353:
302:
288:
232:
189:
1658:
1003:
858:
823:
780:
737:
647:
609:
553:
459:
125:
An illustration of the different components of the
Elementary Osmotic Pump.
932:
901:
691:
639:
507:
176:
An illustration of the different components of the Push-Pull Osmotic Pump.
1950:
1939:
1731:
1711:
1706:
1680:
1624:
1517:
1507:
1502:
1259:
1205:
1170:
1155:
1097:
367:
281:
161:
1736:
1665:
1604:
1549:
1544:
1512:
1352:
1308:
1185:
866:
683:
201:
185:
64:
1726:
1646:
1559:
1379:
1290:
1180:
1145:
1132:
600:
583:
17:
1362:
773:
10.1002/1099-081x(200001)21:1<23::aid-bdd212>3.0.co;2-v
584:"Osmotically controlled drug delivery system with associated drugs"
83:, which pioneered the use of osmotic pumps for oral drug delivery.
1614:
1339:
1241:
1200:
1079:
247:
214:
171:
141:), but unexpectedly severe issues with GI irritation and cases of
120:
29:
915:
Heimlich, KR (1983). "The evolution of precision drug delivery".
130:
75:
through the laser drilled opening(s) in the tablet and into the
72:
1017:
1478:
1423:
1657:
1595:
1488:
582:
Gupta, BP; Thakur, N; Jain, NP; Banweer, J; Jain, S (2010).
204:
are added to both the drug and push layers to increase the
97:
51:
with a semi-permeable outer membrane and one or more small
212:) was one of the first drugs to utilize this PPOP design.
845:
Theeuwes, F (December 1975). "Elementary osmotic pump".
440:
European Journal of Pharmaceutics and Biopharmaceutics
41:
osmotic-controlled release oral delivery system (OROS)
27:
Advanced controlled release oral drug delivery system
1900:
1872:
1844:
1797:
1764:
1645:
1583:
1536:
1472:
1422:
1378:
1338:
1324:
1289:
1240:
1218:
1131:
1078:
1064:
1055:
129:The Elementary Osmotic Pump (EOP) was developed by
588:Journal of Pharmacy & Pharmaceutical Sciences
47:oral drug delivery system in the form of a rigid
971:
969:
433:
711:
709:
621:
619:
577:
575:
573:
571:
527:
525:
431:
429:
427:
425:
423:
421:
419:
417:
415:
413:
1029:
473:
471:
469:
8:
954:. United States Patent and Trademark Office
1794:
1335:
1237:
1075:
1061:
1036:
1022:
1014:
480:Annals of the New York Academy of Sciences
993:
672:European Journal of Clinical Pharmacology
599:
628:Drug Development and Industrial Pharmacy
229:attention deficit hyperactivity disorder
761:Biopharmaceutics & Drug Disposition
409:
79:. OROS is a trademarked name owned by
7:
917:Current Medical Research and Opinion
804:Current Medical Research and Opinion
534:Current Medical Research and Opinion
1393:Heated humidified high-flow therapy
227:for the treatment of children with
948:"Controlled porosity osmotic pump"
847:Journal of Pharmaceutical Sciences
500:10.1111/j.1749-6632.1991.tb27262.x
145:led to the withdrawal of Osmosin.
25:
946:Haslam, John L.; Rork, Gerald S.
1835:
1361:
1122:
1116:
730:10.2165/00002018-200225140-00004
231:(ADHD). Methylphenidate's short
59:, water is absorbed through the
982:Archives of General Psychiatry
1:
1161:Effervescent powder or tablet
1945:Patient-controlled analgesia
1250:Orally disintegrating tablet
894:10.1016/0142-9612(81)90005-3
112:Oral osmotic release systems
71:is used to push the active
55:holes in it. As the tablet
2017:
1456:Relative analgesia machine
816:10.1185/030079902125000840
452:10.1016/j.ejpb.2009.07.002
261:OROS medications include:
1833:
1359:
1114:
995:10.1001/archpsyc.60.2.204
925:10.1185/03007998309109821
1045:Routes of administration
546:10.1185/030079906x132613
308:Ditropan XL/Lyrinel XL (
257:List of OROS medications
1976:Pharmaceutical industry
1571:Extra-amniotic infusion
1176:Molecular encapsulation
1108:Osmotic delivery system
1103:Time release technology
57:passes through the body
1874:Central nervous system
1662:
1600:
1528:Mucoadhesive microdisc
1493:
859:10.1002/jps.2600641218
253:
220:
177:
126:
77:gastrointestinal tract
61:semipermeable membrane
36:
1981:Drug delivery devices
1717:Transfersome vesicles
1661:
1599:
1576:Intravesical infusion
1492:
640:10.1081/ddc-100101287
251:
218:
175:
124:
33:
1403:Metered-dose inhaler
1388:Anesthetic vaporizer
919:. 8 Suppl 2: 28–37.
67:, and the resulting
1828:Transdermal implant
1788:(into tissue/blood)
1565:Intrauterine device
1446:Anaesthetic machine
1441:Oxygen concentrator
492:1991NYASA.618..428T
268:phenylpropanolamine
139:phenylpropanolamine
1940:Nanocell injection
1663:
1601:
1494:
1348:Dry-powder inhaler
684:10.1007/bf00315121
254:
221:
198:potassium chloride
178:
127:
45:controlled release
37:
1963:
1962:
1959:
1958:
1790:
1752:Transdermal spray
1747:Transdermal patch
1737:Medicated shampoo
1468:
1467:
1464:
1463:
1451:Medical inhalants
1398:Medical inhalants
1326:Respiratory tract
1320:
1319:
1214:
1213:
345:Exalgo/Jurnista (
160:additive such as
16:(Redirected from
2008:
2001:Pharmacokinetics
1839:
1795:
1786:
1373:
1369:
1365:
1336:
1265:Sublingual drops
1238:
1126:
1120:
1076:
1062:
1038:
1031:
1024:
1015:
1008:
1007:
997:
973:
964:
963:
961:
959:
943:
937:
936:
912:
906:
905:
877:
871:
870:
842:
836:
835:
799:
793:
792:
756:
750:
749:
713:
704:
703:
666:
660:
659:
623:
614:
613:
603:
579:
566:
565:
529:
520:
519:
475:
464:
463:
435:
336:chlorpheniramine
206:osmotic pressure
106:pharmacokinetics
81:ALZA Corporation
69:osmotic pressure
21:
2016:
2015:
2011:
2010:
2009:
2007:
2006:
2005:
1966:
1965:
1964:
1955:
1935:Intraperitoneal
1907:musculoskeletal
1905:
1896:
1868:
1864:Intra-articular
1840:
1831:
1791:
1785:
1784:
1760:
1641:
1579:
1532:
1477:
1460:
1418:
1374:
1371:
1370:
1367:
1366:
1357:
1316:
1285:
1210:
1127:
1121:
1112:
1066:Digestive tract
1051:
1042:
1012:
1011:
975:
974:
967:
957:
955:
945:
944:
940:
914:
913:
909:
879:
878:
874:
853:(12): 1987–91.
844:
843:
839:
801:
800:
796:
758:
757:
753:
724:(14): 1021–33.
715:
714:
707:
668:
667:
663:
625:
624:
617:
601:10.18433/j38w25
581:
580:
569:
540:(10): 1879–92.
531:
530:
523:
477:
476:
467:
437:
436:
411:
406:
401:
382:pseudoephedrine
340:pseudoephedrine
332:pseudoephedrine
328:brompheniramine
324:pseudoephedrine
296:methylphenidate
259:
237:pharmacokinetic
225:methylphenidate
194:sodium chloride
170:
149:Merck & Co.
137:) and Acutrim (
119:
114:
100:, food intake,
94:
89:
43:is an advanced
28:
23:
22:
15:
12:
11:
5:
2014:
2012:
2004:
2003:
1998:
1993:
1988:
1983:
1978:
1968:
1967:
1961:
1960:
1957:
1956:
1954:
1953:
1948:
1942:
1937:
1932:
1927:
1922:
1917:
1911:
1909:
1898:
1897:
1895:
1894:
1889:
1884:
1878:
1876:
1870:
1869:
1867:
1866:
1861:
1856:
1854:Intracavernous
1850:
1848:
1842:
1841:
1834:
1832:
1830:
1825:
1824:
1823:
1813:
1808:
1806:
1792:
1783:
1782:
1777:
1772:
1766:
1765:
1762:
1761:
1759:
1754:
1749:
1744:
1739:
1734:
1729:
1724:
1719:
1714:
1709:
1704:
1699:
1693:
1688:
1683:
1678:
1673:
1668:
1656:
1654:
1643:
1642:
1640:
1639:
1637:Nutrient enema
1634:
1629:
1628:
1627:
1622:
1612:
1607:
1594:
1592:
1581:
1580:
1578:
1573:
1568:
1562:
1557:
1552:
1547:
1542:
1540:
1534:
1533:
1531:
1530:
1525:
1520:
1515:
1510:
1505:
1500:
1487:
1485:
1470:
1469:
1466:
1465:
1462:
1461:
1459:
1458:
1453:
1448:
1443:
1438:
1428:
1426:
1420:
1419:
1417:
1416:
1411:
1406:
1400:
1395:
1390:
1384:
1382:
1376:
1375:
1360:
1358:
1356:
1355:
1350:
1344:
1342:
1333:
1322:
1321:
1318:
1317:
1315:
1314:
1311:
1306:
1301:
1295:
1293:
1287:
1286:
1284:
1283:
1278:
1272:
1267:
1262:
1257:
1252:
1246:
1244:
1235:
1216:
1215:
1212:
1211:
1209:
1208:
1203:
1198:
1193:
1188:
1183:
1178:
1173:
1168:
1163:
1158:
1153:
1148:
1143:
1137:
1135:
1129:
1128:
1115:
1113:
1111:
1110:
1105:
1100:
1095:
1090:
1084:
1082:
1073:
1059:
1053:
1052:
1043:
1041:
1040:
1033:
1026:
1018:
1010:
1009:
965:
952:Google Patents
938:
907:
872:
837:
794:
751:
705:
661:
634:(7): 695–708.
615:
567:
521:
465:
408:
407:
405:
402:
400:
399:
392:
385:
378:
373:Procardia XL (
371:
366:Minipress XL (
364:
357:
352:Glucotrol XL (
350:
343:
320:
313:
306:
299:
292:
285:
278:
271:
263:
258:
255:
169:
166:
143:GI perforation
118:
115:
113:
110:
93:
90:
88:
85:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
2013:
2002:
1999:
1997:
1994:
1992:
1989:
1987:
1984:
1982:
1979:
1977:
1974:
1973:
1971:
1952:
1949:
1946:
1943:
1941:
1938:
1936:
1933:
1931:
1928:
1926:
1925:Intramuscular
1923:
1921:
1918:
1916:
1913:
1912:
1910:
1908:
1903:
1899:
1893:
1890:
1888:
1885:
1883:
1882:Intracerebral
1880:
1879:
1877:
1875:
1871:
1865:
1862:
1860:
1857:
1855:
1852:
1851:
1849:
1847:
1843:
1838:
1829:
1826:
1822:
1819:
1818:
1817:
1814:
1812:
1809:
1807:
1804:
1800:
1796:
1793:
1789:
1781:
1778:
1776:
1773:
1771:
1768:
1767:
1763:
1758:
1755:
1753:
1750:
1748:
1745:
1743:
1740:
1738:
1735:
1733:
1730:
1728:
1725:
1723:
1720:
1718:
1715:
1713:
1710:
1708:
1705:
1703:
1702:Iontophoresis
1700:
1697:
1694:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1671:Topical cream
1669:
1667:
1664:
1660:
1655:
1652:
1648:
1644:
1638:
1635:
1633:
1630:
1626:
1623:
1621:
1618:
1617:
1616:
1613:
1611:
1608:
1606:
1603:
1602:
1598:
1593:
1590:
1586:
1582:
1577:
1574:
1572:
1569:
1566:
1563:
1561:
1558:
1556:
1553:
1551:
1548:
1546:
1543:
1541:
1539:
1535:
1529:
1526:
1524:
1521:
1519:
1516:
1514:
1511:
1509:
1506:
1504:
1501:
1499:
1496:
1495:
1491:
1486:
1484:
1480:
1475:
1471:
1457:
1454:
1452:
1449:
1447:
1444:
1442:
1439:
1437:
1436:Nasal cannula
1433:
1430:
1429:
1427:
1425:
1421:
1415:
1412:
1410:
1407:
1404:
1401:
1399:
1396:
1394:
1391:
1389:
1386:
1385:
1383:
1381:
1377:
1364:
1354:
1351:
1349:
1346:
1345:
1343:
1341:
1337:
1334:
1331:
1327:
1323:
1312:
1310:
1307:
1305:
1302:
1300:
1297:
1296:
1294:
1292:
1288:
1282:
1279:
1276:
1273:
1271:
1268:
1266:
1263:
1261:
1258:
1256:
1253:
1251:
1248:
1247:
1245:
1243:
1239:
1236:
1233:
1229:
1225:
1221:
1217:
1207:
1204:
1202:
1199:
1197:
1194:
1192:
1189:
1187:
1184:
1182:
1179:
1177:
1174:
1172:
1169:
1167:
1164:
1162:
1159:
1157:
1154:
1152:
1149:
1147:
1144:
1142:
1139:
1138:
1136:
1134:
1130:
1125:
1119:
1109:
1106:
1104:
1101:
1099:
1096:
1094:
1091:
1089:
1086:
1085:
1083:
1081:
1077:
1074:
1071:
1067:
1063:
1060:
1058:
1054:
1050:
1046:
1039:
1034:
1032:
1027:
1025:
1020:
1019:
1016:
1005:
1001:
996:
991:
988:(2): 204–11.
987:
983:
979:
972:
970:
966:
953:
949:
942:
939:
934:
930:
926:
922:
918:
911:
908:
903:
899:
895:
891:
887:
883:
876:
873:
868:
864:
860:
856:
852:
848:
841:
838:
833:
829:
825:
821:
817:
813:
809:
805:
798:
795:
790:
786:
782:
778:
774:
770:
766:
762:
755:
752:
747:
743:
739:
735:
731:
727:
723:
719:
712:
710:
706:
701:
697:
693:
689:
685:
681:
677:
673:
665:
662:
657:
653:
649:
645:
641:
637:
633:
629:
622:
620:
616:
611:
607:
602:
597:
594:(4): 571–88.
593:
589:
585:
578:
576:
574:
572:
568:
563:
559:
555:
551:
547:
543:
539:
535:
528:
526:
522:
517:
513:
509:
505:
501:
497:
493:
489:
486:(1): 428–40.
485:
481:
474:
472:
470:
466:
461:
457:
453:
449:
446:(3): 311–23.
445:
441:
434:
432:
430:
428:
426:
424:
422:
420:
418:
416:
414:
410:
403:
397:
393:
390:
389:carbamazepine
387:Tegretol XR (
386:
383:
379:
376:
372:
369:
365:
362:
358:
355:
351:
348:
347:hydromorphone
344:
341:
337:
333:
329:
325:
321:
318:
315:Dynacirc CR (
314:
311:
307:
304:
300:
297:
293:
290:
286:
283:
279:
276:
273:Adalat OROS (
272:
269:
265:
264:
262:
256:
250:
246:
243:
238:
234:
230:
226:
217:
213:
211:
207:
203:
199:
195:
191:
187:
183:
174:
167:
165:
163:
159:
155:
150:
146:
144:
140:
136:
132:
123:
116:
111:
109:
107:
103:
99:
92:Pros and cons
91:
86:
84:
82:
78:
74:
70:
66:
62:
58:
54:
53:laser drilled
50:
46:
42:
32:
19:
1996:Pharmacology
1986:Dosage forms
1930:Intraosseous
1920:Intracardiac
1859:Intravitreal
1821:Injector pen
1816:Subcutaneous
1787:
1757:Jet injector
1742:Dermal patch
1555:Vaginal ring
1523:Insufflation
1275:Effervescent
1107:
1049:dosage forms
985:
981:
956:. Retrieved
951:
941:
916:
910:
888:(2): 89–97.
885:
882:Biomaterials
881:
875:
850:
846:
840:
810:(5): 311–6.
807:
803:
797:
767:(1): 23–31.
764:
760:
754:
721:
717:
678:(3): 315–6.
675:
671:
664:
631:
627:
591:
587:
537:
533:
483:
479:
443:
439:
380:Sudafed 24 (
361:paliperidone
287:Cardura XL (
280:Alpress LP (
260:
222:
179:
147:
135:indomethacin
128:
117:Single-layer
95:
40:
38:
1991:Alza brands
1915:Intravenous
1902:Circulatory
1887:Intrathecal
1811:Intradermal
1803:transdermal
1770:Parenterals
1676:Topical gel
1632:Murphy drip
1610:Suppository
1498:Nasal spray
1432:Oxygen mask
1281:Chewing gum
1220:Oral mucosa
718:Drug Safety
322:Efidac 24 (
301:Covera HS (
168:Multi-layer
158:dissolvable
102:GI motility
1970:Categories
1775:Injections
1538:Urogenital
1474:Ophthalmic
1330:inhalation
1304:Toothpaste
1232:sublingual
1196:Suspension
1166:Herbal tea
404:References
396:salbutamol
375:nifedipine
317:isradipine
310:oxybutynin
294:Concerta (
275:nifedipine
242:hypothesis
210:nifedipine
1780:infusions
1712:Liposomes
1508:Eye drops
1503:Ear drops
1414:Vaporizer
1409:Nebulizer
1299:Mouthwash
1228:sublabial
1151:Electuary
1141:Decoction
354:glipizide
303:verapamil
289:doxazosin
266:Acutrim (
233:half-life
154:leachable
87:Rationale
1951:PIC line
1892:Epidural
1732:Lip balm
1707:Hydrogel
1698:solution
1681:Liniment
1666:Ointment
1625:Hydrogel
1620:Solution
1605:Ointment
1545:Ointment
1518:Hydrogel
1513:Ointment
1309:Ointment
1270:Lozenges
1260:Lollipop
1206:Tincture
1191:Solution
1171:Hydrogel
1156:Emulsion
1098:Pastille
1004:12578439
958:19 March
832:25994524
824:12240794
789:33413277
781:11038435
746:35424637
738:12408733
656:35670161
648:10872087
610:21486532
562:42490425
554:17022845
516:31442663
460:19602438
394:Volmax (
368:prazosin
359:Invega (
282:prazosin
162:sorbitol
1651:topical
1589:enteral
1550:Pessary
1380:Liquids
1353:Smoking
1291:Liquids
1186:Softgel
1133:Liquids
1093:Capsule
1070:enteral
933:6851623
902:7248427
700:1838636
692:2257873
508:2006800
488:Bibcode
202:xylitol
190:viscous
186:polymer
182:soluble
65:osmosis
1846:Organs
1727:Lotion
1647:Dermal
1585:Rectal
1560:Douche
1340:Solids
1277:tablet
1242:Solids
1224:buccal
1181:Powder
1146:Elixir
1088:Tablet
1080:Solids
1002:
931:
900:
865:
830:
822:
787:
779:
744:
736:
698:
690:
654:
646:
608:
560:
552:
514:
506:
458:
49:tablet
1722:Cream
1686:Paste
1615:Enema
1567:(IUD)
1483:nasal
1405:(MDI)
1313:Spray
1201:Syrup
828:S2CID
785:S2CID
742:S2CID
696:S2CID
652:S2CID
558:S2CID
512:S2CID
200:, or
1947:pump
1799:Skin
1696:DMSO
1691:Film
1479:otic
1434:and
1255:Film
1057:Oral
1000:PMID
960:2016
929:PMID
898:PMID
867:1510
863:PMID
820:PMID
777:PMID
734:PMID
688:PMID
644:PMID
606:PMID
550:PMID
504:PMID
456:PMID
131:ALZA
73:drug
63:via
39:The
18:OROS
1424:Gas
990:doi
921:doi
890:doi
855:doi
812:doi
769:doi
726:doi
680:doi
636:doi
596:doi
542:doi
496:doi
484:618
448:doi
156:or
1972::
1481:,
1230:,
1226:,
1047:,
998:.
986:60
984:.
980:.
968:^
950:.
927:.
896:.
884:.
861:.
851:64
849:.
826:.
818:.
808:18
806:.
783:.
775:.
765:21
763:.
740:.
732:.
722:25
720:.
708:^
694:.
686:.
676:39
674:.
650:.
642:.
632:26
630:.
618:^
604:.
592:13
590:.
586:.
570:^
556:.
548:.
538:22
536:.
524:^
510:.
502:.
494:.
482:.
468:^
454:.
444:73
442:.
412:^
338:,
330:,
196:,
164:.
98:pH
1904:,
1805:)
1801:(
1653:)
1649:(
1591:)
1587:(
1476:,
1372:0
1368:0
1332:)
1328:(
1234:)
1222:(
1072:)
1068:(
1037:e
1030:t
1023:v
1006:.
992::
962:.
935:.
923::
904:.
892::
886:2
869:.
857::
834:.
814::
791:.
771::
748:.
728::
702:.
682::
658:.
638::
612:.
598::
564:.
544::
518:.
498::
490::
462:.
450::
398:)
391:)
384:)
377:)
370:)
363:)
356:)
349:)
342:)
334:/
326:/
319:)
312:)
305:)
298:)
291:)
284:)
277:)
270:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.