169:
157:
146:
119:
103:. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was originally discovered by Desai, McKelvie and Ramirez. The phosphine can be partially substituted by zinc dust, which can improve yields and simplify product separation. The second step of the reaction to convert dibromoolefins to alkynes is known as
164:
The second part of the reaction converts the isolable gem-dibromoalkene intermediate to the alkyne. Deuterium-labelling studies show that this step proceeds through a carbene mechanism. Lithium-Bromide exchange is followed by α-elimination to afford the carbene. 1,2-shift then affords the
168:
4000:
156:
3116:
713:
104:
3061:
3829:
3171:
145:
246:
3321:
1955:
4149:
4050:
3824:
2926:
1650:
2696:
847:
3496:
1440:
3591:
1695:
3571:
3066:
2233:
2114:
1670:
3661:
187:
165:
deuterium-labelled terminal alkyne. The 50% H-incorporation could be explained by deprotonation of the (acidic) terminal deuterium with excess BuLi.
379:
3416:
883:
126:
By suitable choice of base, it is often possible to stop the reaction at the 1-bromoalkyne, a useful functional group for further transformation.
2248:
3894:
3351:
3844:
3456:
3436:
3396:
2203:
3990:
3915:
3799:
2411:
1815:
1146:
3985:
3814:
3471:
3326:
2956:
70:
2801:
2038:
3161:
2651:
2326:
4065:
3849:
2871:
3426:
4060:
3774:
3636:
3391:
794:
3950:
3421:
3336:
3306:
3286:
3151:
3146:
2521:
2446:
2089:
2043:
1910:
1171:
723:
3889:
428:
230:
4055:
4015:
3965:
3441:
3191:
3121:
1610:
3651:
3256:
2149:
1870:
153:
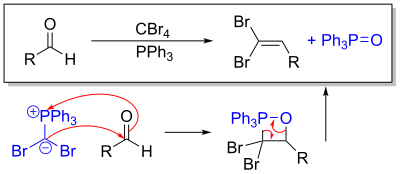
This ylide undergoes a Wittig reaction when exposed to an aldehyde. Alternatively, using a ketone generates a gem-dibromoalkene.
107:. The overall combined transformation of an aldehyde to an alkyne by this method is named after its developers, American chemists
4154:
3641:
1181:
363:
339:
3809:
3566:
3516:
2306:
2238:
2129:
1705:
1460:
1385:
1166:
804:
4144:
3521:
3331:
2806:
2716:
840:
118:
4095:
3879:
3819:
3221:
3196:
3106:
2686:
2566:
1600:
1096:
3980:
3466:
3261:
1530:
4085:
3671:
3181:
2691:
2636:
2481:
2441:
2273:
2028:
1745:
1595:
262:"Synthetic and Mechanistic Investigations on the Rearrangement of 2,3-Unsaturated 1,4-Bis(alkylidene)carbenes to Enediynes"
4045:
3606:
3561:
3051:
2906:
192:
4080:
3995:
3854:
3769:
3666:
2741:
2396:
2064:
1475:
1036:
3970:
3945:
3930:
3626:
3491:
3446:
3211:
2756:
2606:
1820:
1500:
1445:
3975:
3920:
3451:
2866:
2581:
2576:
2069:
1885:
1875:
1590:
1450:
1400:
1395:
1370:
1276:
4030:
3631:
3551:
3166:
3131:
2976:
2401:
2361:
2258:
2033:
1785:
1730:
1330:
1041:
1031:
1006:
4005:
3706:
3511:
2946:
2511:
2486:
2426:
2018:
1725:
1375:
1560:
3296:
2831:
2283:
1505:
1470:
1066:
1001:
833:
63:
3864:
3486:
2546:
2471:
1995:
1830:
1515:
1291:
1251:
996:
789:
4105:
4010:
3744:
3716:
3686:
3601:
3531:
3461:
3381:
3281:
3241:
2936:
2556:
1855:
1850:
1312:
1176:
4070:
3940:
3804:
3646:
3506:
2000:
1550:
1261:
784:
3960:
3556:
3526:
3401:
3356:
3186:
3096:
2911:
2901:
2731:
2288:
2228:
2193:
1980:
1940:
1715:
1585:
1101:
1091:
1021:
3536:
2516:
1540:
1086:
966:
3749:
55:
4159:
4040:
3899:
3691:
3616:
3596:
3316:
3266:
3126:
3091:
3031:
2961:
2263:
2243:
1975:
1895:
1790:
1750:
1720:
1655:
1525:
1435:
1425:
1301:
1011:
3779:
3501:
3251:
3231:
3206:
3156:
3071:
3046:
3001:
2971:
2951:
2921:
2886:
2841:
2816:
2791:
2676:
2601:
2381:
2074:
2010:
1810:
1535:
1455:
1141:
1116:
893:
888:
4115:
2861:
1485:
3701:
3656:
3371:
3341:
3311:
3246:
3226:
3141:
3136:
3101:
3056:
3041:
3036:
3016:
3006:
2941:
2931:
2811:
2331:
2134:
1710:
1665:
1495:
1231:
951:
913:
421:
39:
1161:
1156:
3754:
3884:
3834:
3784:
3764:
3611:
3586:
3301:
3291:
3176:
2991:
2986:
2916:
2701:
2501:
2461:
2391:
2356:
2311:
2278:
2144:
2119:
2099:
1920:
1880:
1840:
1805:
1735:
1490:
1360:
1335:
873:
799:
779:
677:
172:
Deuterium-labelling shows the involvement of carbenes in the second part of the Corey-Fuchs reaction.
112:
4100:
4090:
4075:
3721:
3696:
3681:
3676:
3406:
3361:
3346:
3236:
3216:
3111:
2996:
2981:
2826:
2771:
2761:
2751:
2726:
2491:
2366:
2341:
2253:
2109:
2094:
2079:
1935:
1900:
1845:
1615:
1465:
1410:
1281:
1196:
1056:
981:
704:
139:
1126:
3839:
3789:
3759:
3621:
3411:
3201:
3086:
3021:
3011:
2776:
2706:
2671:
2666:
2646:
2641:
2586:
2496:
2346:
2208:
2198:
2104:
1890:
1835:
1765:
1685:
1580:
1480:
1415:
1340:
1186:
1051:
986:
814:
774:
754:
240:
971:
3576:
2896:
2781:
2746:
2711:
2656:
2611:
2571:
2526:
2506:
2456:
2451:
2421:
2406:
2316:
2223:
2159:
2124:
1950:
1825:
1700:
1625:
1605:
1520:
1355:
1350:
1296:
1206:
1111:
1071:
1026:
908:
903:
868:
764:
355:
331:
304:
281:
226:
108:
92:
27:
4110:
3955:
3925:
3869:
3794:
3726:
3481:
3431:
3276:
3081:
2856:
2851:
2796:
2786:
2561:
2371:
2351:
2321:
2218:
2154:
2139:
1970:
1925:
1915:
1905:
1800:
1780:
1775:
1760:
1755:
1635:
1630:
1570:
1555:
1545:
1390:
1380:
1246:
1236:
1226:
1136:
1131:
1106:
1046:
898:
857:
414:
384:
316:
273:
4020:
3711:
3546:
3541:
2836:
2821:
2766:
2721:
2681:
2631:
2596:
2591:
2536:
2531:
2466:
2416:
2336:
2164:
2048:
2023:
1985:
1960:
1945:
1930:
1865:
1740:
1690:
1680:
1660:
1620:
1430:
1420:
1405:
1201:
1121:
946:
941:
809:
682:
367:
343:
261:
197:
135:
991:
961:
4025:
3935:
3874:
2966:
2876:
2846:
2621:
2476:
2213:
1990:
1860:
1675:
1645:
1345:
1241:
1016:
878:
182:
360:
336:
320:
4138:
4035:
3736:
3581:
3476:
3271:
2661:
2626:
2616:
2551:
2541:
2431:
2268:
2084:
1795:
1770:
1640:
1286:
1271:
1256:
1151:
1081:
1061:
976:
759:
744:
3076:
2436:
2188:
1965:
1565:
1365:
1216:
1211:
1076:
931:
769:
749:
708:
1575:
1221:
1191:
956:
3859:
3386:
2736:
825:
695:
260:
Sahu, Bichismita; Muruganantham, Rajendran; Namboothiri, Irishi N. N. (2007).
160:
Step 2 in the Corey-Fuchs reaction, doing the Wittig to make the dibromoalkene
285:
739:
699:
533:
528:
448:
353:
Marshall, J. A.; Yanik, M. M.; Adams, N. D.; Ellis, K. C.; Chobanian, H. R.
277:
1266:
936:
659:
654:
613:
608:
567:
562:
557:
504:
499:
401:
222:
96:
388:
926:
574:
511:
465:
690:
686:
637:
620:
591:
540:
482:
437:
100:
1310:
829:
410:
149:
Step 1 of the Corey-Fuchs reaction, generating the active ylide
219:
Strategic
Applications of Named Reactions in Organic Synthesis
167:
155:
144:
138:, where two equivalents of triphenylphosphine are used with
117:
142:
to produce the triphenylphosphine-dibromomethylene ylide.
134:
The Corey–Fuchs reaction is based on a special case of the
406:
217:
Kurti 1 Czako 2, Laszlo 1 Barbara 2 (15 September 2005).
4001:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
3908:
3735:
3370:
2885:
2380:
2297:
2177:
2057:
2009:
1319:
732:
670:
3062:Divinylcyclopropane-cycloheptadiene rearrangement
3322:Thermal rearrangement of aromatic hydrocarbons
1956:Thermal rearrangement of aromatic hydrocarbons
4051:Lectka enantioselective beta-lactam synthesis
841:
422:
8:
3830:Inverse electron-demand Diels–Alder reaction
1651:Heterogeneous metal catalyzed cross-coupling
3172:Lobry de Bruyn–Van Ekenstein transformation
245:: CS1 maint: numeric names: authors list (
3732:
2006:
1307:
848:
834:
826:
429:
415:
407:
15:
3662:Petrenko-Kritschenko piperidone synthesis
3117:Fritsch–Buttenberg–Wiechell rearrangement
714:Fritsch–Buttenberg–Wiechell rearrangement
188:Fritsch-Buttenberg-Wiechell rearrangement
105:Fritsch–Buttenberg–Wiechell rearrangement
3825:Intramolecular Diels–Alder cycloaddition
647:
643:
630:
626:
601:
597:
584:
580:
550:
546:
521:
517:
492:
488:
475:
471:
458:
454:
209:
3845:Metal-centered cycloaddition reactions
3497:Debus–Radziszewski imidazole synthesis
1441:Bodroux–Chichibabin aldehyde synthesis
329:Mori, M.; Tonogaki, K.; Kinoshita, A.
238:
3991:Diazoalkane 1,3-dipolar cycloaddition
3895:Vinylcyclopropane (5+2) cycloaddition
3800:Diazoalkane 1,3-dipolar cycloaddition
3572:Hurd–Mori 1,2,3-thiadiazole synthesis
3067:Dowd–Beckwith ring-expansion reaction
2234:Hurd–Mori 1,2,3-thiadiazole synthesis
1147:LFER solvent coefficients (data page)
383:, Vol. 84, p. 1745-1747 (1962).
377:N. B. Desai, N. McKelvie, F. Ramirez
266:European Journal of Organic Chemistry
7:
4150:Carbon-carbon bond forming reactions
2802:Sharpless asymmetric dihydroxylation
2039:Methoxymethylenetriphenylphosphorane
2927:Allen–Millar–Trippett rearrangement
4066:Nitrone-olefin (3+2) cycloaddition
4061:Niementowski quinazoline synthesis
3850:Nitrone-olefin (3+2) cycloaddition
3775:Azide-alkyne Huisgen cycloaddition
3637:Niementowski quinazoline synthesis
3392:Azide-alkyne Huisgen cycloaddition
2697:Meerwein–Ponndorf–Verley reduction
2249:Leimgruber–Batcho indole synthesis
795:Azide-alkyne Huisgen cycloaddition
14:
3890:Trimethylenemethane cycloaddition
3592:Johnson–Corey–Chaykovsky reaction
3457:Cadogan–Sundberg indole synthesis
3437:Bohlmann–Rahtz pyridine synthesis
3397:Baeyer–Emmerling indole synthesis
2204:Cadogan–Sundberg indole synthesis
1696:Johnson–Corey–Chaykovsky reaction
3986:Cook–Heilbron thiazole synthesis
3815:Hexadehydro Diels–Alder reaction
3642:Niementowski quinoline synthesis
3472:Cook–Heilbron thiazole synthesis
3417:Bischler–Möhlau indole synthesis
3327:Tiffeneau–Demjanov rearrangement
2957:Baker–Venkataraman rearrangement
2115:Horner–Wadsworth–Emmons reaction
1786:Mizoroki-Heck vs. Reductive Heck
1671:Horner–Wadsworth–Emmons reaction
1182:Neighbouring group participation
359:, Vol. 81, p. 157 (2005). (
3522:Fiesselmann thiophene synthesis
3352:Westphalen–Lettré rearrangement
3332:Vinylcyclopropane rearrangement
3162:Kornblum–DeLaMare rearrangement
2807:Epoxidation of allylic alcohols
2717:Noyori asymmetric hydrogenation
2652:Kornblum–DeLaMare rearrangement
2327:Gallagher–Hollander degradation
3981:Chichibabin pyridine synthesis
3467:Chichibabin pyridine synthesis
3427:Blum–Ittah aziridine synthesis
3262:Ring expansion and contraction
1531:Cross dehydrogenative coupling
335:, Vol. 81, p. 1 (2005). (
1:
3951:Bischler–Napieralski reaction
3909:Heterocycle forming reactions
3562:Hemetsberger indole synthesis
3422:Bischler–Napieralski reaction
3337:Wagner–Meerwein rearrangement
3307:Sommelet–Hauser rearrangement
3287:Seyferth–Gilbert homologation
3152:Ireland–Claisen rearrangement
3147:Hofmann–Martius rearrangement
2907:2,3-sigmatropic rearrangement
2522:Corey–Winter olefin synthesis
2447:Barton–McCombie deoxygenation
2090:Corey–Winter olefin synthesis
2044:Seyferth–Gilbert homologation
1911:Seyferth–Gilbert homologation
724:Seyferth–Gilbert homologation
321:10.1016/S0040-4039(01)94157-7
193:Seyferth-Gilbert homologation
4056:Lehmstedt–Tanasescu reaction
4016:Gabriel–Colman rearrangement
3971:Bucherer carbazole synthesis
3966:Borsche–Drechsel cyclization
3946:Bernthsen acridine synthesis
3931:Bamberger triazine synthesis
3916:Algar–Flynn–Oyamada reaction
3627:Nazarov cyclization reaction
3492:De Kimpe aziridine synthesis
3447:Bucherer carbazole synthesis
3442:Borsche–Drechsel cyclization
3212:Nazarov cyclization reaction
3192:Meyer–Schuster rearrangement
3122:Gabriel–Colman rearrangement
2872:Wolffenstein–Böters reaction
2757:Reduction of nitro compounds
2607:Grundmann aldehyde synthesis
2412:Algar–Flynn–Oyamada reaction
1821:Olefin conversion technology
1816:Nozaki–Hiyama–Kishi reaction
1611:Gabriel–Colman rearrangement
1501:Claisen-Schmidt condensation
1446:Bouveault aldehyde synthesis
402:Corey-Fuchs Alkyne Synthesis
89:Ramirez–Corey–Fuchs reaction
4031:Hantzsch pyridine synthesis
3810:Enone–alkene cycloadditions
3632:Nenitzescu indole synthesis
3552:Hantzsch pyridine synthesis
3517:Ferrario–Ackermann reaction
3167:Kowalski ester homologation
3132:Halogen dance rearrangement
2977:Benzilic acid rearrangement
2402:Akabori amino-acid reaction
2362:Von Braun amide degradation
2307:Barbier–Wieland degradation
2259:Nenitzescu indole synthesis
2239:Kharasch–Sosnovsky reaction
2130:Julia–Kocienski olefination
2034:Kowalski ester homologation
1731:Kowalski ester homologation
1706:Julia–Kocienski olefination
1461:Cadiot–Chodkiewicz coupling
1386:Aza-Baylis–Hillman reaction
1331:Acetoacetic ester synthesis
1042:Dynamic binding (chemistry)
1032:Conrotatory and disrotatory
1007:Charge remote fragmentation
805:Cadiot–Chodkiewicz coupling
302:Corey, E. J.; Fuchs, P. L.
4176:
4096:Robinson–Gabriel synthesis
4046:Kröhnke pyridine synthesis
3880:Retro-Diels–Alder reaction
3820:Imine Diels–Alder reaction
3607:Kröhnke pyridine synthesis
3222:Newman–Kwart rearrangement
3197:Mislow–Evans rearrangement
3107:Fischer–Hepp rearrangement
3052:Di-π-methane rearrangement
2832:Stephen aldehyde synthesis
2567:Eschweiler–Clarke reaction
2284:Williamson ether synthesis
1601:Fujiwara–Moritani reaction
1506:Combes quinoline synthesis
1471:Carbonyl olefin metathesis
1172:More O'Ferrall–Jencks plot
1097:Grunwald–Winstein equation
1067:Electron-withdrawing group
1002:Catalytic resonance theory
4106:Urech hydantoin synthesis
4086:Pomeranz–Fritsch reaction
4011:Fischer oxazole synthesis
3745:1,3-Dipolar cycloaddition
3717:Urech hydantoin synthesis
3687:Reissert indole synthesis
3672:Pomeranz–Fritsch reaction
3602:Knorr quinoline synthesis
3532:Fischer oxazole synthesis
3462:Camps quinoline synthesis
3382:1,3-Dipolar cycloaddition
3282:Semipinacol rearrangement
3257:Ramberg–Bäcklund reaction
3242:Piancatelli rearrangement
3182:McFadyen–Stevens reaction
2937:Alpha-ketol rearrangement
2692:McFadyen–Stevens reaction
2637:Kiliani–Fischer synthesis
2557:Elbs persulfate oxidation
2482:Bouveault–Blanc reduction
2442:Baeyer–Villiger oxidation
2274:Schotten–Baumann reaction
2150:Ramberg–Bäcklund reaction
2029:Kiliani–Fischer synthesis
1871:Ramberg–Bäcklund reaction
1856:Pinacol coupling reaction
1851:Piancatelli rearrangement
1746:Liebeskind–Srogl coupling
1596:Fujimoto–Belleau reaction
1313:List of organic reactions
1177:Negative hyperconjugation
922:
864:
444:
95:designed to transform an
77:
51:Organic Chemistry Portal
45:
18:
4081:Pictet–Spengler reaction
3996:Einhorn–Brunner reaction
3961:Boger pyridine synthesis
3855:Oxo-Diels–Alder reaction
3770:Aza-Diels–Alder reaction
3667:Pictet–Spengler reaction
3567:Hofmann–Löffler reaction
3557:Hegedus indole synthesis
3527:Fischer indole synthesis
3402:Bartoli indole synthesis
3357:Willgerodt rearrangement
3187:McLafferty rearrangement
3097:Ferrier carbocyclization
2912:2,3-Wittig rearrangement
2902:1,2-Wittig rearrangement
2742:Parikh–Doering oxidation
2732:Oxygen rebound mechanism
2397:Adkins–Peterson reaction
2289:Yamaguchi esterification
2229:Hegedus indole synthesis
2194:Bartoli indole synthesis
2065:Bamford–Stevens reaction
1981:Weinreb ketone synthesis
1941:Stork enamine alkylation
1716:Knoevenagel condensation
1586:Ferrier carbocyclization
1476:Castro–Stephens coupling
1102:Hammett acidity function
1092:Free-energy relationship
1037:Curtin–Hammett principle
1022:Conformational isomerism
122:The Corey–Fuchs reaction
4155:Rearrangement reactions
4041:Knorr pyrrole synthesis
3976:Bucherer–Bergs reaction
3921:Allan–Robinson reaction
3900:Wagner-Jauregg reaction
3692:Ring-closing metathesis
3617:Larock indole synthesis
3597:Knorr pyrrole synthesis
3452:Bucherer–Bergs reaction
3317:Stieglitz rearrangement
3297:Skattebøl rearrangement
3267:Ring-closing metathesis
3127:Group transfer reaction
3092:Favorskii rearrangement
3032:Cornforth rearrangement
2962:Bamberger rearrangement
2867:Wolff–Kishner reduction
2687:Markó–Lam deoxygenation
2582:Fleming–Tamao oxidation
2577:Fischer–Tropsch process
2264:Oxymercuration reaction
2244:Knorr pyrrole synthesis
2070:Barton–Kellogg reaction
1976:Wagner-Jauregg reaction
1896:Ring-closing metathesis
1886:Reimer–Tiemann reaction
1876:Rauhut–Currier reaction
1791:Nef isocyanide reaction
1751:Malonic ester synthesis
1721:Knorr pyrrole synthesis
1656:High dilution principle
1591:Friedel–Crafts reaction
1526:Cross-coupling reaction
1451:Bucherer–Bergs reaction
1436:Blanc chloromethylation
1426:Blaise ketone synthesis
1401:Baylis–Hillman reaction
1396:Barton–Kellogg reaction
1371:Allan–Robinson reaction
1277:Woodward–Hoffmann rules
1012:Charge-transfer complex
4145:Substitution reactions
4006:Feist–Benary synthesis
3780:Bradsher cycloaddition
3750:4+4 Photocycloaddition
3707:Simmons–Smith reaction
3652:Paternò–Büchi reaction
3512:Feist–Benary synthesis
3502:Dieckmann condensation
3252:Pummerer rearrangement
3232:Oxy-Cope rearrangement
3207:Myers allene synthesis
3157:Jacobsen rearrangement
3072:Electrocyclic reaction
3047:Demjanov rearrangement
3002:Buchner ring expansion
2972:Beckmann rearrangement
2952:Aza-Cope rearrangement
2947:Arndt–Eistert reaction
2922:Alkyne zipper reaction
2842:Transfer hydrogenation
2817:Sharpless oxyamination
2792:Selenoxide elimination
2677:Lombardo methylenation
2602:Griesbaum coozonolysis
2512:Corey–Itsuno reduction
2487:Boyland–Sims oxidation
2427:Angeli–Rimini reaction
2075:Boord olefin synthesis
2019:Arndt–Eistert reaction
2011:Homologation reactions
1811:Nitro-Mannich reaction
1726:Kolbe–Schmitt reaction
1536:Cross-coupling partner
1456:Buchner ring expansion
1376:Arndt–Eistert reaction
1142:Kinetic isotope effect
889:Rearrangement reaction
278:10.1002/ejoc.200601137
173:
161:
150:
123:
3865:Pauson–Khand reaction
3702:Sharpless epoxidation
3657:Pechmann condensation
3537:Friedländer synthesis
3487:Davis–Beirut reaction
3342:Wallach rearrangement
3312:Stevens rearrangement
3247:Pinacol rearrangement
3227:Overman rearrangement
3142:Hofmann rearrangement
3137:Hayashi rearrangement
3102:Ferrier rearrangement
3057:Dimroth rearrangement
3042:Curtius rearrangement
3037:Criegee rearrangement
3017:Claisen rearrangement
3007:Carroll rearrangement
2942:Amadori rearrangement
2932:Allylic rearrangement
2812:Sharpless epoxidation
2547:Dess–Martin oxidation
2472:Bohn–Schmidt reaction
2332:Hofmann rearrangement
2135:Kauffmann olefination
2058:Olefination reactions
1996:Wurtz–Fittig reaction
1831:Palladium–NHC complex
1711:Kauffmann olefination
1666:Homologation reaction
1516:Corey–House synthesis
1496:Claisen rearrangement
1292:Yukawa–Tsuno equation
1252:Swain–Lupton equation
1232:Spherical aromaticity
1167:Möbius–Hückel concept
952:Aromatic ring current
914:Substitution reaction
790:Pauson–Khand reaction
171:
159:
148:
121:
40:Substitution reaction
19:Corey–Fuchs reaction
4071:Paal–Knorr synthesis
3941:Barton–Zard reaction
3885:Staudinger synthesis
3835:Ketene cycloaddition
3805:Diels–Alder reaction
3785:Cheletropic reaction
3765:Alkyne trimerisation
3647:Paal–Knorr synthesis
3612:Kulinkovich reaction
3587:Jacobsen epoxidation
3507:Diels–Alder reaction
3302:Smiles rearrangement
3292:Sigmatropic reaction
3177:Lossen rearrangement
3027:Corey–Fuchs reaction
2992:Boekelheide reaction
2987:Bergmann degradation
2917:Achmatowicz reaction
2702:Methionine sulfoxide
2502:Clemmensen reduction
2462:Bergmann degradation
2392:Acyloin condensation
2357:Strecker degradation
2312:Bergmann degradation
2279:Ullmann condensation
2145:Peterson olefination
2120:Hydrazone iodination
2100:Elimination reaction
2001:Zincke–Suhl reaction
1921:Sonogashira coupling
1881:Reformatsky reaction
1841:Peterson olefination
1806:Nierenstein reaction
1736:Kulinkovich reaction
1551:Diels–Alder reaction
1511:Corey–Fuchs reaction
1491:Claisen condensation
1361:Alkyne trimerisation
1336:Acyloin condensation
1302:Σ-bishomoaromaticity
1262:Thorpe–Ingold effect
874:Elimination reaction
800:Sonogashira coupling
785:Diels–Alder reaction
780:Alkyne trimerisation
719:Corey–Fuchs reaction
225:. pp. 104–105.
87:, also known as the
85:Corey–Fuchs reaction
56:corey-fuchs-reaction
4091:Prilezhaev reaction
4076:Pellizzari reaction
3755:(4+3) cycloaddition
3722:Van Leusen reaction
3697:Robinson annulation
3682:Pschorr cyclization
3677:Prilezhaev reaction
3407:Bergman cyclization
3362:Wolff rearrangement
3347:Weerman degradation
3237:Pericyclic reaction
3217:Neber rearrangement
3112:Fries rearrangement
2997:Brook rearrangement
2982:Bergman cyclization
2827:Staudinger reaction
2772:Rosenmund reduction
2762:Reductive amination
2727:Oppenauer oxidation
2517:Corey–Kim oxidation
2492:Cannizzaro reaction
2367:Weerman degradation
2342:Isosaccharinic acid
2254:Mukaiyama hydration
2110:Hofmann elimination
2095:Dehydrohalogenation
2080:Chugaev elimination
1901:Robinson annulation
1846:Pfitzinger reaction
1616:Gattermann reaction
1561:Wulff–Dötz reaction
1541:Dakin–West reaction
1466:Carbonyl allylation
1411:Bergman cyclization
1197:Kennedy J. P. Orton
1117:Hammond's postulate
1087:Flippin–Lodge angle
1057:Electromeric effect
982:Beta-silicon effect
967:Baker–Nathan effect
705:Dehydrohalogenation
389:10.1021/ja00868a057
140:carbon tetrabromide
3840:McCormack reaction
3790:Conia-ene reaction
3622:Madelung synthesis
3412:Biginelli reaction
3202:Mumm rearrangement
3087:Favorskii reaction
3022:Cope rearrangement
3012:Chan rearrangement
2777:Rubottom oxidation
2707:Miyaura borylation
2672:Lipid peroxidation
2667:Lindgren oxidation
2647:Kornblum oxidation
2642:Kolbe electrolysis
2587:Fukuyama reduction
2497:Carbonyl reduction
2347:Marker degradation
2209:Diazonium compound
2199:Boudouard reaction
2178:Carbon-heteroatom
2105:Grieco elimination
1891:Rieche formylation
1836:Passerini reaction
1766:Meerwein arylation
1686:Hydroxymethylation
1581:Favorskii reaction
1481:Chan rearrangement
1416:Biginelli reaction
1341:Aldol condensation
1187:2-Norbornyl cation
1162:Möbius aromaticity
1157:Markovnikov's rule
1052:Effective molarity
997:Bürgi–Dunitz angle
987:Bicycloaromaticity
815:Favorskii reaction
775:Thiol-yne reaction
366:2011-05-14 at the
342:2011-05-14 at the
174:
162:
151:
130:Reaction mechanism
124:
93:chemical reactions
4132:
4131:
4128:
4127:
4124:
4123:
4116:Wohl–Aue reaction
3760:6+4 Cycloaddition
3577:Iodolactonization
2897:1,2-rearrangement
2862:Wohl–Aue reaction
2782:Sabatier reaction
2747:Pinnick oxidation
2712:Mozingo reduction
2657:Leuckart reaction
2612:Haloform reaction
2527:Criegee oxidation
2507:Collins oxidation
2457:Benkeser reaction
2452:Bechamp reduction
2422:Andrussow process
2407:Alcohol oxidation
2317:Edman degradation
2224:Haloform reaction
2173:
2172:
2160:Takai olefination
2125:Julia olefination
1951:Takai olefination
1826:Olefin metathesis
1701:Julia olefination
1626:Grignard reaction
1606:Fukuyama coupling
1521:Coupling reaction
1486:Chan–Lam coupling
1356:Alkyne metathesis
1351:Alkane metathesis
1207:Phosphaethynolate
1112:George S. Hammond
1072:Electronic effect
1027:Conjugated system
909:Stereospecificity
904:Stereoselectivity
869:Addition reaction
858:organic reactions
823:
822:
765:Hydrohalogenation
356:Organic Syntheses
332:Organic Syntheses
305:Tetrahedron Lett.
272:(15): 2477–2489.
109:Elias James Corey
91:, is a series of
81:
80:
28:Elias James Corey
4167:
4111:Wenker synthesis
4101:Stollé synthesis
3956:Bobbitt reaction
3926:Auwers synthesis
3870:Povarov reaction
3795:Cyclopropanation
3733:
3727:Wenker synthesis
3482:Darzens reaction
3432:Bobbitt reaction
3277:Schmidt reaction
3082:Enyne metathesis
2857:Whiting reaction
2852:Wharton reaction
2797:Shapiro reaction
2787:Sarett oxidation
2752:Prévost reaction
2562:Emde degradation
2372:Wohl degradation
2352:Ruff degradation
2322:Emde degradation
2219:Grignard reagent
2155:Shapiro reaction
2140:McMurry reaction
2007:
1971:Ullmann reaction
1936:Stollé synthesis
1926:Stetter reaction
1916:Shapiro reaction
1906:Sakurai reaction
1801:Negishi coupling
1781:Minisci reaction
1776:Michael reaction
1761:McMurry reaction
1756:Mannich reaction
1636:Hammick reaction
1631:Grignard reagent
1571:Enyne metathesis
1556:Doebner reaction
1546:Darzens reaction
1391:Barbier reaction
1381:Auwers synthesis
1308:
1282:Woodward's rules
1247:Superaromaticity
1237:Spiroaromaticity
1137:Inductive effect
1132:Hyperconjugation
1107:Hammett equation
1047:Edwards equation
899:Regioselectivity
850:
843:
836:
827:
650:
633:
604:
587:
553:
524:
495:
478:
461:
431:
424:
417:
408:
376:
352:
328:
301:
290:
289:
257:
251:
250:
244:
236:
214:
73:
58:
31:Philip L. Fuchs
16:
4175:
4174:
4170:
4169:
4168:
4166:
4165:
4164:
4135:
4134:
4133:
4120:
4021:Gewald reaction
3904:
3731:
3712:Skraup reaction
3547:Graham reaction
3542:Gewald reaction
3373:
3366:
2888:
2881:
2837:Swern oxidation
2822:Stahl oxidation
2767:Riley oxidation
2722:Omega oxidation
2682:Luche reduction
2632:Jones oxidation
2597:Glycol cleavage
2592:Ganem oxidation
2537:Davis oxidation
2532:Dakin oxidation
2467:Birch reduction
2417:Amide reduction
2383:
2376:
2337:Hooker reaction
2299:
2293:
2181:
2179:
2169:
2165:Wittig reaction
2053:
2049:Wittig reaction
2024:Hooker reaction
2005:
1986:Wittig reaction
1961:Thorpe reaction
1946:Suzuki reaction
1931:Stille reaction
1866:Quelet reaction
1741:Kumada coupling
1691:Ivanov reaction
1681:Hydrovinylation
1661:Hiyama coupling
1621:Glaser coupling
1431:Blaise reaction
1421:Bingel reaction
1406:Benary reaction
1323:
1321:
1315:
1306:
1202:Passive binding
1122:Homoaromaticity
972:Baldwin's rules
947:Antiaromaticity
942:Anomeric effect
918:
860:
854:
824:
819:
810:Glaser coupling
728:
683:Dehydrogenation
666:
649:
645:
641:
632:
628:
624:
603:
599:
595:
586:
582:
578:
552:
548:
544:
523:
519:
515:
494:
490:
486:
477:
473:
469:
460:
456:
452:
440:
435:
398:
393:
373:
368:Wayback Machine
349:
344:Wayback Machine
325:
298:
294:
293:
259:
258:
254:
237:
233:
216:
215:
211:
206:
198:Wittig reaction
179:
136:Wittig reaction
132:
113:Philip L. Fuchs
69:
54:
30:
12:
11:
5:
4173:
4171:
4163:
4162:
4160:Name reactions
4157:
4152:
4147:
4137:
4136:
4130:
4129:
4126:
4125:
4122:
4121:
4119:
4118:
4113:
4108:
4103:
4098:
4093:
4088:
4083:
4078:
4073:
4068:
4063:
4058:
4053:
4048:
4043:
4038:
4033:
4028:
4026:Hantzsch ester
4023:
4018:
4013:
4008:
4003:
3998:
3993:
3988:
3983:
3978:
3973:
3968:
3963:
3958:
3953:
3948:
3943:
3938:
3936:Banert cascade
3933:
3928:
3923:
3918:
3912:
3910:
3906:
3905:
3903:
3902:
3897:
3892:
3887:
3882:
3877:
3875:Prato reaction
3872:
3867:
3862:
3857:
3852:
3847:
3842:
3837:
3832:
3827:
3822:
3817:
3812:
3807:
3802:
3797:
3792:
3787:
3782:
3777:
3772:
3767:
3762:
3757:
3752:
3747:
3741:
3739:
3730:
3729:
3724:
3719:
3714:
3709:
3704:
3699:
3694:
3689:
3684:
3679:
3674:
3669:
3664:
3659:
3654:
3649:
3644:
3639:
3634:
3629:
3624:
3619:
3614:
3609:
3604:
3599:
3594:
3589:
3584:
3579:
3574:
3569:
3564:
3559:
3554:
3549:
3544:
3539:
3534:
3529:
3524:
3519:
3514:
3509:
3504:
3499:
3494:
3489:
3484:
3479:
3474:
3469:
3464:
3459:
3454:
3449:
3444:
3439:
3434:
3429:
3424:
3419:
3414:
3409:
3404:
3399:
3394:
3389:
3384:
3378:
3376:
3368:
3367:
3365:
3364:
3359:
3354:
3349:
3344:
3339:
3334:
3329:
3324:
3319:
3314:
3309:
3304:
3299:
3294:
3289:
3284:
3279:
3274:
3269:
3264:
3259:
3254:
3249:
3244:
3239:
3234:
3229:
3224:
3219:
3214:
3209:
3204:
3199:
3194:
3189:
3184:
3179:
3174:
3169:
3164:
3159:
3154:
3149:
3144:
3139:
3134:
3129:
3124:
3119:
3114:
3109:
3104:
3099:
3094:
3089:
3084:
3079:
3074:
3069:
3064:
3059:
3054:
3049:
3044:
3039:
3034:
3029:
3024:
3019:
3014:
3009:
3004:
2999:
2994:
2989:
2984:
2979:
2974:
2969:
2967:Banert cascade
2964:
2959:
2954:
2949:
2944:
2939:
2934:
2929:
2924:
2919:
2914:
2909:
2904:
2899:
2893:
2891:
2887:Rearrangement
2883:
2882:
2880:
2879:
2877:Zinin reaction
2874:
2869:
2864:
2859:
2854:
2849:
2847:Wacker process
2844:
2839:
2834:
2829:
2824:
2819:
2814:
2809:
2804:
2799:
2794:
2789:
2784:
2779:
2774:
2769:
2764:
2759:
2754:
2749:
2744:
2739:
2734:
2729:
2724:
2719:
2714:
2709:
2704:
2699:
2694:
2689:
2684:
2679:
2674:
2669:
2664:
2659:
2654:
2649:
2644:
2639:
2634:
2629:
2624:
2622:Hydrogenolysis
2619:
2614:
2609:
2604:
2599:
2594:
2589:
2584:
2579:
2574:
2572:Étard reaction
2569:
2564:
2559:
2554:
2549:
2544:
2539:
2534:
2529:
2524:
2519:
2514:
2509:
2504:
2499:
2494:
2489:
2484:
2479:
2477:Bosch reaction
2474:
2469:
2464:
2459:
2454:
2449:
2444:
2439:
2434:
2429:
2424:
2419:
2414:
2409:
2404:
2399:
2394:
2388:
2386:
2382:Organic redox
2378:
2377:
2375:
2374:
2369:
2364:
2359:
2354:
2349:
2344:
2339:
2334:
2329:
2324:
2319:
2314:
2309:
2303:
2301:
2295:
2294:
2292:
2291:
2286:
2281:
2276:
2271:
2266:
2261:
2256:
2251:
2246:
2241:
2236:
2231:
2226:
2221:
2216:
2214:Esterification
2211:
2206:
2201:
2196:
2191:
2185:
2183:
2175:
2174:
2171:
2170:
2168:
2167:
2162:
2157:
2152:
2147:
2142:
2137:
2132:
2127:
2122:
2117:
2112:
2107:
2102:
2097:
2092:
2087:
2082:
2077:
2072:
2067:
2061:
2059:
2055:
2054:
2052:
2051:
2046:
2041:
2036:
2031:
2026:
2021:
2015:
2013:
2004:
2003:
1998:
1993:
1991:Wurtz reaction
1988:
1983:
1978:
1973:
1968:
1963:
1958:
1953:
1948:
1943:
1938:
1933:
1928:
1923:
1918:
1913:
1908:
1903:
1898:
1893:
1888:
1883:
1878:
1873:
1868:
1863:
1861:Prins reaction
1858:
1853:
1848:
1843:
1838:
1833:
1828:
1823:
1818:
1813:
1808:
1803:
1798:
1793:
1788:
1783:
1778:
1773:
1768:
1763:
1758:
1753:
1748:
1743:
1738:
1733:
1728:
1723:
1718:
1713:
1708:
1703:
1698:
1693:
1688:
1683:
1678:
1676:Hydrocyanation
1673:
1668:
1663:
1658:
1653:
1648:
1646:Henry reaction
1643:
1638:
1633:
1628:
1623:
1618:
1613:
1608:
1603:
1598:
1593:
1588:
1583:
1578:
1573:
1568:
1563:
1558:
1553:
1548:
1543:
1538:
1533:
1528:
1523:
1518:
1513:
1508:
1503:
1498:
1493:
1488:
1483:
1478:
1473:
1468:
1463:
1458:
1453:
1448:
1443:
1438:
1433:
1428:
1423:
1418:
1413:
1408:
1403:
1398:
1393:
1388:
1383:
1378:
1373:
1368:
1363:
1358:
1353:
1348:
1346:Aldol reaction
1343:
1338:
1333:
1327:
1325:
1320:Carbon-carbon
1317:
1316:
1311:
1305:
1304:
1299:
1297:Zaitsev's rule
1294:
1289:
1284:
1279:
1274:
1269:
1264:
1259:
1254:
1249:
1244:
1242:Steric effects
1239:
1234:
1229:
1224:
1219:
1214:
1209:
1204:
1199:
1194:
1189:
1184:
1179:
1174:
1169:
1164:
1159:
1154:
1149:
1144:
1139:
1134:
1129:
1124:
1119:
1114:
1109:
1104:
1099:
1094:
1089:
1084:
1079:
1074:
1069:
1064:
1059:
1054:
1049:
1044:
1039:
1034:
1029:
1024:
1019:
1014:
1009:
1004:
999:
994:
989:
984:
979:
974:
969:
964:
959:
954:
949:
944:
939:
934:
929:
923:
920:
919:
917:
916:
911:
906:
901:
896:
894:Redox reaction
891:
886:
881:
879:Polymerization
876:
871:
865:
862:
861:
855:
853:
852:
845:
838:
830:
821:
820:
818:
817:
812:
807:
802:
797:
792:
787:
782:
777:
772:
767:
762:
757:
752:
747:
742:
736:
734:
730:
729:
727:
726:
721:
716:
711:
702:
693:
680:
674:
672:
668:
667:
665:
664:
663:
662:
657:
635:
618:
617:
616:
611:
589:
572:
571:
570:
565:
560:
538:
537:
536:
531:
509:
508:
507:
502:
480:
463:
445:
442:
441:
436:
434:
433:
426:
419:
411:
405:
404:
397:
396:External links
394:
392:
391:
371:
347:
323:
295:
292:
291:
252:
231:
208:
207:
205:
202:
201:
200:
195:
190:
185:
183:Appel reaction
178:
175:
131:
128:
79:
78:
75:
74:
67:
60:
59:
52:
48:
47:
43:
42:
37:
36:Reaction type
33:
32:
25:
21:
20:
13:
10:
9:
6:
4:
3:
2:
4172:
4161:
4158:
4156:
4153:
4151:
4148:
4146:
4143:
4142:
4140:
4117:
4114:
4112:
4109:
4107:
4104:
4102:
4099:
4097:
4094:
4092:
4089:
4087:
4084:
4082:
4079:
4077:
4074:
4072:
4069:
4067:
4064:
4062:
4059:
4057:
4054:
4052:
4049:
4047:
4044:
4042:
4039:
4037:
4036:Herz reaction
4034:
4032:
4029:
4027:
4024:
4022:
4019:
4017:
4014:
4012:
4009:
4007:
4004:
4002:
3999:
3997:
3994:
3992:
3989:
3987:
3984:
3982:
3979:
3977:
3974:
3972:
3969:
3967:
3964:
3962:
3959:
3957:
3954:
3952:
3949:
3947:
3944:
3942:
3939:
3937:
3934:
3932:
3929:
3927:
3924:
3922:
3919:
3917:
3914:
3913:
3911:
3907:
3901:
3898:
3896:
3893:
3891:
3888:
3886:
3883:
3881:
3878:
3876:
3873:
3871:
3868:
3866:
3863:
3861:
3858:
3856:
3853:
3851:
3848:
3846:
3843:
3841:
3838:
3836:
3833:
3831:
3828:
3826:
3823:
3821:
3818:
3816:
3813:
3811:
3808:
3806:
3803:
3801:
3798:
3796:
3793:
3791:
3788:
3786:
3783:
3781:
3778:
3776:
3773:
3771:
3768:
3766:
3763:
3761:
3758:
3756:
3753:
3751:
3748:
3746:
3743:
3742:
3740:
3738:
3737:Cycloaddition
3734:
3728:
3725:
3723:
3720:
3718:
3715:
3713:
3710:
3708:
3705:
3703:
3700:
3698:
3695:
3693:
3690:
3688:
3685:
3683:
3680:
3678:
3675:
3673:
3670:
3668:
3665:
3663:
3660:
3658:
3655:
3653:
3650:
3648:
3645:
3643:
3640:
3638:
3635:
3633:
3630:
3628:
3625:
3623:
3620:
3618:
3615:
3613:
3610:
3608:
3605:
3603:
3600:
3598:
3595:
3593:
3590:
3588:
3585:
3583:
3582:Isay reaction
3580:
3578:
3575:
3573:
3570:
3568:
3565:
3563:
3560:
3558:
3555:
3553:
3550:
3548:
3545:
3543:
3540:
3538:
3535:
3533:
3530:
3528:
3525:
3523:
3520:
3518:
3515:
3513:
3510:
3508:
3505:
3503:
3500:
3498:
3495:
3493:
3490:
3488:
3485:
3483:
3480:
3478:
3477:Cycloaddition
3475:
3473:
3470:
3468:
3465:
3463:
3460:
3458:
3455:
3453:
3450:
3448:
3445:
3443:
3440:
3438:
3435:
3433:
3430:
3428:
3425:
3423:
3420:
3418:
3415:
3413:
3410:
3408:
3405:
3403:
3400:
3398:
3395:
3393:
3390:
3388:
3385:
3383:
3380:
3379:
3377:
3375:
3372:Ring forming
3369:
3363:
3360:
3358:
3355:
3353:
3350:
3348:
3345:
3343:
3340:
3338:
3335:
3333:
3330:
3328:
3325:
3323:
3320:
3318:
3315:
3313:
3310:
3308:
3305:
3303:
3300:
3298:
3295:
3293:
3290:
3288:
3285:
3283:
3280:
3278:
3275:
3273:
3272:Rupe reaction
3270:
3268:
3265:
3263:
3260:
3258:
3255:
3253:
3250:
3248:
3245:
3243:
3240:
3238:
3235:
3233:
3230:
3228:
3225:
3223:
3220:
3218:
3215:
3213:
3210:
3208:
3205:
3203:
3200:
3198:
3195:
3193:
3190:
3188:
3185:
3183:
3180:
3178:
3175:
3173:
3170:
3168:
3165:
3163:
3160:
3158:
3155:
3153:
3150:
3148:
3145:
3143:
3140:
3138:
3135:
3133:
3130:
3128:
3125:
3123:
3120:
3118:
3115:
3113:
3110:
3108:
3105:
3103:
3100:
3098:
3095:
3093:
3090:
3088:
3085:
3083:
3080:
3078:
3075:
3073:
3070:
3068:
3065:
3063:
3060:
3058:
3055:
3053:
3050:
3048:
3045:
3043:
3040:
3038:
3035:
3033:
3030:
3028:
3025:
3023:
3020:
3018:
3015:
3013:
3010:
3008:
3005:
3003:
3000:
2998:
2995:
2993:
2990:
2988:
2985:
2983:
2980:
2978:
2975:
2973:
2970:
2968:
2965:
2963:
2960:
2958:
2955:
2953:
2950:
2948:
2945:
2943:
2940:
2938:
2935:
2933:
2930:
2928:
2925:
2923:
2920:
2918:
2915:
2913:
2910:
2908:
2905:
2903:
2900:
2898:
2895:
2894:
2892:
2890:
2884:
2878:
2875:
2873:
2870:
2868:
2865:
2863:
2860:
2858:
2855:
2853:
2850:
2848:
2845:
2843:
2840:
2838:
2835:
2833:
2830:
2828:
2825:
2823:
2820:
2818:
2815:
2813:
2810:
2808:
2805:
2803:
2800:
2798:
2795:
2793:
2790:
2788:
2785:
2783:
2780:
2778:
2775:
2773:
2770:
2768:
2765:
2763:
2760:
2758:
2755:
2753:
2750:
2748:
2745:
2743:
2740:
2738:
2735:
2733:
2730:
2728:
2725:
2723:
2720:
2718:
2715:
2713:
2710:
2708:
2705:
2703:
2700:
2698:
2695:
2693:
2690:
2688:
2685:
2683:
2680:
2678:
2675:
2673:
2670:
2668:
2665:
2663:
2662:Ley oxidation
2660:
2658:
2655:
2653:
2650:
2648:
2645:
2643:
2640:
2638:
2635:
2633:
2630:
2628:
2627:Hydroxylation
2625:
2623:
2620:
2618:
2617:Hydrogenation
2615:
2613:
2610:
2608:
2605:
2603:
2600:
2598:
2595:
2593:
2590:
2588:
2585:
2583:
2580:
2578:
2575:
2573:
2570:
2568:
2565:
2563:
2560:
2558:
2555:
2553:
2552:DNA oxidation
2550:
2548:
2545:
2543:
2542:Deoxygenation
2540:
2538:
2535:
2533:
2530:
2528:
2525:
2523:
2520:
2518:
2515:
2513:
2510:
2508:
2505:
2503:
2500:
2498:
2495:
2493:
2490:
2488:
2485:
2483:
2480:
2478:
2475:
2473:
2470:
2468:
2465:
2463:
2460:
2458:
2455:
2453:
2450:
2448:
2445:
2443:
2440:
2438:
2435:
2433:
2432:Aromatization
2430:
2428:
2425:
2423:
2420:
2418:
2415:
2413:
2410:
2408:
2405:
2403:
2400:
2398:
2395:
2393:
2390:
2389:
2387:
2385:
2379:
2373:
2370:
2368:
2365:
2363:
2360:
2358:
2355:
2353:
2350:
2348:
2345:
2343:
2340:
2338:
2335:
2333:
2330:
2328:
2325:
2323:
2320:
2318:
2315:
2313:
2310:
2308:
2305:
2304:
2302:
2296:
2290:
2287:
2285:
2282:
2280:
2277:
2275:
2272:
2270:
2269:Reed reaction
2267:
2265:
2262:
2260:
2257:
2255:
2252:
2250:
2247:
2245:
2242:
2240:
2237:
2235:
2232:
2230:
2227:
2225:
2222:
2220:
2217:
2215:
2212:
2210:
2207:
2205:
2202:
2200:
2197:
2195:
2192:
2190:
2187:
2186:
2184:
2180:bond forming
2176:
2166:
2163:
2161:
2158:
2156:
2153:
2151:
2148:
2146:
2143:
2141:
2138:
2136:
2133:
2131:
2128:
2126:
2123:
2121:
2118:
2116:
2113:
2111:
2108:
2106:
2103:
2101:
2098:
2096:
2093:
2091:
2088:
2086:
2085:Cope reaction
2083:
2081:
2078:
2076:
2073:
2071:
2068:
2066:
2063:
2062:
2060:
2056:
2050:
2047:
2045:
2042:
2040:
2037:
2035:
2032:
2030:
2027:
2025:
2022:
2020:
2017:
2016:
2014:
2012:
2008:
2002:
1999:
1997:
1994:
1992:
1989:
1987:
1984:
1982:
1979:
1977:
1974:
1972:
1969:
1967:
1964:
1962:
1959:
1957:
1954:
1952:
1949:
1947:
1944:
1942:
1939:
1937:
1934:
1932:
1929:
1927:
1924:
1922:
1919:
1917:
1914:
1912:
1909:
1907:
1904:
1902:
1899:
1897:
1894:
1892:
1889:
1887:
1884:
1882:
1879:
1877:
1874:
1872:
1869:
1867:
1864:
1862:
1859:
1857:
1854:
1852:
1849:
1847:
1844:
1842:
1839:
1837:
1834:
1832:
1829:
1827:
1824:
1822:
1819:
1817:
1814:
1812:
1809:
1807:
1804:
1802:
1799:
1797:
1796:Nef synthesis
1794:
1792:
1789:
1787:
1784:
1782:
1779:
1777:
1774:
1772:
1771:Methylenation
1769:
1767:
1764:
1762:
1759:
1757:
1754:
1752:
1749:
1747:
1744:
1742:
1739:
1737:
1734:
1732:
1729:
1727:
1724:
1722:
1719:
1717:
1714:
1712:
1709:
1707:
1704:
1702:
1699:
1697:
1694:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1669:
1667:
1664:
1662:
1659:
1657:
1654:
1652:
1649:
1647:
1644:
1642:
1641:Heck reaction
1639:
1637:
1634:
1632:
1629:
1627:
1624:
1622:
1619:
1617:
1614:
1612:
1609:
1607:
1604:
1602:
1599:
1597:
1594:
1592:
1589:
1587:
1584:
1582:
1579:
1577:
1574:
1572:
1569:
1567:
1564:
1562:
1559:
1557:
1554:
1552:
1549:
1547:
1544:
1542:
1539:
1537:
1534:
1532:
1529:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1507:
1504:
1502:
1499:
1497:
1494:
1492:
1489:
1487:
1484:
1482:
1479:
1477:
1474:
1472:
1469:
1467:
1464:
1462:
1459:
1457:
1454:
1452:
1449:
1447:
1444:
1442:
1439:
1437:
1434:
1432:
1429:
1427:
1424:
1422:
1419:
1417:
1414:
1412:
1409:
1407:
1404:
1402:
1399:
1397:
1394:
1392:
1389:
1387:
1384:
1382:
1379:
1377:
1374:
1372:
1369:
1367:
1364:
1362:
1359:
1357:
1354:
1352:
1349:
1347:
1344:
1342:
1339:
1337:
1334:
1332:
1329:
1328:
1326:
1322:bond forming
1318:
1314:
1309:
1303:
1300:
1298:
1295:
1293:
1290:
1288:
1287:Y-aromaticity
1285:
1283:
1280:
1278:
1275:
1273:
1272:Walsh diagram
1270:
1268:
1265:
1263:
1260:
1258:
1257:Taft equation
1255:
1253:
1250:
1248:
1245:
1243:
1240:
1238:
1235:
1233:
1230:
1228:
1227:Σ-aromaticity
1225:
1223:
1220:
1218:
1215:
1213:
1210:
1208:
1205:
1203:
1200:
1198:
1195:
1193:
1190:
1188:
1185:
1183:
1180:
1178:
1175:
1173:
1170:
1168:
1165:
1163:
1160:
1158:
1155:
1153:
1152:Marcus theory
1150:
1148:
1145:
1143:
1140:
1138:
1135:
1133:
1130:
1128:
1127:Hückel's rule
1125:
1123:
1120:
1118:
1115:
1113:
1110:
1108:
1105:
1103:
1100:
1098:
1095:
1093:
1090:
1088:
1085:
1083:
1082:Evelyn effect
1080:
1078:
1075:
1073:
1070:
1068:
1065:
1063:
1062:Electron-rich
1060:
1058:
1055:
1053:
1050:
1048:
1045:
1043:
1040:
1038:
1035:
1033:
1030:
1028:
1025:
1023:
1020:
1018:
1015:
1013:
1010:
1008:
1005:
1003:
1000:
998:
995:
993:
990:
988:
985:
983:
980:
978:
977:Bema Hapothle
975:
973:
970:
968:
965:
963:
960:
958:
955:
953:
950:
948:
945:
943:
940:
938:
935:
933:
930:
928:
925:
924:
921:
915:
912:
910:
907:
905:
902:
900:
897:
895:
892:
890:
887:
885:
882:
880:
877:
875:
872:
870:
867:
866:
863:
859:
851:
846:
844:
839:
837:
832:
831:
828:
816:
813:
811:
808:
806:
803:
801:
798:
796:
793:
791:
788:
786:
783:
781:
778:
776:
773:
771:
768:
766:
763:
761:
760:Hydroboration
758:
756:
753:
751:
748:
746:
745:Hydrogenation
743:
741:
740:Deprotonation
738:
737:
735:
731:
725:
722:
720:
717:
715:
712:
710:
706:
703:
701:
700:alkynyl anion
697:
694:
692:
688:
684:
681:
679:
676:
675:
673:
669:
661:
658:
656:
653:
652:
639:
636:
622:
619:
615:
612:
610:
607:
606:
593:
590:
576:
573:
569:
566:
564:
561:
559:
556:
555:
542:
539:
535:
532:
530:
527:
526:
513:
510:
506:
503:
501:
498:
497:
484:
481:
467:
464:
450:
447:
446:
443:
439:
432:
427:
425:
420:
418:
413:
412:
409:
403:
400:
399:
395:
390:
386:
382:
381:
375:
372:
369:
365:
362:
358:
357:
351:
348:
345:
341:
338:
334:
333:
327:
324:
322:
318:
315:, 3769–3772.
314:
310:
307:
306:
300:
297:
296:
287:
283:
279:
275:
271:
267:
263:
256:
253:
248:
242:
234:
232:0-12-429785-4
228:
224:
220:
213:
210:
203:
199:
196:
194:
191:
189:
186:
184:
181:
180:
176:
170:
166:
158:
154:
147:
143:
141:
137:
129:
127:
120:
116:
114:
110:
106:
102:
98:
94:
90:
86:
76:
72:
68:
65:
62:
61:
57:
53:
50:
49:
44:
41:
38:
35:
34:
29:
26:
23:
22:
17:
3077:Ene reaction
3026:
2437:Autoxidation
2298:Degradation
2189:Azo coupling
1966:Ugi reaction
1566:Ene reaction
1510:
1366:Alkynylation
1217:Polyfluorene
1212:Polar effect
1077:Electrophile
992:Bredt's rule
962:Baird's rule
932:Alpha effect
770:Alkynylation
750:Halogenation
718:
709:dihaloalkane
671:Preparations
378:
374:
354:
350:
330:
326:
312:
308:
303:
299:
269:
265:
255:
218:
212:
163:
152:
133:
125:
88:
84:
82:
71:RXNO:0000146
66:ontology ID
46:Identifiers
24:Named after
1576:Ethenolysis
1222:Ring strain
1192:Nucleophile
1017:Clar's rule
957:Aromaticity
4139:Categories
3860:Ozonolysis
3387:Annulation
2737:Ozonolysis
856:Topics in
696:Alkylation
204:References
3374:reactions
2889:reactions
2384:reactions
2300:reactions
2182:reactions
1324:reactions
755:Hydration
733:Reactions
286:1434-193X
241:cite book
1267:Vinylogy
937:Annulene
884:Reagents
678:Cracking
364:Archived
340:Archived
223:Elsevier
177:See also
99:into an
97:aldehyde
927:A value
575:Heptyne
512:Pentyne
466:Propyne
438:Alkynes
361:Article
337:Article
691:alkene
687:alkane
638:Decyne
621:Nonyne
592:Octyne
541:Hexyne
483:Butyne
449:Ethyne
284:
229:
101:alkyne
380:JACS
309:1972
282:ISSN
270:2007
247:link
227:ISBN
111:and
83:The
707:of
698:of
685:of
385:doi
317:doi
274:doi
64:RSC
4141::
689:,
651:)
648:18
644:10
631:16
605:)
602:14
585:12
554:)
551:10
525:)
496:)
313:13
311:,
280:.
268:.
264:.
243:}}
239:{{
221:.
115:.
849:e
842:t
835:v
660:5
655:1
646:H
642:C
640:(
634:)
629:H
627:9
625:C
623:(
614:4
609:2
600:H
598:8
596:C
594:(
588:)
583:H
581:7
579:C
577:(
568:3
563:2
558:1
549:H
547:6
545:C
543:(
534:2
529:1
522:8
520:H
518:5
516:C
514:(
505:2
500:1
493:6
491:H
489:4
487:C
485:(
479:)
476:4
474:H
472:3
470:C
468:(
462:)
459:2
457:H
455:2
453:C
451:(
430:e
423:t
416:v
387::
370:)
346:)
319::
288:.
276::
249:)
235:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.