38:
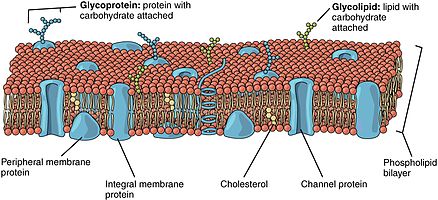
314:. Membranes contain sugar-containing lipid molecules known as glycolipids. In the bilayer, the sugar groups of glycolipids are exposed at the cell surface, where they can form hydrogen bonds. Glycolipids provide the most extreme example of asymmetry in the lipid bilayer. Glycolipids perform a vast number of functions in the biological membrane that are mainly communicative, including cell recognition and cell-cell adhesion. Glycoproteins are integral proteins. They play an important role in the immune response and protection.
163:
179:• Both the plasma membrane and internal membranes have cytosolic and exoplasmic faces • This orientation is maintained during membrane trafficking – proteins, lipids, glycoconjugates facing the lumen of the ER and Golgi get expressed on the extracellular side of the plasma membrane. In eukaryotic cells, new phospholipids are manufactured by enzymes bound to the part of the endoplasmic reticulum membrane that faces the cytosol. These enzymes, which use free fatty acids as
489:. It permits membrane lipids and proteins to diffuse from sites where they are inserted into the bilayer after their synthesis to other regions of the cell. It allows membranes to fuse with one another and mix their molecules, and it ensures that membrane molecules are distributed evenly between daughter cells when a cell divides. If biological membranes were not fluid, it is hard to imagine how cells could live, grow, and reproduce.
481:. This molecule is present in especially large amounts in the plasma membrane, where it constitutes approximately 20% of the lipids in the membrane by weight. Because cholesterol molecules are short and rigid, they fill the spaces between neighboring phospholipid molecules left by the kinks in their unsaturated hydrocarbon tails. In this way, cholesterol tends to stiffen the bilayer, making it more rigid and less permeable.
1186:
330:, where hydrophobic ends come into contact with each other and are sequestered away from water. This arrangement maximises hydrogen bonding between hydrophilic heads and water while minimising unfavorable contact between hydrophobic tails and water. The increase in available hydrogen bonding increases the entropy of the system, creating a spontaneous process.
461:, melanosomes, and chromaffin granules). Different types of biological membranes have diverse lipid and protein compositions. The content of membranes defines their physical and biological properties. Some components of membranes play a key role in medicine, such as the efflux pumps that pump drugs out of a cell.
223:
have various functions and characteristics and catalyze different chemical reactions. Integral proteins span the membranes with different domains on either side. Integral proteins hold strong association with the lipid bilayer and cannot easily become detached. They will dissociate only with chemical
170:
The lipid bilayer consists of two layers- an outer leaflet and an inner leaflet. The components of bilayers are distributed unequally between the two surfaces to create asymmetry between the outer and inner surfaces. This asymmetric organization is important for cell functions such as cell signaling.
378:
Probably the most important feature of a biomembrane is that it is a selectively permeable structure. This means that the size, charge, and other chemical properties of the atoms and molecules attempting to cross it will determine whether they succeed in doing so. Selective permeability is essential
207:
Red blood cells, or erythrocytes, have a unique lipid composition. The bilayer of red blood cells is composed of cholesterol and phospholipids in equal proportions by weight. Erythrocyte membrane plays a crucial role in blood clotting. In the bilayer of red blood cells is phosphatidylserine. This is
365:
shields the rest of the cell from peroxides, chemicals that can be toxic to the cell, and the cell membrane separates a cell from its surrounding medium. Peroxisomes are one form of vacuole found in the cell that contain by-products of chemical reactions within the cell. Most organelles are defined
224:
treatment that breaks the membrane. Peripheral proteins are unlike integral proteins in that they hold weak interactions with the surface of the bilayer and can easily become dissociated from the membrane. Peripheral proteins are located on only one face of a membrane and create membrane asymmetry.
469:
The hydrophobic core of the phospholipid bilayer is constantly in motion because of rotations around the bonds of lipid tails. Hydrophobic tails of a bilayer bend and lock together. However, because of hydrogen bonding with water, the hydrophilic head groups exhibit less movement as their rotation
203:
The biological membrane is made up of lipids with hydrophobic tails and hydrophilic heads. The hydrophobic tails are hydrocarbon tails whose length and saturation is important in characterizing the cell. Lipid rafts occur when lipid species and proteins aggregate in domains in the membrane. These
473:
Below a transition temperature, a lipid bilayer loses fluidity when the highly mobile lipids exhibits less movement becoming a gel-like solid. The transition temperature depends on such components of the lipid bilayer as the hydrocarbon chain length and the saturation of its fatty acids.
393:, where the membrane allows for a vacuole to join onto it and push its contents into the cell. Many types of specialized plasma membranes can separate cell from external environment: apical, basolateral, presynaptic and postsynaptic ones, membranes of flagella, cilia,
183:, deposit all newly made phospholipids into the cytosolic half of the bilayer. To enable the membrane as a whole to grow evenly, half of the new phospholipid molecules then have to be transferred to the opposite monolayer. This transfer is catalyzed by enzymes called
806:
Dougherty, R. M.; Galli, C.; Ferro-Luzzi, A.; Iacono, J. M. (1987). "Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA".
474:
Temperature-dependence fluidity constitutes an important physiological attribute for bacteria and cold-blooded organisms. These organisms maintain a constant fluidity by modifying membrane lipid fatty acid composition in accordance with differing temperatures.
432:
Distinct types of membranes also create intracellular organelles: endosome; smooth and rough endoplasmic reticulum; sarcoplasmic reticulum; Golgi apparatus; lysosome; mitochondrion (inner and outer membranes); nucleus (inner and outer membranes);
190:
Using selective flippases is not the only way to produce asymmetry in lipid bilayers, however. In particular, a different mechanism operates for glycolipids—the lipids that show the most striking and consistent asymmetric distribution in
175:
of the phospholipid bilayer, the outer leaflet and inner leaflet of the membrane are asymmetrical in their composition. Certain proteins and lipids rest only on one surface of the membrane and not the other.
484:
For all cells, membrane fluidity is important for many reasons. It enables membrane proteins to diffuse rapidly in the plane of the bilayer and to interact with one another, as is crucial, for example, in
361:
Membranes in cells typically define enclosed spaces or compartments in which cells may maintain a chemical or biochemical environment that differs from the outside. For example, the membrane around
379:
for effective separation of a cell or organelle from its surroundings. Biological membranes also have certain mechanical or elastic properties that allow them to change shape and move as required.
389:
Particles that are required for cellular function but are unable to diffuse freely across a membrane enter through a membrane transport protein or are taken in by means of
751:
1228:
338:
Biological molecules are amphiphilic or amphipathic, i.e. are simultaneously hydrophobic and hydrophilic. The phospholipid bilayer contains charged
350:
tails, which meet with the hydrophobic tails of the complementary layer. The hydrophobic tails are usually fatty acids that differ in lengths. The
1041:
187:. In the plasma membrane, flippases transfer specific phospholipids selectively, so that different types become concentrated in each monolayer.
1358:
1054:
787:
735:
726:
Alberts, Bray, Hopkin, Johnson, Lewis, Raff, Roberts, Walter, Bruce, Dennis, Karen, Alexander, Julian, Martin, Keith, Peter (2010).
171:
The asymmetry of the biological membrane reflects the different functions of the two leaflets of the membrane. As seen in the fluid
1221:
208:
usually in the cytoplasmic side of the membrane. However, it is flipped to the outer membrane to be used during blood clotting.
1190:
273:
204:
help organize membrane components into localized areas that are involved in specific processes, such as signal transduction.
1378:
470:
and mobility are constrained. This results in increasing viscosity of the lipid bilayer closer to the hydrophilic heads.
545:
Murate, Motohide; Kobayashi, Toshihide (2016). "Revisiting transbilayer distribution of lipids in the plasma membrane".
493:
355:
279:
binds extracellular PDGF and, as a consequence, generates intracellular signals that cause the cell to grow and divide
1214:
92:
1427:
1326:
120:
73:
162:
1422:
88:
37:
1321:
1200:
367:
945:
Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002-01-01).
292:
catalyzes the production of intracellular signaling molecule cyclic AMP in response to extracellular signals
983:"General N-and O-Linked Glycosylation of Lipoproteins in Mycoplasmas and Role of Exogenous Oligosaccharide"
981:
Daubenspeck, James M.; Jordan, David S.; Simmons, Warren; Renfrow, Matthew B.; Dybvig, Kevin (2015-11-23).
413:
membranes of neurons. Plasma membranes can also form different types of "supramembrane" structures such as
58:
626:"Osmoporin OmpC forms a complex with MlaA to maintain outer membrane lipid asymmetry in Escherichia coli"
1288:
1273:
1087:
Vitrac, Heidi; MacLean, David M.; Jayaraman, Vasanthi; Bogdanov, Mikhail; Dowhan, William (2015-11-10).
954:
891:
Lein, Max; deRonde, Brittany M.; Sgolastra, Federica; Tew, Gregory N.; Holden, Matthew A. (2015-11-01).
853:
Lentz, Barry R. (2003). "Exposure of platelet membrane phosphatidylserine regulates blood coagulation".
429:, focal adhesion, and cell junctions. These types of membranes differ in lipid and protein composition.
180:
76:
by serving as a boundary between one part of the cell and another. Biological membranes, in the form of
1060:
1146:
Rojko, Nejc; Anderluh, Gregor (2015-12-07). "How Lipid
Membranes Affect Pore Forming Toxin Activity".
1432:
1100:
994:
351:
216:
84:
343:
116:
1267:
1237:
832:
745:
510:
327:
41:
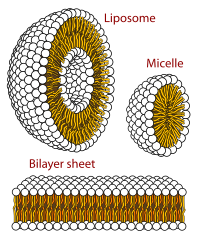
Cross-sectional view of the structures that can be formed by phospholipids in an aqueous solution
730:. New York: Garland Science, Taylor & Francis Group, LLC, an informa business. p. 370.
306:
are sugar containing polymers. In the membrane, they can be covalently bound to lipids to form
30:
This article is about various membranes in organisms. For the membranes surrounding cells, see
1255:
1163:
1128:
1050:
1022:
922:
870:
824:
783:
731:
699:
647:
606:
562:
132:
108:
893:"Protein transport across membranes: Comparison between lysine and guanidinium-rich carriers"
585:"Lateral organization, bilayer asymmetry, and inter-leaflet coupling of biological membranes"
1298:
1263:
1155:
1118:
1108:
1012:
1002:
912:
904:
862:
816:
689:
681:
637:
596:
554:
520:
287:
220:
124:
107:
to rotate and laterally diffuse for physiological functioning. Proteins are adapted to high
967:
685:
410:
303:
247:
172:
136:
128:
1089:"Dynamic membrane protein topological switching upon changes in phospholipid environment"
382:
Generally, small hydrophobic molecules can readily cross phospholipid bilayers by simple
1104:
998:
496:
which allows for calculating the energy cost of an elastic deformation to the membrane.
27:
Enclosing or separating membrane in organisms acting as selective semi-permeable barrier
1123:
1088:
1017:
982:
917:
892:
694:
669:
486:
323:
65:
866:
601:
584:
558:
1416:
1399:
1353:
1196:
505:
438:
426:
152:
148:
112:
80:
69:
31:
1383:
1363:
836:
422:
402:
311:
192:
1159:
1007:
908:
1373:
1368:
1345:
1283:
525:
478:
454:
394:
390:
347:
339:
946:
477:
In animal cells, membrane fluidity is modulated by the inclusion of the sterol
1393:
1316:
434:
406:
362:
307:
17:
1311:
1306:
1113:
820:
398:
383:
77:
1167:
1132:
1026:
926:
874:
703:
651:
610:
566:
1185:
828:
458:
442:
418:
414:
260:
184:
61:
1336:
1278:
515:
104:
642:
625:
1206:
100:
265:
link intracellular actin filaments to extracellular matrix proteins
450:
36:
780:
Fundamentals of
Biochemistry: Life at the Molecular Level (4 ed.)
446:
119:, consisting of lipid molecules bound tightly to the surface of
1210:
583:
Nickels, Jonathan D.; Smith, Jeremy C.; Cheng, Xiaolin (2015).
228:
SOME EXAMPLES OF PLASMA MEMBRANE PROTEINS AND THEIR FUNCTIONS
96:
322:
The phospholipid bilayer is formed due to the aggregation of
624:
Chong, Zhi-Soon; Woo, Wei-Fen; Chng, Shu-Sin (2015-12-01).
437:; vacuole; cytoplasmic granules; cell vesicles (phagosome,
354:
of lipids, especially the hydrophobic tails, determine the
95:
used in communication and transportation of chemicals and
492:
The fluidity property is at the center of the
Helfrich
123:. The cell membranes are different from the isolating
1049:. London, U.K.: The Biochemical Society. p. 21.
453:-coated vesicles) and secretory vesicles (including
1392:
1344:
1335:
1297:
1254:
409:of muscle cells, as well as specialized myelin and
326:in aqueous solutions. Aggregation is caused by the
166:
A fluid membrane model of the phospholipid bilayer.
897:Biochimica et Biophysica Acta (BBA) - Biomembranes
1093:Proceedings of the National Academy of Sciences
103:in a cell membrane provides a fluid matrix for
1222:
8:
750:: CS1 maint: multiple names: authors list (
1341:
1246:Mechanisms for chemical transport through
1229:
1215:
1207:
809:The American Journal of Clinical Nutrition
670:"Structural Symmetry in Membrane Proteins"
252:actively pumps Na+ out of cells and K+ in
226:
1199:at the U.S. National Library of Medicine
1122:
1112:
1016:
1006:
916:
693:
641:
600:
310:or covalently bound to proteins to form
161:
537:
963:
952:
743:
342:headgroups, which interact with polar
1082:
1080:
940:
938:
936:
686:10.1146/annurev-biophys-051013-023008
7:
1359:Non-specific, adsorptive pinocytosis
886:
884:
848:
846:
801:
799:
773:
771:
769:
767:
765:
763:
761:
728:Essential Cell Biology third edition
721:
719:
717:
715:
713:
663:
661:
578:
576:
127:formed by layers of cells, such as
366:by such membranes, and are called
219:contain different proteins. These
25:
602:10.1016/j.chemphyslip.2015.07.012
559:10.1016/j.chemphyslip.2015.08.009
356:lipid bilayer physical properties
64:that separates the interior of a
1184:
668:Forrest, Lucy R. (2015-01-01).
589:Chemistry and Physics of Lipids
547:Chemistry and Physics of Lipids
274:platelet-derived growth factor
1:
1379:Receptor-mediated endocytosis
1148:Accounts of Chemical Research
867:10.1016/s0163-7827(03)00025-0
1160:10.1021/acs.accounts.5b00403
1008:10.1371/journal.pone.0143362
909:10.1016/j.bbamem.2015.09.004
674:Annual Review of Biophysics
1449:
1327:Secondary active transport
855:Progress in Lipid Research
346:. The layers also contain
146:
121:integral membrane proteins
74:intracellular compartments
29:
1244:
903:(11, Part A): 2980–2984.
368:membrane-bound organelles
1322:Primary active transport
1201:Medical Subject Headings
417:, postsynaptic density,
115:with the presence of an
1114:10.1073/pnas.1512994112
1040:Brown, Bernard (1996).
72:environment or creates
962:Cite journal requires
630:Molecular Microbiology
374:Selective permeability
167:
42:
1274:Facilitated diffusion
821:10.1093/ajcn/45.2.443
778:Voet, Donald (2012).
217:Phospholipid bilayers
165:
59:selectively permeable
40:
1248:biological membranes
1193:at Wikimedia Commons
1191:Biological membranes
1043:Biological Membranes
85:phospholipid bilayer
1105:2015PNAS..11213874V
1099:(45): 13874–13879.
999:2015PLoSO..1043362D
947:"The Lipid Bilayer"
229:
117:annular lipid shell
111:environment of the
93:peripheral proteins
47:biological membrane
1268:mediated transport
1238:Membrane transport
511:Fluid mosaic model
445:-coated vesicles,
358:such as fluidity.
328:hydrophobic effect
239:SPECIFIC FUNCTION
227:
168:
133:basement membranes
43:
1428:Biological matter
1410:
1409:
1406:
1405:
1256:Passive transport
1189:Media related to
1154:(12): 3073–3079.
643:10.1111/mmi.13202
296:
295:
233:FUNCTIONAL CLASS
221:membrane proteins
109:membrane fluidity
16:(Redirected from
1440:
1423:Membrane biology
1342:
1299:Active transport
1264:Simple diffusion
1231:
1224:
1217:
1208:
1188:
1172:
1171:
1143:
1137:
1136:
1126:
1116:
1084:
1075:
1074:
1072:
1071:
1065:
1059:. Archived from
1048:
1037:
1031:
1030:
1020:
1010:
993:(11): e0143362.
978:
972:
971:
965:
960:
958:
950:
942:
931:
930:
920:
888:
879:
878:
850:
841:
840:
803:
794:
793:
775:
756:
755:
749:
741:
723:
708:
707:
697:
665:
656:
655:
645:
636:(6): 1133–1146.
621:
615:
614:
604:
580:
571:
570:
542:
521:Membrane biology
304:Oligosaccharides
299:Oligosaccharides
288:adenylyl cyclase
236:PROTEIN EXAMPLE
230:
137:serous membranes
129:mucous membranes
21:
1448:
1447:
1443:
1442:
1441:
1439:
1438:
1437:
1413:
1412:
1411:
1402:
1388:
1331:
1293:
1250:
1240:
1235:
1181:
1176:
1175:
1145:
1144:
1140:
1086:
1085:
1078:
1069:
1067:
1063:
1057:
1046:
1039:
1038:
1034:
980:
979:
975:
961:
951:
944:
943:
934:
890:
889:
882:
852:
851:
844:
805:
804:
797:
790:
777:
776:
759:
742:
738:
725:
724:
711:
667:
666:
659:
623:
622:
618:
582:
581:
574:
544:
543:
539:
534:
502:
467:
411:dendritic spine
376:
336:
324:membrane lipids
320:
301:
214:
201:
160:
155:
147:Main articles:
145:
87:with embedded,
83:, consist of a
35:
28:
23:
22:
15:
12:
11:
5:
1446:
1444:
1436:
1435:
1430:
1425:
1415:
1414:
1408:
1407:
1404:
1403:
1398:
1396:
1390:
1389:
1387:
1386:
1381:
1376:
1371:
1366:
1361:
1356:
1350:
1348:
1339:
1333:
1332:
1330:
1329:
1324:
1319:
1314:
1309:
1303:
1301:
1295:
1294:
1292:
1291:
1286:
1281:
1276:
1271:
1260:
1258:
1252:
1251:
1245:
1242:
1241:
1236:
1234:
1233:
1226:
1219:
1211:
1205:
1204:
1194:
1180:
1179:External links
1177:
1174:
1173:
1138:
1076:
1056:978-0904498325
1055:
1032:
973:
964:|journal=
932:
880:
861:(5): 423–438.
842:
815:(2): 443–455.
795:
789:978-1118129180
788:
757:
737:978-0815341291
736:
709:
680:(1): 311–337.
657:
616:
572:
536:
535:
533:
530:
529:
528:
523:
518:
513:
508:
501:
498:
487:cell signaling
466:
463:
375:
372:
335:
332:
319:
316:
300:
297:
294:
293:
290:
285:
281:
280:
277:
271:
267:
266:
263:
258:
254:
253:
250:
245:
241:
240:
237:
234:
213:
210:
200:
197:
173:membrane model
159:
156:
144:
141:
99:. The bulk of
81:cell membranes
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1445:
1434:
1431:
1429:
1426:
1424:
1421:
1420:
1418:
1401:
1400:Degranulation
1397:
1395:
1391:
1385:
1382:
1380:
1377:
1375:
1372:
1370:
1367:
1365:
1362:
1360:
1357:
1355:
1354:Efferocytosis
1352:
1351:
1349:
1347:
1343:
1340:
1338:
1334:
1328:
1325:
1323:
1320:
1318:
1315:
1313:
1310:
1308:
1305:
1304:
1302:
1300:
1296:
1290:
1287:
1285:
1282:
1280:
1277:
1275:
1272:
1269:
1265:
1262:
1261:
1259:
1257:
1253:
1249:
1243:
1239:
1232:
1227:
1225:
1220:
1218:
1213:
1212:
1209:
1202:
1198:
1195:
1192:
1187:
1183:
1182:
1178:
1169:
1165:
1161:
1157:
1153:
1149:
1142:
1139:
1134:
1130:
1125:
1120:
1115:
1110:
1106:
1102:
1098:
1094:
1090:
1083:
1081:
1077:
1066:on 2015-11-06
1062:
1058:
1052:
1045:
1044:
1036:
1033:
1028:
1024:
1019:
1014:
1009:
1004:
1000:
996:
992:
988:
984:
977:
974:
969:
956:
948:
941:
939:
937:
933:
928:
924:
919:
914:
910:
906:
902:
898:
894:
887:
885:
881:
876:
872:
868:
864:
860:
856:
849:
847:
843:
838:
834:
830:
826:
822:
818:
814:
810:
802:
800:
796:
791:
785:
781:
774:
772:
770:
768:
766:
764:
762:
758:
753:
747:
739:
733:
729:
722:
720:
718:
716:
714:
710:
705:
701:
696:
691:
687:
683:
679:
675:
671:
664:
662:
658:
653:
649:
644:
639:
635:
631:
627:
620:
617:
612:
608:
603:
598:
594:
590:
586:
579:
577:
573:
568:
564:
560:
556:
552:
548:
541:
538:
531:
527:
524:
522:
519:
517:
514:
512:
509:
507:
506:Collodion bag
504:
503:
499:
497:
495:
490:
488:
482:
480:
475:
471:
464:
462:
460:
456:
452:
448:
444:
440:
439:autophagosome
436:
430:
428:
427:hemidesmosome
425:, desmosome,
424:
420:
416:
412:
408:
404:
400:
396:
392:
387:
385:
380:
373:
371:
369:
364:
359:
357:
353:
349:
345:
341:
333:
331:
329:
325:
317:
315:
313:
312:glycoproteins
309:
305:
298:
291:
289:
286:
283:
282:
278:
275:
272:
269:
268:
264:
262:
259:
256:
255:
251:
249:
246:
244:Transporters
243:
242:
238:
235:
232:
231:
225:
222:
218:
211:
209:
205:
198:
196:
194:
188:
186:
182:
177:
174:
164:
157:
154:
153:Lipid bilayer
150:
149:Cell membrane
142:
140:
138:
134:
130:
126:
122:
118:
114:
113:lipid bilayer
110:
106:
102:
98:
94:
90:
86:
82:
79:
75:
71:
67:
63:
60:
56:
55:cell membrane
52:
48:
39:
33:
32:cell membrane
19:
18:Inner leaflet
1384:Transcytosis
1364:Phagocytosis
1247:
1151:
1147:
1141:
1096:
1092:
1068:. Retrieved
1061:the original
1042:
1035:
990:
986:
976:
955:cite journal
900:
896:
858:
854:
812:
808:
779:
727:
677:
673:
633:
629:
619:
592:
588:
550:
546:
540:
491:
483:
476:
472:
468:
449:-coated and
431:
423:invadopodium
403:lamellipodia
388:
381:
377:
360:
352:interactions
337:
321:
302:
215:
206:
202:
193:animal cells
189:
178:
169:
54:
50:
46:
44:
1433:Soft matter
1374:Potocytosis
1369:Pinocytosis
1346:Endocytosis
526:Soft matter
479:cholesterol
455:synaptosome
395:microvillus
391:endocytosis
363:peroxisomes
348:hydrophobic
340:hydrophilic
308:glycolipids
143:Composition
51:biomembrane
1417:Categories
1394:Exocytosis
1317:Antiporter
1070:2014-05-01
532:References
435:peroxisome
407:sarcolemma
270:Receptors
181:substrates
78:eukaryotic
1312:Symporter
1307:Uniporter
1197:Membranes
782:. Wiley.
746:cite book
595:: 87–99.
553:: 58–71.
459:acrosomes
399:filopodia
384:diffusion
318:Formation
276:receptor
261:integrins
185:flippases
158:Asymmetry
68:from the
1289:Carriers
1284:Channels
1266:(or non-
1168:26641659
1133:26512118
1027:26599081
987:PLOS ONE
927:26342679
875:12814644
704:26098517
652:26314242
611:26232661
567:26319805
500:See also
465:Fluidity
443:clathrin
419:podosome
415:caveolae
334:Function
284:Enzymes
257:Anchors
248:Na+ Pump
212:Proteins
105:proteins
89:integral
70:external
62:membrane
1337:Cytosis
1279:Osmosis
1124:4653158
1101:Bibcode
1018:4657876
995:Bibcode
918:4704449
837:4436467
829:3812343
695:5500171
516:Osmosis
125:tissues
1203:(MeSH)
1166:
1131:
1121:
1053:
1025:
1015:
925:
915:
873:
835:
827:
786:
734:
702:
692:
650:
609:
565:
405:, the
199:Lipids
135:, and
101:lipids
1064:(PDF)
1047:(PDF)
833:S2CID
494:model
451:COPII
344:water
57:is a
1164:PMID
1129:PMID
1051:ISBN
1023:PMID
968:help
923:PMID
901:1848
871:PMID
825:PMID
784:ISBN
752:link
732:ISBN
700:PMID
648:PMID
607:PMID
563:PMID
447:COPI
401:and
151:and
97:ions
91:and
66:cell
1156:doi
1119:PMC
1109:doi
1097:112
1013:PMC
1003:doi
913:PMC
905:doi
863:doi
817:doi
690:PMC
682:doi
638:doi
597:doi
593:192
555:doi
551:194
53:or
1419::
1162:.
1152:48
1150:.
1127:.
1117:.
1107:.
1095:.
1091:.
1079:^
1021:.
1011:.
1001:.
991:10
989:.
985:.
959::
957:}}
953:{{
935:^
921:.
911:.
899:.
895:.
883:^
869:.
859:42
857:.
845:^
831:.
823:.
813:45
811:.
798:^
760:^
748:}}
744:{{
712:^
698:.
688:.
678:44
676:.
672:.
660:^
646:.
634:98
632:.
628:.
605:.
591:.
587:.
575:^
561:.
549:.
457:,
441:,
421:,
397:,
386:.
370:.
195:.
139:.
131:,
49:,
45:A
1270:)
1230:e
1223:t
1216:v
1170:.
1158::
1135:.
1111::
1103::
1073:.
1029:.
1005::
997::
970:)
966:(
949:.
929:.
907::
877:.
865::
839:.
819::
792:.
754:)
740:.
706:.
684::
654:.
640::
613:.
599::
569:.
557::
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.