295:
may be absorbed again. Another inner filter effect occurs because of high concentrations of absorbing molecules, including the fluorophore. The result is that the intensity of the excitation light is not constant throughout the solution. Resultingly, only a small percentage of the excitation light reaches the fluorophores that are visible for the detection system. The inner filter effects change the spectrum and intensity of the emitted light and they must therefore be considered when analysing the emission spectrum of fluorescent light.
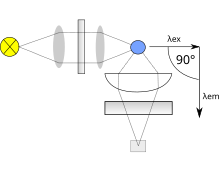
205:, that is, light with other wavelengths than the targeted. An ideal monochromator would only transmit light in the specified range and have a high wavelength-independent transmission. When measuring at a 90° angle, only the light scattered by the sample causes stray light. This results in a better signal-to-noise ratio, and lowers the detection limit by approximately a factor 10000, when compared to the 180° geometry. Furthermore, the fluorescence can also be measured from the front, which is often done for turbid or opaque samples .
333:
or a few tryptophan residues. Therefore, tryptophan fluorescence can be a very sensitive measurement of the conformational state of individual tryptophan residues. The advantage compared to extrinsic probes is that the protein itself is not changed. The use of intrinsic fluorescence for the study of protein conformation is in practice limited to cases with few (or perhaps only one) tryptophan residues, since each experiences a different local environment, which gives rise to different emission spectra.
263:
either instrument- or sample-related. Firstly, the distortion arising from the instrument is discussed. As a start, the light source intensity and wavelength characteristics varies over time during each experiment and between each experiment. Furthermore, no lamp has a constant intensity at all wavelengths. To correct this, a beam splitter can be applied after the excitation monochromator or filter to direct a portion of the light to a reference detector.
222:
233:
1577:
172:
213:
absorption, and the emission monochromator scans the spectrum. For measuring excitation spectra, the wavelength passing through the emission filter or monochromator is kept constant and the excitation monochromator is scanning. The excitation spectrum generally is identical to the absorption spectrum as the fluorescence intensity is proportional to the absorption.
1589:
267:
excitation monochromator or filter. The percentage of the fluorescence picked up by the detector is also dependent upon the system. Furthermore, the detector quantum efficiency, that is, the percentage of photons detected, varies between different detectors, with wavelength and with time, as the detector inevitably deteriorates.
31:
271:
to select materials that have relatively little absorption in the wavelength range of interest. Quartz is ideal because it transmits from 200 nm-2500 nm; higher grade quartz can even transmit up to 3500 nm, whereas the absorption properties of other materials can mask the fluorescence from the sample.
112:, from its ground electronic state to one of the various vibrational states in the excited electronic state. Collisions with other molecules cause the excited molecule to lose vibrational energy until it reaches the lowest vibrational state from the excited electronic state. This process is often visualized with a
291:, the molecules may relax back to a vibrational level other than the vibrational ground state. In fluorescence spectra, it is always seen at a constant wavenumber difference relative to the excitation wavenumber e.g. the peak appears at a wavenumber 3600 cm lower than the excitation light in water.
200:
As mentioned before, the fluorescence is most often measured at a 90° angle relative to the excitation light. This geometry is used instead of placing the sensor at the line of the excitation light at a 180° angle in order to avoid interference of the transmitted excitation light. No monochromator is
196:
light illuminates a grating and exits with a different angle depending on the wavelength. The monochromator can then be adjusted to select which wavelengths to transmit. For allowing anisotropy measurements, the addition of two polarization filters is necessary: One after the excitation monochromator
294:
Other aspects to consider are the inner filter effects. These include reabsorption. Reabsorption happens because another molecule or part of a macromolecule absorbs at the wavelengths at which the fluorophore emits radiation. If this is the case, some or all of the photons emitted by the fluorophore
270:
Two other topics that must be considered include the optics used to direct the radiation and the means of holding or containing the sample material (called a cuvette or cell). For most UV, visible, and NIR measurements the use of precision quartz cuvettes is necessary. In both cases, it is important
266:
Additionally, the transmission efficiency of monochromators and filters must be taken into account. These may also change over time. The transmission efficiency of the monochromator also varies depending on wavelength. This is the reason that an optional reference detector should be placed after the
262:
Unlike in UV/visible spectroscopy, ‘standard’, device independent spectra are not easily attained. Several factors influence and distort the spectra, and corrections are necessary to attain ‘true’, i.e. machine-independent, spectra. The different types of distortions will here be classified as being
119:
The molecule then drops down to one of the various vibrational levels of the ground electronic state again, emitting a photon in the process. As molecules may drop down into any of several vibrational levels in the ground state, the emitted photons will have different energies, and thus frequencies.
332:
of Trp fluorescence). Also, energy transfer between tryptophan and the other fluorescent amino acids is possible, which would affect the analysis, especially in cases where the Förster acidic approach is taken. In addition, tryptophan is a relatively rare amino acid; many proteins contain only one
278:
As mentioned earlier, distortions arise from the sample as well. Therefore, some aspects of the sample must be taken into account too. Firstly, photodecomposition may decrease the intensity of fluorescence over time. Scattering of light must also be taken into account. The most significant types of
123:
For atomic species, the process is similar; however, since atomic species do not have vibrational energy levels, the emitted photons are often at the same wavelength as the incident radiation. This process of re-emitting the absorbed photon is "resonance fluorescence" and while it is characteristic
187:
in particular. A laser only emits light of high irradiance at a very narrow wavelength interval, typically under 0.01 nm, which makes an excitation monochromator or filter unnecessary. The disadvantage of this method is that the wavelength of a laser cannot be changed by much. A mercury vapor
274:
Correction of all these instrumental factors for getting a ‘standard’ spectrum is a tedious process, which is only applied in practice when it is strictly necessary. This is the case when measuring the quantum yield or when finding the wavelength with the highest emission intensity for instance.
212:
The most versatile fluorimeters with dual monochromators and a continuous excitation light source can record both an excitation spectrum and a fluorescence spectrum. When measuring fluorescence spectra, the wavelength of the excitation light is kept constant, preferably at a wavelength of high
208:
The detector can either be single-channeled or multichanneled. The single-channeled detector can only detect the intensity of one wavelength at a time, while the multichanneled one detects the intensity of all wavelengths simultaneously, making the emission monochromator or filter unnecessary.
347:
molecules, the microenvironment of the tryptophan might change. For example, if a protein containing a single tryptophan in its 'hydrophobic' core is denatured with increasing temperature, a red-shifted emission spectrum will appear. This is due to the exposure of the tryptophan to an aqueous
167:
Both types use the following scheme: the light from an excitation source passes through a filter or monochromator, and strikes the sample. A proportion of the incident light is absorbed by the sample, and some of the molecules in the sample fluoresce. The fluorescent light is emitted in all
132:
is measured by recording the emission spectra resulting from a range of excitation wavelengths and combining them all together. This is a three dimensional surface data set: emission intensity as a function of excitation and emission wavelengths, and is typically depicted as a contour map.
320:, ranging from ca. 300 to 350 nm depending in the polarity of the local environment Hence, protein fluorescence may be used as a diagnostic of the conformational state of a protein. Furthermore, tryptophan fluorescence is strongly influenced by the proximity of other residues (
127:
In a typical fluorescence (emission) measurement, the excitation wavelength is fixed and the detection wavelength varies, while in a fluorescence excitation measurement the detection wavelength is fixed and the excitation wavelength is varied across a region of interest. An
348:
environment as opposed to a hydrophobic protein interior. In contrast, the addition of a surfactant to a protein which contains a tryptophan which is exposed to the aqueous solvent will cause a blue-shifted emission spectrum if the tryptophan is embedded in the surfactant
188:
lamp is a line lamp, meaning it emits light near peak wavelengths. By contrast, a xenon arc has a continuous emission spectrum with nearly constant intensity in the range from 300-800 nm and a sufficient irradiance for measurements down to just above 200 nm.
909:
Nakar, Amir; Schmilovitch, Ze’ev; Vaizel-Ohayon, Dalit; Kroupitski, Yulia; Borisover, Mikhail; Sela (Saldinger), Shlomo (2020-02-01). "Quantification of bacteria in water using PLS analysis of emission spectra of fluorescence and excitation-emission matrices".
168:
directions. Some of this fluorescent light passes through a second filter or monochromator and reaches a detector, which is usually placed at 90° to the incident light beam to minimize the risk of transmitted or reflected incident light reaching the detector.
413:
In the field of water research, fluorescence spectroscopy can be used to monitor water quality by detecting organic pollutants. Recent advances in computer science and machine learning have even enabled detection of bacterial contamination of water.
315:
residues, with some emissions due to tyrosine and phenylalanine; but disulfide bonds also have appreciable absorption in this wavelength range. Typically, tryptophan has a wavelength of maximum absorption of 280 nm and an emission peak that is
191:
Filters and/or monochromators may be used in fluorimeters. A monochromator transmits light of an adjustable wavelength with an adjustable tolerance. The most common type of monochromator utilizes a diffraction grating, that is,
120:
Therefore, by analysing the different frequencies of light emitted in fluorescent spectroscopy, along with their relative intensities, the structure of the different vibrational levels can be determined.
681:"On the origin and correction for inner filter effects in fluorescence. Part II: secondary inner filter effect -the proper use of front-face configuration for highly absorbing and scattering samples"
616:
Kimball, Joseph; Chavez, Jose; Ceresa, Luca; Kitchner, Emma; Nurekeyev, Zhangatay; Doan, Hung; Szabelski, Mariusz; Borejdo, Julian; Gryczynski, Ignacy; Gryczynski, Zygmunt (2020-06-01).
76:. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores.
1408:
971:"Quantifying uptake and distribution of arginine rich peptides at therapeutic concentrations using fluorescence correlation spectroscopy and image correlation spectroscopy techniques"
339:
is an important intrinsic fluorescent (amino acid), which can be used to estimate the nature of microenvironment of the tryptophan. When performing experiments with denaturants,
287:
the scattered light changes wavelength usually to longer wavelengths. Raman scattering is the result of a virtual electronic state induced by the excitation light. From this
417:
In biomedical research, fluorescence spectroscopy is used to evaluate the efficiency of drug distribution through the cross-linking of fluorescent agents to various drugs.
1081:
753:
679:
Ceresa, Luca; Kimball, Joseph; Chavez, Jose; Kitchner, Emma; Nurekeyev, Zhangatay; Doan, Hung; Borejdo, Julian; Gryczynski, Ignacy; Gryczynski, Zygmunt (2021-05-24).
386:
Atomic
Fluorescence Spectroscopy (AFS) techniques are useful in other kinds of analysis/measurement of a compound present in air or water, or other media, such as
105:(a low energy state) of interest, and an excited electronic state of higher energy. Within each of these electronic states there are various vibrational states.
618:"On the origin and correction for inner filter effects in fluorescence Part I: primary inner filter effect-the proper approach for sample absorbance correction"
1299:
311:
is a mixture of the fluorescence from individual aromatic residues. Most of the intrinsic fluorescence emissions of a folded protein are due to excitation of
1232:
1177:
1146:
1141:
831:
Cumulative effects of amino acid substitutions and hydrophobic mismatch upon the transmembrane stability and conformation of hydrophobic alpha-helices.
1514:
1332:
1194:
1463:
1282:
1126:
1403:
1205:
1106:
463:
1349:
1327:
1074:
844:
420:
Fluorescence spectroscopy in biophysical research enables individuals to visualize and characterize lipid domains within cellular membranes.
1415:
1337:
1019:
555:
1167:
1272:
1217:
101:. Fluorescence spectroscopy is primarily concerned with electronic and vibrational states. Generally, the species being examined has a
1499:
1251:
1067:
770:
1504:
1322:
1519:
1489:
1420:
1354:
1448:
1239:
1136:
750:
1246:
1151:
1380:
1227:
1116:
53:
1536:
1375:
1344:
1277:
379:
Fluorescence spectroscopy is used in, among others, biochemical, medical, and chemical research fields for analyzing
1003:
Fluorescence
Spectroscopy in Biology: Advanced Methods and their Applications to Membranes, Proteins, DNA, and Cells
1526:
1468:
1317:
1189:
439:
1552:
1531:
1294:
394:
572:
329:
1593:
1425:
1121:
288:
73:
1212:
1620:
1615:
1581:
1453:
1184:
1098:
919:
859:
782:
692:
629:
363:
With fluorescence excitation at 295 nm, the tryptophan emission spectrum is dominant over the weaker
1038:
741:
Lakowicz, J. R. (1999). Principles of
Fluorescence Spectroscopy. Kluwer Academic / Plenum Publishers
573:"OpenFluor– an online spectral library of auto-fluorescence by organic compounds in the environment"
1509:
1222:
1131:
280:
248:
158:
1557:
1494:
1473:
1289:
1267:
1200:
1111:
970:
951:
891:
724:
661:
184:
154:
142:
1006:. Springer Series on Fluorescence. Vol. 3. Berlin, Heidelberg: Springer Berlin Heidelberg.
383:. There has also been a report of its use in differentiating malignant skin tumors from benign.
1458:
1385:
1359:
1015:
943:
935:
883:
875:
808:
716:
708:
653:
645:
551:
521:
513:
434:
429:
349:
113:
61:
35:
1007:
982:
927:
867:
798:
790:
700:
637:
587:
505:
401:
380:
284:
102:
221:
179:
Various light sources may be used as excitation sources, including lasers, LED, and lamps;
757:
816:
923:
863:
786:
696:
633:
232:
803:
544:
317:
308:
180:
794:
400:
Additionally, Fluorescence spectroscopy can be adapted to the microscopic level using
1609:
955:
895:
843:
Carstea, Elfrida M.; Bridgeman, John; Baker, Andy; Reynolds, Darren M. (2016-05-15).
728:
665:
509:
368:
252:
161:
98:
69:
1055:
Database of fluorescent minerals with pictures, activators and spectra (fluomin.org)
68:
of certain compounds and causes them to emit light; typically, but not necessarily,
1090:
1048:
496:
Eisinger, Josef; Flores, Jorge (1979). "Front-face fluorometry of liquid samples".
304:
245:
150:
92:
57:
17:
146:
986:
931:
871:
279:
scattering in this context are
Rayleigh and Raman scattering. Light scattered by
357:
344:
256:
202:
80:
704:
641:
340:
336:
312:
193:
939:
879:
712:
649:
517:
1001:
237:
65:
947:
887:
812:
720:
680:
657:
617:
571:
Murphy, Kathleen R.; Stedmon, Colin A.; Wenig, Philip; Bro, Rasmus (2014).
606:
Gauglitz, G. and Vo-Dinh, T. (2003). Handbook of spectroscopy. Wiley-VCH.
525:
364:
171:
592:
353:
1054:
464:
Animation for the principle of fluorescence and UV-visible absorbance
109:
1011:
124:
of atomic fluorescence, is seen in molecular fluorescence as well.
387:
231:
225:
220:
170:
29:
407:
197:
or filter, and one before the emission monochromator or filter.
30:
1063:
1059:
845:"Fluorescence spectroscopy for wastewater monitoring: A review"
34:
Atomic fluorescence spectroscopy analyzer for determination of
406:
In analytical chemistry, fluorescence detectors are used with
108:
In fluorescence, the species is first excited, by absorbing a
477:
F.James Holler, Douglas A. Skoog & Stanley R. Crouch 2006
1044:
486:
390:
which is used for heavy metals detection, such as mercury.
771:"Mechanisms of tryptophan fluorescence shifts in proteins"
283:
has the same wavelength as the incident light, whereas in
60:
from a sample. It involves using a beam of light, usually
393:
Fluorescence can also be used to redirect photons, see
175:
A simplistic design of the components of a fluorimeter
969:
Staley, Ben; Zindy, Egor; Pluen, Alain (2010-12-25).
164:
to isolate the incident light and fluorescent light.
542:
Ashutosh Sharma; Stephen G. Schulman (21 May 1999).
356:. Proteins that lack tryptophan may be coupled to a
1545:
1482:
1441:
1434:
1396:
1368:
1310:
1260:
1160:
1097:
543:
1000:Hof, M.; Hutterer, R.; Fidler, V., eds. (2005).
537:
535:
751:Intrinsic Fluorescence of Proteins and Peptides
1075:
97:Molecules have various states referred to as
79:Devices that measure fluorescence are called
8:
1147:Vibrational spectroscopy of linear molecules
1438:
1142:Nuclear resonance vibrational spectroscopy
1082:
1068:
1060:
1515:Inelastic electron tunneling spectroscopy
1195:Resonance-enhanced multiphoton ionization
833:Biochemistry. 2003 Mar 25;42(11):3275-85.
802:
591:
546:Introduction to Fluorescence Spectroscopy
1283:Extended X-ray absorption fine structure
685:Methods and Applications in Fluorescence
622:Methods and Applications in Fluorescence
141:Two general types of instruments exist:
1051:analysis of organic matter fluorescence
459:
457:
455:
451:
251:will generally be proportional to the
7:
1588:
328:groups such as Asp or Glu can cause
27:Type of electromagnetic spectroscopy
475:Principles Of Instrumental Analysis
1041:, the database of fluorescent dyes
201:perfect and it will transmit some
25:
1500:Deep-level transient spectroscopy
1252:Saturated absorption spectroscopy
1587:
1576:
1575:
1505:Dual-polarization interferometry
145:that use filters to isolate the
64:, that excites the electrons in
1520:Scanning tunneling spectroscopy
1495:Circular dichroism spectroscopy
1490:Acoustic resonance spectroscopy
72:. A complementary technique is
1449:Fourier-transform spectroscopy
1137:Vibrational circular dichroism
1:
1247:Cavity ring-down spectroscopy
1152:Thermal infrared spectroscopy
1047:, Community tools supporting
795:10.1016/S0006-3495(01)76183-8
769:Vivian JT, Callis PR (2001).
1381:Inelastic neutron scattering
987:10.1016/j.drudis.2010.09.402
932:10.1016/j.watres.2019.115197
872:10.1016/j.watres.2016.03.021
510:10.1016/0003-2697(79)90783-8
54:electromagnetic spectroscopy
1442:Data collection, processing
1318:Photoelectron/photoemission
395:fluorescent solar collector
240:showing substance matchings
1637:
1527:Photoacoustic spectroscopy
1469:Time-resolved spectroscopy
440:Laser-induced fluorescence
244:At low concentrations the
90:
1571:
1553:Astronomical spectroscopy
1532:Photothermal spectroscopy
42:Fluorescence spectroscopy
705:10.1088/2050-6120/ac0243
642:10.1088/2050-6120/ab947c
1537:Pump–probe spectroscopy
1426:Ferromagnetic resonance
1218:Laser-induced breakdown
498:Analytical Biochemistry
299:Tryptophan fluorescence
103:ground electronic state
74:absorption spectroscopy
1233:Glow-discharge optical
1213:Raman optical activity
1127:Rotational–vibrational
241:
229:
176:
38:
1454:Hyperspectral imaging
829:Caputo GA, London E.
819:on September 6, 2008.
235:
228:export from OpenChrom
224:
174:
33:
1206:Coherent anti-Stokes
1161:UV–Vis–NIR "Optical"
981:(23–24): 1099–1099.
975:Drug Discovery Today
236:OpenFluor plugin in
1510:Hadron spectroscopy
1300:Conversion electron
1261:X-ray and Gamma ray
1168:Ultraviolet–visible
924:2020WatRe.16915197N
864:2016WatRe..95..205C
787:2001BpJ....80.2093V
697:2021MApFl...9c5005C
634:2020MApFl...8c3002K
281:Rayleigh scattering
185:mercury-vapor lamps
159:diffraction grating
155:spectrofluorometers
143:filter fluorometers
18:Excitation spectrum
1558:Force spectroscopy
1483:Measured phenomena
1474:Video spectroscopy
1178:Cold vapour atomic
756:2010-05-16 at the
593:10.1039/C3AY41935E
242:
230:
177:
50:spectrofluorometry
39:
1603:
1602:
1567:
1566:
1459:Spectrophotometry
1386:Neutron spin echo
1360:Beta spectroscopy
1273:Energy-dispersive
1021:978-3-540-22338-2
557:978-0-471-11098-9
435:Photoluminescence
430:Lanthanide probes
381:organic compounds
114:Jablonski diagram
62:ultraviolet light
16:(Redirected from
1628:
1591:
1590:
1579:
1578:
1439:
1350:phenomenological
1099:Vibrational (IR)
1084:
1077:
1070:
1061:
1039:Fluorophores.org
1026:
1025:
997:
991:
990:
966:
960:
959:
906:
900:
899:
849:
840:
834:
827:
821:
820:
815:. Archived from
806:
766:
760:
748:
742:
739:
733:
732:
676:
670:
669:
613:
607:
604:
598:
597:
595:
577:
568:
562:
561:
549:
539:
530:
529:
493:
487:
484:
478:
472:
466:
461:
402:microfluorimetry
285:Raman scattering
217:Analysis of data
21:
1636:
1635:
1631:
1630:
1629:
1627:
1626:
1625:
1606:
1605:
1604:
1599:
1563:
1541:
1478:
1430:
1392:
1364:
1306:
1256:
1156:
1117:Resonance Raman
1093:
1088:
1035:
1030:
1029:
1022:
1012:10.1007/b138383
999:
998:
994:
968:
967:
963:
908:
907:
903:
847:
842:
841:
837:
828:
824:
781:(5): 2093–109.
768:
767:
763:
758:Wayback Machine
749:
745:
740:
736:
678:
677:
673:
615:
614:
610:
605:
601:
575:
570:
569:
565:
558:
541:
540:
533:
495:
494:
490:
485:
481:
473:
469:
462:
453:
448:
426:
377:
301:
219:
139:
137:Instrumentation
95:
89:
52:) is a type of
44:(also known as
28:
23:
22:
15:
12:
11:
5:
1634:
1632:
1624:
1623:
1618:
1608:
1607:
1601:
1600:
1598:
1597:
1585:
1572:
1569:
1568:
1565:
1564:
1562:
1561:
1555:
1549:
1547:
1543:
1542:
1540:
1539:
1534:
1529:
1524:
1523:
1522:
1512:
1507:
1502:
1497:
1492:
1486:
1484:
1480:
1479:
1477:
1476:
1471:
1466:
1461:
1456:
1451:
1445:
1443:
1436:
1432:
1431:
1429:
1428:
1423:
1418:
1413:
1412:
1411:
1400:
1398:
1394:
1393:
1391:
1390:
1389:
1388:
1378:
1372:
1370:
1366:
1365:
1363:
1362:
1357:
1352:
1347:
1342:
1341:
1340:
1335:
1333:Angle-resolved
1330:
1325:
1314:
1312:
1308:
1307:
1305:
1304:
1303:
1302:
1292:
1287:
1286:
1285:
1280:
1275:
1264:
1262:
1258:
1257:
1255:
1254:
1249:
1244:
1243:
1242:
1237:
1236:
1235:
1220:
1215:
1210:
1209:
1208:
1198:
1192:
1187:
1182:
1181:
1180:
1170:
1164:
1162:
1158:
1157:
1155:
1154:
1149:
1144:
1139:
1134:
1129:
1124:
1119:
1114:
1109:
1103:
1101:
1095:
1094:
1089:
1087:
1086:
1079:
1072:
1064:
1058:
1057:
1052:
1042:
1034:
1033:External links
1031:
1028:
1027:
1020:
992:
961:
912:Water Research
901:
852:Water Research
835:
822:
761:
743:
734:
671:
608:
599:
586:(3): 658–661.
563:
556:
531:
488:
479:
467:
450:
449:
447:
444:
443:
442:
437:
432:
425:
422:
376:
373:
371:fluorescence.
318:solvatochromic
309:folded protein
300:
297:
218:
215:
162:monochromators
138:
135:
91:Main article:
88:
85:
56:that analyzes
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1633:
1622:
1619:
1617:
1614:
1613:
1611:
1596:
1595:
1586:
1584:
1583:
1574:
1573:
1570:
1559:
1556:
1554:
1551:
1550:
1548:
1544:
1538:
1535:
1533:
1530:
1528:
1525:
1521:
1518:
1517:
1516:
1513:
1511:
1508:
1506:
1503:
1501:
1498:
1496:
1493:
1491:
1488:
1487:
1485:
1481:
1475:
1472:
1470:
1467:
1465:
1462:
1460:
1457:
1455:
1452:
1450:
1447:
1446:
1444:
1440:
1437:
1433:
1427:
1424:
1422:
1419:
1417:
1414:
1410:
1407:
1406:
1405:
1402:
1401:
1399:
1395:
1387:
1384:
1383:
1382:
1379:
1377:
1374:
1373:
1371:
1367:
1361:
1358:
1356:
1353:
1351:
1348:
1346:
1343:
1339:
1336:
1334:
1331:
1329:
1326:
1324:
1321:
1320:
1319:
1316:
1315:
1313:
1309:
1301:
1298:
1297:
1296:
1293:
1291:
1288:
1284:
1281:
1279:
1276:
1274:
1271:
1270:
1269:
1266:
1265:
1263:
1259:
1253:
1250:
1248:
1245:
1241:
1238:
1234:
1231:
1230:
1229:
1226:
1225:
1224:
1221:
1219:
1216:
1214:
1211:
1207:
1204:
1203:
1202:
1199:
1196:
1193:
1191:
1190:Near-infrared
1188:
1186:
1183:
1179:
1176:
1175:
1174:
1171:
1169:
1166:
1165:
1163:
1159:
1153:
1150:
1148:
1145:
1143:
1140:
1138:
1135:
1133:
1130:
1128:
1125:
1123:
1120:
1118:
1115:
1113:
1110:
1108:
1105:
1104:
1102:
1100:
1096:
1092:
1085:
1080:
1078:
1073:
1071:
1066:
1065:
1062:
1056:
1053:
1050:
1046:
1043:
1040:
1037:
1036:
1032:
1023:
1017:
1013:
1009:
1005:
1004:
996:
993:
988:
984:
980:
976:
972:
965:
962:
957:
953:
949:
945:
941:
937:
933:
929:
925:
921:
917:
913:
905:
902:
897:
893:
889:
885:
881:
877:
873:
869:
865:
861:
857:
853:
846:
839:
836:
832:
826:
823:
818:
814:
810:
805:
800:
796:
792:
788:
784:
780:
776:
772:
765:
762:
759:
755:
752:
747:
744:
738:
735:
730:
726:
722:
718:
714:
710:
706:
702:
698:
694:
691:(3): 035005.
690:
686:
682:
675:
672:
667:
663:
659:
655:
651:
647:
643:
639:
635:
631:
628:(3): 033002.
627:
623:
619:
612:
609:
603:
600:
594:
589:
585:
581:
580:Anal. Methods
574:
567:
564:
559:
553:
548:
547:
538:
536:
532:
527:
523:
519:
515:
511:
507:
503:
499:
492:
489:
483:
480:
476:
471:
468:
465:
460:
458:
456:
452:
445:
441:
438:
436:
433:
431:
428:
427:
423:
421:
418:
415:
411:
409:
404:
403:
398:
396:
391:
389:
384:
382:
374:
372:
370:
369:phenylalanine
366:
361:
359:
355:
351:
346:
342:
338:
334:
331:
327:
323:
319:
314:
310:
306:
298:
296:
292:
290:
289:virtual state
286:
282:
276:
272:
268:
264:
260:
258:
254:
253:concentration
250:
247:
239:
234:
227:
223:
216:
214:
210:
206:
204:
198:
195:
189:
186:
182:
173:
169:
165:
163:
160:
156:
152:
148:
144:
136:
134:
131:
125:
121:
117:
115:
111:
106:
104:
100:
99:energy levels
94:
86:
84:
82:
77:
75:
71:
70:visible light
67:
63:
59:
55:
51:
47:
43:
37:
32:
19:
1621:Spectroscopy
1616:Fluorescence
1592:
1580:
1560:(a misnomer)
1546:Applications
1464:Time-stretch
1355:paramagnetic
1173:Fluorescence
1172:
1091:Spectroscopy
1002:
995:
978:
974:
964:
915:
911:
904:
855:
851:
838:
830:
825:
817:the original
778:
774:
764:
746:
737:
688:
684:
674:
625:
621:
611:
602:
583:
579:
566:
545:
504:(1): 15–21.
501:
497:
491:
482:
474:
470:
419:
416:
412:
405:
399:
392:
385:
378:
375:Applications
362:
335:
325:
321:
305:fluorescence
302:
293:
277:
273:
269:
265:
261:
246:fluorescence
243:
211:
207:
199:
190:
178:
166:
140:
130:emission map
129:
126:
122:
118:
107:
96:
93:Fluorescence
81:fluorometers
78:
58:fluorescence
49:
45:
41:
40:
1132:Vibrational
1049:chemometric
858:: 205–219.
358:fluorophore
345:amphiphilic
341:surfactants
257:fluorophore
203:stray light
151:fluorescent
46:fluorimetry
1610:Categories
1338:Two-photon
1240:absorption
1122:Rotational
918:: 115197.
775:Biophys. J
446:References
337:Tryptophan
326:protonated
313:tryptophan
194:collimated
181:xenon arcs
157:that use
153:light and
149:light and
1416:Terahertz
1397:Radiowave
1295:Mössbauer
1045:OpenFluor
956:204967767
940:0043-1354
896:205696150
880:0043-1354
729:235201243
713:2050-6120
666:218758981
650:2050-6120
550:. Wiley.
518:0003-2697
343:or other
330:quenching
324:, nearby
249:intensity
238:OpenChrom
66:molecules
1582:Category
1311:Electron
1278:Emission
1228:emission
1185:Vibronic
948:31670087
888:26999254
813:11325713
754:Archived
721:34032610
658:32428893
424:See also
365:tyrosine
147:incident
1594:Commons
1421:ESR/EPR
1369:Nucleon
1197:(REMPI)
920:Bibcode
860:Bibcode
804:1301402
783:Bibcode
693:Bibcode
630:Bibcode
354:micelle
350:vesicle
255:of the
36:mercury
1435:Others
1223:Atomic
1018:
954:
946:
938:
894:
886:
878:
811:
801:
727:
719:
711:
664:
656:
648:
554:
526:464277
524:
516:
110:photon
87:Theory
1376:Alpha
1345:Auger
1323:X-ray
1290:Gamma
1268:X-ray
1201:Raman
1112:Raman
1107:FT-IR
952:S2CID
892:S2CID
848:(PDF)
725:S2CID
662:S2CID
576:(PDF)
388:CVAFS
307:of a
226:GNU R
1016:ISBN
944:PMID
936:ISSN
884:PMID
876:ISSN
809:PMID
717:PMID
709:ISSN
654:PMID
646:ISSN
552:ISBN
522:PMID
514:ISSN
408:HPLC
367:and
322:i.e.
303:The
183:and
1404:NMR
1008:doi
983:doi
928:doi
916:169
868:doi
799:PMC
791:doi
701:doi
638:doi
588:doi
506:doi
352:or
48:or
1612::
1409:2D
1328:UV
1014:.
979:15
977:.
973:.
950:.
942:.
934:.
926:.
914:.
890:.
882:.
874:.
866:.
856:95
854:.
850:.
807:.
797:.
789:.
779:80
777:.
773:.
723:.
715:.
707:.
699:.
687:.
683:.
660:.
652:.
644:.
636:.
624:.
620:.
582:.
578:.
534:^
520:.
512:.
502:94
500:.
454:^
410:.
397:.
360:.
259:.
116:.
83:.
1083:e
1076:t
1069:v
1024:.
1010::
989:.
985::
958:.
930::
922::
898:.
870::
862::
793::
785::
731:.
703::
695::
689:9
668:.
640::
632::
626:8
596:.
590::
584:6
560:.
528:.
508::
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.