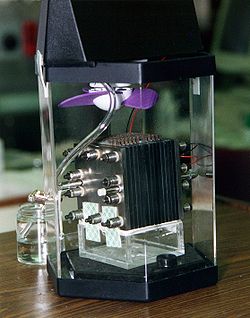
89:
the exact kinetics are debated, the result is a reduction of the cell voltage. Cross-over remains a major factor in inefficiencies, and often half of the methanol is lost to cross-over. Methanol cross-over and/or its effects can be alleviated by (a) developing alternative membranes (e.g.), (b) improving the electro-oxidation process in the catalyst layer and improving the structure of the catalyst and gas diffusion layers (e.g. ), and (c) optimizing the design of the flow field and the membrane electrode assembly (MEA) which can be achieved by studying the current density distributions (e.g. ).
128:. Military applications of DMFCs are an emerging application since they have low noise and thermal signatures and no toxic effluent. These applications include power for man-portable tactical equipment, battery chargers, and autonomous power for test and training instrumentation. Units are available with power outputs between 25 watts and 5 kilowatts with durations up to 100 hours between refuelings. Especially for power output up to 0.3 kW the DMFC is suitable. For a power output of more than 0.3 kW the
85:, i.e. about 3% in mass) to carry the reactant into the cell; common operating temperatures are in the range 50 to 120 °C (122 to 248 °F), where high temperatures are usually pressurized. DMFCs themselves are more efficient at high temperatures and pressures, but these conditions end up causing so many losses in the complete system that the advantage is lost; therefore, atmospheric-pressure configurations are currently preferred.
20:
172:
issued a proposal to allow airline passengers to carry fuel cell cartridges on board. The
Department of Transportation issued a final ruling on April 30, 2008, permitting passengers and crew to carry an approved fuel cell with an installed methanol cartridge and up to two additional spare cartridges.
1011:
Methanol on the anodic side is usually in a weak solution (from 1M to 3M), because methanol in high concentrations has the tendency to diffuse through the membrane to the cathode, where its concentration is about zero because it is rapidly consumed by oxygen. Low concentrations help in reducing the
88:
Because of the methanol cross-over, a phenomenon by which methanol diffuses through the membrane without reacting, methanol is fed as a weak solution: this decreases efficiency significantly, since crossed-over methanol, after reaching the air side (the cathode), immediately reacts with air; though
115:
Current DMFCs are limited in the power they can produce, but can still store a high energy content in a small space. This means they can produce a small amount of power over a long period of time. This makes them ill-suited for powering large vehicles (at least directly), but ideal for smaller
605:
Platinum is used as a catalyst for both half-reactions. This contributes to the loss of cell voltage potential, as any methanol that is present in the cathode chamber will oxidize. If another catalyst could be found for the reduction of oxygen, the problem of methanol crossover would likely be
1024:
The water in the anodic loop is lost because of the anodic reaction, but mostly because of the associated water drag: every proton formed at the anode drags a number of water molecules to the cathode. Depending on temperature and membrane type, this number can be between 2 and 6.
1015:
The practical realization is usually that a solution loop enters the anode, exits, is refilled with methanol, and returns to the anode again. Alternatively, fuel cells with optimized structures can be directly fed with high concentration methanol solutions or even pure methanol.
1443:
Hazardous
Materials: Revision to Requirements for the Transportation of Batteries and Battery-Powered Devices; and Harmonization with the United Nations Recommendations, International Maritime Dangerous Goods Code, and International Civil Aviation Organization's Technical
1033:
A direct methanol fuel cell is usually part of a larger system including all the ancillary units that permit its operation. Compared to most other types of fuel cells, the ancillary system of DMFCs is relatively complex. The main reasons for its complexity are:
132:
presents a higher efficiency and is more cost-efficient. Freezing of the liquid methanol-water mixture in the stack at low ambient temperature can be problematic for the membrane of DMFC (in contrast to indirect methanol fuel cell).
164:. However, the International Civil Aviation Organization's (ICAO) Dangerous Goods Panel (DGP) voted in November 2005 to allow passengers to carry and use micro fuel cells and methanol fuel cartridges when aboard airplanes to power
886:
458:
995:
567:
774:
349:
1425:
53:
of a DMFC is 97%; as of 2014 the achievable energy conversion efficiency for operational cells attains 30% – 40%. There is intensive research on promising approaches to increase the operational efficiency.
173:
It is worth noting that 200 ml maximum methanol cartridge volume allowed in the final ruling is double the 100 ml limit on liquids allowed by the
Transportation Security Administration in carry-on bags.
1447:
613:(CO) is formed, which strongly adsorbs onto the platinum catalyst, reducing the number of available reaction sites and thus the performance of the cell. The addition of other metals, such as
621:, to the platinum catalyst tends to ameliorate this problem. In the case of platinum-ruthenium catalysts, the oxophilic nature of ruthenium is believed to promote the formation of
625:
on its surface, which can then react with carbon monoxide adsorbed on the platinum atoms. The water in the fuel cell is oxidized to a hydroxy radical via the following reaction: H
46:(PEM). Their main advantage is low temperature operation and the ease of transport of methanol, an energy-dense yet reasonably stable liquid at all environmental conditions.
1420:
1353:
Simon Araya, Samuel; Liso, Vincenzo; Cui, Xiaoti; Li, Na; Zhu, Jimin; Sahlin, Simon
Lennart; Jensen, Søren Højgaard; Nielsen, Mads Pagh; Kær, Søren Knudsen (2020).
57:
A more efficient version of a direct fuel cell would play a key role in the theoretical use of methanol as a general energy transport medium, in the hypothesized
1441:
1216:
Dohle, H.; Mergel, J. & Stolten, D.: Heat and power management of a direct-methanol-fuel-cell (DMFC) system, Journal of Power
Sources, 2002, 111, 268-282.
1556:
1048:
water in the anodic loop is slowly consumed by reaction and drag; it is necessary to recover water from the cathodic side to maintain steady operation.
789:
364:
901:
473:
1485:
Motoo, S.; Watanabe, M. (1975). "Electrolysis by Ad-Atoms Part II. Enhancement of the
Oxidation of Methanol on Platinum by Ruthenium Ad-Atoms".
662:
1386:
Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. (December 2008). "Hydrogen and fuel cells: Towards a sustainable energy future".
1253:
246:
1587:
1468:
1625:
1226:
Wei, Yongsheng; et al. (2012). "A novel membrane for DMFC – Na2Ti3O7 Nanotubes/Nafion composite membrane: Performances studies".
35:
1306:
Almheiri, Saif; Hongtan Liu (2014). "Separate measurement of current density under land and channel in Direct
Methanol Fuel Cells".
1198:
Pasha Majidi; et al. (1 May 2016). "Determination of the efficiency of methanol oxidation in a direct methanol fuel cell".
1768:
1094:
169:
606:
significantly lessened. Furthermore, platinum is very expensive and contributes to the high cost per kilowatt of these cells.
1723:
1059:
1763:
1646:
50:
1816:
1733:
1676:
1074:
1038:
providing water along with methanol would make the fuel supply more cumbersome, so water has to be recycled in a loop;
153:. The energy density per mass is a tenth of that of hydrogen, but 10 times higher than that of lithium-ion batteries.
129:
70:
1753:
1615:
1064:
590:
in the reaction, pure methanol cannot be used without provision of water via either passive transport such as back
141:
Methanol is a liquid from −97.6 to 64.7 °C (−143.7 to 148.5 °F) at atmospheric pressure. The volumetric
1743:
1620:
1279:
Matar, Saif; Hongtan Liu (2010). "Effect of cathode catalyst layer thickness on methanol cross-over in a DMFC".
1748:
1656:
1580:
43:
1553:
1738:
1671:
1651:
1125:
Umit B. Demirci (2007). "Review: Direct liquid-feed fuel cells: Thermodynamic and environmental concerns".
1758:
1697:
221:
are transported through an external circuit from anode to cathode, providing power to connected devices.
1666:
1630:
1681:
1395:
1315:
1084:
1837:
1702:
1573:
150:
146:
1610:
1463:
1842:
622:
1803:
1798:
1793:
1788:
1513:
1403:
1366:
1323:
1288:
1235:
1180:
1157:
1134:
1089:
599:
58:
1534:
Merhoff, Henry and Helbig, Peter. Development and
Fielding of a Direct Methanol Fuel Cell;
1560:
1472:
1451:
1429:
1099:
630:
610:
165:
78:
586:
particles, and lose protons until carbon dioxide is formed. As water is consumed at the
1399:
1319:
19:
1549:
Fuel Cell News Today. An internet portal of news and articles of fuel cell developments
1340:
1254:"Safe space: improving the "clean" methanol fuel cells using a protective carbon shell"
1184:
1161:
634:
194:
142:
121:
104:
93:
1554:
12th Small Fuel Cells. Annual conference on portable fuel cell technology developments
1831:
1718:
225:
1292:
1728:
1517:
1327:
1239:
1138:
1079:
161:
117:
100:, the sluggish dynamic behavior, and the ability to maintain the solution water.
1407:
1104:
881:{\displaystyle \mathrm {{\frac {3}{2}}O_{2}+3\ H_{2}O+6\ e^{-}\to 6\ OH^{-}} }
1152:
Ibrahim Dincer, Calin
Zamfirescu (2014). "4.4.7 Direct Methanol Fuel Cells".
453:{\displaystyle \mathrm {{\frac {3}{2}}O_{2}+6\ H^{+}+6\ e^{-}\to 3\ H_{2}O} }
1596:
1422:
US Department of
Transportation moves to approve fuel cells for aircraft use
1069:
614:
591:
583:
990:{\displaystyle \mathrm {CH_{3}OH+{\frac {3}{2}}O_{2}\to 2\ H_{2}O+CO_{2}} }
602:
such as pumping. The need for water limits the energy density of the fuel.
562:{\displaystyle \mathrm {CH_{3}OH+{\frac {3}{2}}O_{2}\to 2\ H_{2}O+CO_{2}} }
769:{\displaystyle \mathrm {CH_{3}OH+6\ OH^{-}\to 5\ H_{2}O+6\ e^{-}+CO_{2}} }
209:(H) are transported across the proton exchange membrane - often made from
1780:
644:
Using these OH groups in the half reactions, they are also expressed as:
579:
218:
190:
186:
157:
149:, about two times greater than liquid hydrogen and 2.6 times higher than
82:
74:
39:
1371:
1354:
595:
202:
344:{\displaystyle \mathrm {CH_{3}OH+H_{2}O\to 6\ H^{+}+6\ e^{-}+CO_{2}} }
214:
210:
206:
125:
42:
is used as the fuel and a special proton-conducting polymer as the
587:
198:
182:
168:
and other consumer electronic devices. On September 24, 2007, the
116:
vehicles such as forklifts and tuggers and consumer goods such as
97:
18:
1548:
618:
1569:
1045:
has to be removed from the solution flow exiting the fuel cell;
578:
Methanol and water are adsorbed on a catalyst usually made of
145:
of methanol is an order of magnitude greater than even highly
1565:
646:
637:, which is released from the surface as a gas: CO + OH• → CO
230:
1012:
cross-over, but also limit the maximum attainable current.
103:
The only waste products with these types of fuel cells are
1355:"A Review of The Methanol Economy: The Fuel Cell Route"
904:
792:
665:
476:
367:
249:
1779:
1711:
1690:
1639:
1603:
629:O → OH• + H + e. The hydroxy radical then oxidizes
1175:Keith Scott, Lei Xing (2012). "3.1 Introduction".
989:
880:
768:
561:
452:
343:
1487:Electrochemistry and Interfacial Electrochemistry
81:, DMFCs use a methanol solution (usually around 1
1341:Tenn. Nissan Plant to Use Methanol to Cut Costs
1475:, by the US transport security administration.
1581:
8:
1588:
1574:
1566:
1454:, by the US department of transportation.
1370:
980:
961:
942:
928:
913:
905:
903:
871:
849:
827:
808:
794:
793:
791:
759:
743:
721:
702:
674:
666:
664:
552:
533:
514:
500:
485:
477:
475:
440:
421:
402:
383:
369:
368:
366:
334:
318:
299:
277:
258:
250:
248:
1228:International Journal of Hydrogen Energy
1117:
609:During the methanol oxidation reaction
213:- to the cathode where they react with
92:Other issues include the management of
49:Whilst the thermodynamic theoretical
7:
1465:3-1-1 Gains International Acceptance
36:proton-exchange membrane fuel cells
1626:Proton-exchange membrane fuel cell
1185:10.1016/B978-0-12-386874-9.00005-1
1162:10.1016/B978-0-12-383860-5.00004-3
977:
973:
967:
958:
939:
922:
919:
910:
906:
868:
864:
846:
833:
824:
805:
756:
752:
740:
727:
718:
699:
695:
683:
680:
671:
667:
549:
545:
539:
530:
511:
494:
491:
482:
478:
446:
437:
418:
399:
380:
331:
327:
315:
296:
283:
274:
267:
264:
255:
251:
14:
1154:Advanced Power Generation Systems
1769:Unitized regenerative fuel cell
1293:10.1016/j.electacta.2010.09.001
1095:Portable fuel cell applications
170:US Department of Transportation
73:, where methanol is reacted to
1518:10.1016/j.jpowsour.2012.10.061
1504:Li, Xianglin; Faghri. "Amir".
1328:10.1016/j.jpowsour.2013.08.029
1240:10.1016/j.ijhydene.2011.08.107
1139:10.1016/j.jpowsour.2007.03.050
1060:Alkali anion-exchange membrane
948:
855:
708:
520:
427:
286:
1:
1764:Solid oxide electrolyzer cell
1647:Direct borohydride fuel cell
71:indirect methanol fuel cells
51:energy conversion efficiency
1734:Membrane electrode assembly
1677:Reformed methanol fuel cell
1408:10.1016/j.enpol.2008.09.036
1075:Glossary of fuel cell terms
197:. Water is consumed at the
130:indirect methanol fuel cell
1859:
1754:Protonic ceramic fuel cell
1724:Electro-galvanic fuel cell
1616:Molten carbonate fuel cell
1065:Dynamic hydrogen electrode
28:Direct methanol fuel cells
1812:
1744:Photoelectrochemical cell
1662:Direct methanol fuel cell
1621:Phosphoric acid fuel cell
181:The DMFC relies upon the
23:Direct methanol fuel cell
1749:Proton-exchange membrane
1657:Direct-ethanol fuel cell
1506:Journal of Power Sources
1308:Journal of Power Sources
1127:Journal of Power Sources
1739:Membraneless Fuel Cells
1672:Metal hydride fuel cell
1652:Direct carbon fuel cell
1759:Regenerative fuel cell
1698:Enzymatic biofuel cell
991:
882:
770:
563:
454:
345:
24:
1667:Formic acid fuel cell
1631:Solid oxide fuel cell
1177:Fuel Cell Engineering
992:
883:
771:
564:
455:
346:
156:Methanol is slightly
151:lithium-ion batteries
34:are a subcategory of
22:
1085:Methanol (data page)
902:
790:
663:
474:
365:
247:
201:and produced at the
1703:Microbial fuel cell
1432:, by FuelCellToday.
1400:2008EnPol..36.4356E
1320:2014JPS...246..899A
1281:Electrochimica Acta
1200:Electrochimica Acta
147:compressed hydrogen
1611:Alkaline fuel cell
1559:2012-03-03 at the
1471:2008-05-09 at the
1450:2011-07-25 at the
1428:2009-02-11 at the
1372:10.3390/en13030596
1007:Cross-over current
987:
878:
766:
559:
450:
341:
217:to produce water.
25:
1825:
1824:
1394:(12): 4356–4362.
1260:. 4 December 2020
1004:
1003:
1000:
956:
936:
896:Overall reaction
891:
863:
844:
822:
802:
779:
738:
716:
694:
623:hydroxyl radicals
576:
575:
572:
528:
508:
468:Overall reaction
463:
435:
416:
397:
377:
354:
313:
294:
16:Type of fuel cell
1850:
1682:Zinc–air battery
1590:
1583:
1576:
1567:
1522:
1521:
1501:
1495:
1494:
1482:
1476:
1461:
1455:
1439:
1433:
1418:
1412:
1411:
1383:
1377:
1376:
1374:
1350:
1344:
1338:
1332:
1331:
1303:
1297:
1296:
1276:
1270:
1269:
1267:
1265:
1250:
1244:
1243:
1234:(2): 1857–1864.
1223:
1217:
1214:
1208:
1207:
1195:
1189:
1188:
1172:
1166:
1165:
1149:
1143:
1142:
1122:
1090:Methanol economy
998:
996:
994:
993:
988:
986:
985:
984:
966:
965:
954:
947:
946:
937:
929:
918:
917:
889:
887:
885:
884:
879:
877:
876:
875:
861:
854:
853:
842:
832:
831:
820:
813:
812:
803:
795:
777:
775:
773:
772:
767:
765:
764:
763:
748:
747:
736:
726:
725:
714:
707:
706:
692:
679:
678:
647:
600:active transport
570:
568:
566:
565:
560:
558:
557:
556:
538:
537:
526:
519:
518:
509:
501:
490:
489:
461:
459:
457:
456:
451:
449:
445:
444:
433:
426:
425:
414:
407:
406:
395:
388:
387:
378:
370:
352:
350:
348:
347:
342:
340:
339:
338:
323:
322:
311:
304:
303:
292:
282:
281:
263:
262:
231:
166:laptop computers
59:methanol economy
1858:
1857:
1853:
1852:
1851:
1849:
1848:
1847:
1828:
1827:
1826:
1821:
1808:
1775:
1707:
1686:
1635:
1599:
1594:
1561:Wayback Machine
1545:
1531:
1529:Further reading
1526:
1525:
1503:
1502:
1498:
1484:
1483:
1479:
1473:Wayback Machine
1462:
1458:
1452:Wayback Machine
1440:
1436:
1430:Wayback Machine
1419:
1415:
1385:
1384:
1380:
1352:
1351:
1347:
1339:
1335:
1305:
1304:
1300:
1278:
1277:
1273:
1263:
1261:
1258:Bioengineer.org
1252:
1251:
1247:
1225:
1224:
1220:
1215:
1211:
1197:
1196:
1192:
1179:. p. 147.
1174:
1173:
1169:
1151:
1150:
1146:
1124:
1123:
1119:
1114:
1109:
1100:Rudolf Schulten
1055:
1044:
1031:
1029:Ancillary units
1022:
1009:
997:
976:
957:
938:
909:
900:
899:
888:
867:
845:
823:
804:
788:
787:
776:
755:
739:
717:
698:
670:
661:
660:
640:
631:carbon monoxide
628:
611:carbon monoxide
569:
548:
529:
510:
481:
472:
471:
460:
436:
417:
398:
379:
363:
362:
351:
330:
314:
295:
273:
254:
245:
244:
179:
139:
122:digital cameras
113:
96:created at the
79:steam reforming
69:In contrast to
67:
17:
12:
11:
5:
1856:
1854:
1846:
1845:
1840:
1830:
1829:
1823:
1822:
1820:
1819:
1813:
1810:
1809:
1807:
1806:
1801:
1796:
1791:
1785:
1783:
1777:
1776:
1774:
1773:
1772:
1771:
1766:
1756:
1751:
1746:
1741:
1736:
1731:
1726:
1721:
1715:
1713:
1709:
1708:
1706:
1705:
1700:
1694:
1692:
1688:
1687:
1685:
1684:
1679:
1674:
1669:
1664:
1659:
1654:
1649:
1643:
1641:
1637:
1636:
1634:
1633:
1628:
1623:
1618:
1613:
1607:
1605:
1604:By electrolyte
1601:
1600:
1595:
1593:
1592:
1585:
1578:
1570:
1564:
1563:
1551:
1544:
1543:External links
1541:
1540:
1539:
1530:
1527:
1524:
1523:
1496:
1477:
1456:
1434:
1413:
1378:
1345:
1333:
1298:
1287:(1): 600–606.
1271:
1245:
1218:
1209:
1190:
1167:
1144:
1116:
1115:
1113:
1110:
1108:
1107:
1102:
1097:
1092:
1087:
1082:
1077:
1072:
1067:
1062:
1056:
1054:
1051:
1050:
1049:
1046:
1042:
1039:
1030:
1027:
1021:
1018:
1008:
1005:
1002:
1001:
999:redox reaction
983:
979:
975:
972:
969:
964:
960:
953:
950:
945:
941:
935:
932:
927:
924:
921:
916:
912:
908:
897:
893:
892:
874:
870:
866:
860:
857:
852:
848:
841:
838:
835:
830:
826:
819:
816:
811:
807:
801:
798:
785:
781:
780:
762:
758:
754:
751:
746:
742:
735:
732:
729:
724:
720:
713:
710:
705:
701:
697:
691:
688:
685:
682:
677:
673:
669:
658:
654:
653:
650:
638:
635:carbon dioxide
626:
574:
573:
571:redox reaction
555:
551:
547:
544:
541:
536:
532:
525:
522:
517:
513:
507:
504:
499:
496:
493:
488:
484:
480:
469:
465:
464:
448:
443:
439:
432:
429:
424:
420:
413:
410:
405:
401:
394:
391:
386:
382:
376:
373:
360:
356:
355:
337:
333:
329:
326:
321:
317:
310:
307:
302:
298:
291:
288:
285:
280:
276:
272:
269:
266:
261:
257:
253:
242:
238:
237:
234:
226:half-reactions
195:carbon dioxide
193:layer to form
178:
175:
143:energy density
138:
135:
112:
109:
105:carbon dioxide
94:carbon dioxide
66:
63:
15:
13:
10:
9:
6:
4:
3:
2:
1855:
1844:
1841:
1839:
1836:
1835:
1833:
1818:
1815:
1814:
1811:
1805:
1802:
1800:
1797:
1795:
1792:
1790:
1787:
1786:
1784:
1782:
1778:
1770:
1767:
1765:
1762:
1761:
1760:
1757:
1755:
1752:
1750:
1747:
1745:
1742:
1740:
1737:
1735:
1732:
1730:
1727:
1725:
1722:
1720:
1717:
1716:
1714:
1710:
1704:
1701:
1699:
1696:
1695:
1693:
1691:Biofuel cells
1689:
1683:
1680:
1678:
1675:
1673:
1670:
1668:
1665:
1663:
1660:
1658:
1655:
1653:
1650:
1648:
1645:
1644:
1642:
1638:
1632:
1629:
1627:
1624:
1622:
1619:
1617:
1614:
1612:
1609:
1608:
1606:
1602:
1598:
1591:
1586:
1584:
1579:
1577:
1572:
1571:
1568:
1562:
1558:
1555:
1552:
1550:
1547:
1546:
1542:
1537:
1533:
1532:
1528:
1519:
1515:
1511:
1507:
1500:
1497:
1492:
1488:
1481:
1478:
1474:
1470:
1467:
1466:
1460:
1457:
1453:
1449:
1446:
1445:
1438:
1435:
1431:
1427:
1424:
1423:
1417:
1414:
1409:
1405:
1401:
1397:
1393:
1389:
1388:Energy Policy
1382:
1379:
1373:
1368:
1364:
1360:
1356:
1349:
1346:
1342:
1337:
1334:
1329:
1325:
1321:
1317:
1313:
1309:
1302:
1299:
1294:
1290:
1286:
1282:
1275:
1272:
1259:
1255:
1249:
1246:
1241:
1237:
1233:
1229:
1222:
1219:
1213:
1210:
1205:
1201:
1194:
1191:
1186:
1182:
1178:
1171:
1168:
1163:
1159:
1155:
1148:
1145:
1140:
1136:
1132:
1128:
1121:
1118:
1111:
1106:
1103:
1101:
1098:
1096:
1093:
1091:
1088:
1086:
1083:
1081:
1078:
1076:
1073:
1071:
1068:
1066:
1063:
1061:
1058:
1057:
1052:
1047:
1040:
1037:
1036:
1035:
1028:
1026:
1019:
1017:
1013:
1006:
981:
970:
962:
951:
943:
933:
930:
925:
914:
898:
895:
894:
872:
858:
850:
839:
836:
828:
817:
814:
809:
799:
796:
786:
783:
782:
760:
749:
744:
733:
730:
722:
711:
703:
689:
686:
675:
659:
656:
655:
651:
649:
648:
645:
642:
636:
632:
624:
620:
616:
612:
607:
603:
601:
597:
593:
589:
585:
581:
553:
542:
534:
523:
515:
505:
502:
497:
486:
470:
467:
466:
441:
430:
422:
411:
408:
403:
392:
389:
384:
374:
371:
361:
358:
357:
335:
324:
319:
308:
305:
300:
289:
278:
270:
259:
243:
240:
239:
235:
233:
232:
229:
227:
222:
220:
216:
212:
208:
204:
200:
196:
192:
188:
184:
176:
174:
171:
167:
163:
159:
154:
152:
148:
144:
136:
134:
131:
127:
123:
119:
118:mobile phones
110:
108:
106:
101:
99:
95:
90:
86:
84:
80:
76:
72:
64:
62:
60:
55:
52:
47:
45:
41:
37:
33:
29:
21:
1729:Flow battery
1661:
1538:, March 2010
1536:ITEA Journal
1535:
1509:
1505:
1499:
1490:
1486:
1480:
1464:
1459:
1444:Instructions
1442:
1437:
1421:
1416:
1391:
1387:
1381:
1362:
1358:
1348:
1343:by ABC News.
1336:
1311:
1307:
1301:
1284:
1280:
1274:
1262:. Retrieved
1257:
1248:
1231:
1227:
1221:
1212:
1203:
1199:
1193:
1176:
1170:
1153:
1147:
1130:
1126:
1120:
1080:Liquid fuels
1032:
1023:
1014:
1010:
643:
608:
604:
577:
223:
180:
155:
140:
114:
102:
91:
87:
68:
56:
48:
31:
27:
26:
1719:Blue energy
1512:: 223–240.
1314:: 899–905.
1264:30 December
633:to produce
160:and highly
111:Application
107:and water.
1838:Fuel cells
1832:Categories
1597:Fuel cells
1493:: 267–273.
1365:(3): 596.
1112:References
1105:SymPowerco
1020:Water drag
1070:Fuel cell
949:→
890:reduction
873:−
856:→
851:−
778:oxidation
745:−
709:→
704:−
652:Equation
641:+ H + e.
615:ruthenium
592:diffusion
584:ruthenium
521:→
462:reduction
428:→
423:−
353:oxidation
320:−
287:→
236:Equation
219:Electrons
183:oxidation
162:flammable
38:in which
1843:Methanol
1817:Glossary
1781:Hydrogen
1557:Archived
1469:Archived
1448:Archived
1426:Archived
1359:Energies
1053:See also
784:Cathode
580:platinum
359:Cathode
191:catalyst
187:methanol
177:Reaction
137:Methanol
75:hydrogen
65:The cell
44:membrane
40:methanol
1804:Vehicle
1799:Storage
1794:Station
1789:Economy
1640:By fuel
1396:Bibcode
1316:Bibcode
596:osmosis
207:Protons
203:cathode
126:laptops
1712:Others
955:
862:
843:
821:
737:
715:
693:
657:Anode
598:), or
527:
434:
415:
396:
312:
293:
241:Anode
215:oxygen
211:Nafion
588:anode
228:are:
199:anode
189:on a
158:toxic
98:anode
32:DMFCs
1266:2020
619:gold
582:and
224:The
1514:doi
1510:226
1404:doi
1367:doi
1324:doi
1312:246
1289:doi
1236:doi
1204:199
1181:doi
1158:doi
1135:doi
1131:169
617:or
185:of
124:or
77:by
30:or
1834::
1508:.
1491:60
1489:.
1402:.
1392:36
1390:.
1363:13
1361:.
1357:.
1322:.
1310:.
1285:56
1283:.
1256:.
1232:37
1230:.
1202:.
1156:.
1133:.
1129:.
1041:CO
205:.
120:,
61:.
1589:e
1582:t
1575:v
1520:.
1516::
1410:.
1406::
1398::
1375:.
1369::
1330:.
1326::
1318::
1295:.
1291::
1268:.
1242:.
1238::
1206:.
1187:.
1183::
1164:.
1160::
1141:.
1137::
1043:2
982:2
978:O
974:C
971:+
968:O
963:2
959:H
952:2
944:2
940:O
934:2
931:3
926:+
923:H
920:O
915:3
911:H
907:C
869:H
865:O
859:6
847:e
840:6
837:+
834:O
829:2
825:H
818:3
815:+
810:2
806:O
800:2
797:3
761:2
757:O
753:C
750:+
741:e
734:6
731:+
728:O
723:2
719:H
712:5
700:H
696:O
690:6
687:+
684:H
681:O
676:3
672:H
668:C
639:2
627:2
594:(
554:2
550:O
546:C
543:+
540:O
535:2
531:H
524:2
516:2
512:O
506:2
503:3
498:+
495:H
492:O
487:3
483:H
479:C
447:O
442:2
438:H
431:3
419:e
412:6
409:+
404:+
400:H
393:6
390:+
385:2
381:O
375:2
372:3
336:2
332:O
328:C
325:+
316:e
309:6
306:+
301:+
297:H
290:6
284:O
279:2
275:H
271:+
268:H
265:O
260:3
256:H
252:C
83:M
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.