29:
285:
217:
121:. Also known as pulsed direct current (PDC) electrolysis, the increased number of variables that it introduces to the electrolysis method can change the application of the current to the electrodes and the resulting outcome. This varies from direct current (DC) electrolysis, which only allows the variation of one value, the voltage applied. By utilising conventional
281:
or producing a cubic meter of hydrogen. It was discovered a magnetic field of 11T was needed for effective electrolysis, more than 16 times greater than what was originally used. Since superconducting magnets would be required, and they can become too expensive to justify their use, ruling this out as a possible method.
384:
In theoretical electrolysis of water, a voltage of only 1.23 V is required to split water into hydrogen and oxygen, The formation of an EDL increases this to its thermo-neutral voltage of 1.45 V. Minimising the EDL formed during pulse electrolysis is advantageous, as it can reduce the thermo-neutral
325:
Initial observations revealed that the off-period resulted in a reversal in polarity, causing the reaction to reverse. This effected the cathode, which displayed a 2g loss after experimentation. A diode was input into the circuit to rectify the polarity. However, the cell was prevented from dropping
280:
created by the electrolyte, that slowed down the motion of the disc. The two ways they could fix this is to rotate the disc and solution together or increase the magnetic field used. The latter being most practicable, the required magnetic field was calculated according to the power consumption rate
156:
The various and alterable effects of using intermittent pulses in PDC electrolysis has resulted in an area of interest that could benefit industry. However, as it is still being researched and has produced conflicting results, a consistent and reliable answer to how dependent electrolysis efficiency
248:
Poláčik and Pospíšil believe that by manipulating the dependent variables, such as the duty cycle, can increase or decrease the effectiveness of pulse electrolysis at reducing this layer. A theoretical equation, the Sand equation, is used to calculate the amount of time required to allow the EDL to
212:
replacement. For this to be feasible, the production of hydrogen, through methods such as electrolysis, must be efficient in terms of the energy, cost and time required. Whilst multiple methods of pulse electrolysis have been studied, and experimental results are mixed, the underlying theory behind
173:
PDC electrolysis was first considered theoretically in 1952, and experimental research began as early as 1960 however it was originally focused on its technical applications to industry and the possibilities of improving the quality and rate of metal deposition. It partially succeeded, providing
393:
Whilst the method of PDC electrolysis has been proven by
Ghoroghichian and Bockris in 1952 and 1985 to work extremely well in theory, it is difficult to replicate with consistently positive results in practical experimentation. Hence, the many mechanisms that have been patented are unable to be
375:
These effects were first researched on zinc by Coehn. It was discovered a pulsed current at a high frequency can produce deposits of higher quality, with properties ranging from a smoother finish by the reduction in grain size, as well as lowering its corrosion rate. This is beneficial as it is
371:
of the deposition metal is directly affected and can have favourable or unfavourable circumstances if specific conditions are not met. It is reported that pulse plating can encourage nucleation causing grain refinement, and reducing grain size, as well as increasing the deposit density that can
362:
A pulsed current can be varied in many ways that increases the possible outcomes and can vary the properties of deposited metals during electroplating. Hansel and Roy, in their review of the third
European Pulse Plating Seminar, concluded that each deposition system must have a unique sequence
326:
to 0 V during the off-period, maintaining a higher value of 2.3V. This further impacted the experiment, distorting the square wave produced by the function generator
Shaaban used, as the electrical potential provided needed to overcome the cell voltage of 2.3V before current could flow. Bokris
299:
The generator was constructed with a magnetic flux density of 0.6T, a propeller radius of 30 cm and a loop coated with copper strips. To increase the output potential, and reducing the rotation speed required, these were connected in series. Pulses of 2-3V that were sustained for 1ms were
401:
can reverse. This can cause the cathode to deteriorate. In electrolysis, the cathode is where the reduction of hydrogen occurs, forming the desired hydrogen gas. Any loss in mass can reduce the speed and effectiveness of the electrolytic reaction, reducing the overall efficiency of the pulse
350:
The possible increased effect a pulsed current will have on the corrodibility of metals was first looked at by de la Rive in 1837. It was investigated around 60 years later by Coehn regarding the effect of a current with a rectangular waveform, on the plating of zinc deposits, resulting in a
145:
Although theoretical research has made large promise for the efficiencies and benefits of utilising pulse electrolysis, it has many contradictions including a common issue that it is difficult to replicate the successes of patents experimentally and produces its own negative effects on the
196:, discovering an electrolysis method with the greatest efficiency is valued. With early experimental and theoretical success, many patents began to be developed until as recent as 2002, but since 1985, it has only been researched intermittently with varying levels of success.
316:
The experimental electrolyser separated the anolyte and catholyte compartments and used a 324-Naflon membrane to allow the ion exchange. The distance between the anode, made with platinum coated titanium, and the cathode, stainless steel, was 3mm and was immersed in a 10
245:. One of the aims of PDC electrolysis is to overcome this, and theoretically, when the PMW switches the current on, a capacitance will be stored, and when the duty cycle is over, it will be released, continuing the flow of current whilst reducing the EDL that is formed.
241:, or can cause the electrolyser to act as a capacitor. When this is present, excess voltage must be supplied by the direct current to compensate for the loss in the 'capacitor', which rises the required voltage supplied to what is called the
321:
sulfuric acid electrolyte. He conducted tests under several different frequencies that included '0.01 Hz, 0.5 kHz, 5 kHz, i kHz, 10 kHz, 25 kHz, and 40 kHz' and with four duty cycles, '10, 25, 50, and 80%'.
141:. Past research has demonstrated that there is a possibility it can result in a higher electrical efficiency in comparison to DC electrolysis. This would allow electrolysis procedures to produce greater volumes of hydrogen with a reduced
330:
records that current would continue to flow, discharging ions from the EDL, but this was contradicted in this experiment. This only occurred when the diode was in place but it prevented a current spike in the duty cycle as well.
312:
A comparison between a pulsed and non-pulsed dc current electrolysers was explored in 1993 by
Shaaban, that demonstrated a non-pulsed current used the least electrical power. This opposes the previous and future works conducted.
303:
This was the first instance of a successful application of pulse electrolysis for the production of hydrogen. However, it still presents its own limitations in the possibility for it to be used in industry.
945:
Clément, N.; Nishiguchi, K.; Dufreche, J. F.; Guerin, D.; Fujiwara, A.; Vuillaume, D. (2013-08-14). "Water
Electrolysis and Energy Harvesting with Zero-Dimensional Ion-Sensitive Field-Effect Transistors".
334:
With a 10% duty cycle at a 1 kHz pulse, temperature increases of nearly 7 °C greater than in the non-pulsed experimental electrolysis, were found. Temperature increases can prevent the circuit
295:
In this method, a pulse potential was created to take advantage of previous studies that give an effectiveness factor of 2 when either a nickel electrode or a Teflon-bonded platinum electrode was used.
338:
Calculating the power consumption, it was determined a non-pulsed current had power demand losses of 3.5%, and a pulsed current resulted in 13 - 16% losses. It also opposes the idea from
Bockris
405:
Shaaban also states that due to expected internal losses, such as through heat, the current density required will increase, which increases the required voltage. As a result, greater
1288:
Giurlani, Walter; Zangari, Giovanni; Gambinossi, Filippo; Passaponti, Maurizio; Salvietti, Emanuele; Di
Benedetto, Francesco; Caporali, Stefano; Innocenti, Massimo (2018-07-25).
185:. Ghoroghichian and Bockris conducted this experimental research to determine how a pulsed current can impact the rate of hydrogen production and provide economic advantages. A
273:
for electrolysis. The difference between
Faraday's original model and Bockris and Ghorogchian's is that their disc will only rotate when it is in contact with an electrolyte.
177:
The first instance it was considered to initialise the electrolysis of water was from the perspective of magnetolysis in 1985, where high strength magnets, or in this case
1018:
Tseung, A.C.C.; Vassie, P.R. (1976). "A study of gas evolution in teflon bonded porous electrodes—III. Performance of teflon bonded Pt black electrodes for H2 evolution".
1242:
Ramanauskas, R.; Gudavičiūtė, L.; Ščit, O.; Bučinskienė, D.; Juškėnas, R. (2008). "Pulse plating effect on composition and corrosion properties of zinc alloy coatings".
1339:
Fang, YaHui; Liu, Zhi-Pan (2010). "Electrochemical reactions at the electrode/solution interface: Theory and applications to water electrolysis and oxygen reduction".
189:
ratio of 2.07 was observed, demonstrating, for the first time, that a pulsed current can double the production of hydrogen, in comparison to a steady state current.
363:
developed in order to optimise the process and gain the desired results, opposing the inability of traditional plating to be as freely tailored to a situation. The
853:
Nicoletti, Giovanni; Arcuri, Natale; Nicoletti, Gerardo; Bruno, Roberto (2015). "A technical and environmental comparison between hydrogen and some fossil fuels".
757:
Ehrenhaft, Felix (1944-05-01). "The
Decomposition of Water by the So-Called Permanent Magnet and the Measurement of the Intensity of the Magnetic Current".
158:
481:
Kireev, S. Yu. (2017-03-01). "Intensification of processes of electrodeposition of metals by use of various modes of pulse electrolysis".
28:
192:
Since hydrogen gas cannot be collected in its free form, and it can be used to provide a source of renewable and clean energy through
133:, and the frequency. Currently, there has been a focus on theoretical and experimental research into PDC electrolysis in terms of the
1109:
490:
292:
Their final decision was to use a homopolar generator as an external source of power. This follows
Faraday's method more closely.
359:', was only published in 1954 by Baeyens, this being the first area of research into the use of pulse electrolysis in industry.
261:
has the ability to do this, so in Bockris and Ghoroghchian's original experiment in 1985, they followed Faraday's idea. Using a
174:
promising results its ability to create smoother, denser deposits, and reducing the amount of metal required in electroplating.
450:
Morita, K.; Furuya, Etuo. (1994-07-01). "Pulse electrolysis within a solution boundary layer to minimize convective effects".
1196:
Hansal, Wolfgang; Roy, Sudipta (2008). "Pulse plating gaining importance – Review of the 3rdEuropean Pulse Plating Seminar".
1045:
Carmo, Marcelo; Fritz, David L.; Mergel, Jürgen; Stolten, Detlef (2013). "A comprehensive review on PEM water electrolysis".
230:
342:
that the effectiveness of non-pulsed dc current electrolysis increases by a factor of 2 when a pulsed current is applied.
679:
Bockris, J. O'M.; Potter, E. C. (1952). "The Mechanism of Hydrogen Evolution at Nickel Cathodes in Aqueous Solutions".
1388:
162:
142:
427:"Pulse electrolysis of alkaline solutions as highly efficient method of production of hydrogen/oxygen gas mixtures"
269:, they placed a stainless-steel disc in between. The disc needed a rotation speed of 2000 rpm to reach the correct
153:
and electrocrystallisation are also undergoing research due to the wider range of properties that can be achieved.
722:
Arouete, S.; Blurton, K. F.; Oswin, H. G. (1969). "Controlled Current Deposition of Zinc from Alkaline Solution".
1398:
459:
270:
157:
is on the properties of an electrical pulse has not been determined, hence, other forms of electrolysis such as
397:
According to Shabaan, during the pulse-off period, if the electrolytic cell is not constructed properly, the
205:
122:
1393:
242:
134:
815:
Ghoroghchian, J; Bockris, J (1985). "Use of a homopolar generator in hydrogen production from water".
965:
824:
766:
731:
688:
618:
544:
398:
258:
182:
138:
118:
1364:
1267:
1221:
997:
955:
568:
502:
208:
sources is a main cause of global environmental problems, hydrogen is being viewed as a possible
125:(PMW), multiple dependent variables can be altered, including the type of waveform, typically a
216:
1356:
1321:
1259:
1213:
1173:
1105:
989:
981:
782:
704:
560:
266:
149:
PDC electrolysis is not only confined to the electrolysis of water. Uses in industry such as
1348:
1311:
1301:
1251:
1205:
1165:
1097:
1092:
Pesco, Anthony M.; Cheh, Huk Y. (1989), "Theory and Applications of Periodic Electrolysis",
1054:
1027:
973:
927:
889:
862:
832:
774:
739:
696:
656:
647:
Ibl, N.; Puippe, J.Cl.; Angerer, H. (1978). "Electrocrystallization in pulse electrolysis".
626:
552:
494:
463:
209:
368:
234:
186:
969:
828:
770:
735:
692:
622:
548:
1290:"Electroplating for Decorative Applications: Recent Trends in Research and Development"
352:
318:
284:
277:
262:
150:
1382:
1225:
1031:
836:
660:
426:
406:
178:
1368:
1271:
1001:
572:
506:
1169:
1058:
931:
866:
110:
893:
351:
successful application for a patent. A full review on using PDC electrolysis in
229:
When a voltage is applied to an electrolysis cell, immediately following this an
1101:
238:
535:
Poláčik, Ján; Pospíšil, Jiří (2016-10-01). "Some Aspects of PDC Electrolysis".
1352:
880:
Shinnar, Reuel (2003). "The hydrogen economy, fuel cells, and electric cars".
498:
364:
249:
fall to zero, and allow PDC electrolysis to achieve its highest efficiencies.
130:
126:
1360:
1325:
1263:
1255:
1217:
1209:
1177:
985:
786:
778:
708:
564:
1306:
1289:
193:
114:
993:
556:
1316:
915:
609:
Shaaban, Aly H. (1993). "Water Electrolysis and Pulsed Direct Current".
467:
356:
977:
743:
700:
630:
385:
voltage and the energy input required, increasing energy efficiency.
257:
Electrolysers require high currents produced by very low voltages. A
960:
283:
1156:
Baeyens, P. (1954). "Electroplating with Modulated Current".
215:
288:
Faraday disk generator the Magnetolysis design was based on
916:"Review of pulsed power for efficient hydrogen production"
213:
this experimental approach seems to remain consistent.
96:
88:
80:
72:
67:
59:
51:
43:
38:
21:
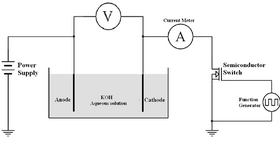
33:A Simplified Experimental Pulse Electrolysis Cell
376:mainly used as a sacrificial anode in industry.
237:, is theoretically formed. This can create a
204:With the perspective that the current use of
8:
355:, also known as electrodeposition or 'pulse
409:are needed that further converts to heat.
1315:
1305:
959:
1047:International Journal of Hydrogen Energy
920:International Journal of Hydrogen Energy
817:International Journal of Hydrogen Energy
418:
1283:
1281:
1237:
1235:
1191:
1189:
1187:
1151:
1149:
1147:
1122:
1120:
1087:
1085:
724:Journal of the Electrochemical Society
611:Journal of the Electrochemical Society
276:They encountered one large problem, a
18:
1013:
1011:
909:
907:
905:
903:
848:
846:
810:
808:
806:
804:
802:
800:
798:
796:
604:
602:
483:Inorganic Materials: Applied Research
7:
674:
672:
670:
642:
640:
600:
598:
596:
594:
592:
590:
588:
586:
584:
582:
530:
528:
526:
524:
522:
520:
518:
516:
914:Monk, Nigel; Watson, Simon (2016).
1094:Modern Aspects of Electrochemistry
119:non-spontaneous chemical reactions
14:
1096:, Springer US, pp. 251–293,
68:State-of-the-art Operating Ranges
855:Energy Conversion and Management
60:Catalyst material on the cathode
27:
681:The Journal of Chemical Physics
491:Springer Science+Business Media
394:repeated and used in industry.
181:, are used in conjunction with
1170:10.1080/00202967.1954.11869655
1059:10.1016/j.ijhydene.2013.01.151
932:10.1016/j.ijhydene.2015.12.086
867:10.1016/j.enconman.2014.09.057
143:electrical energy consumption.
52:Catalyst material on the anode
1:
894:10.1016/j.techsoc.2003.09.024
1032:10.1016/0013-4686(76)80026-6
837:10.1016/0360-3199(85)90042-4
661:10.1016/0376-4583(78)90044-4
165:are being used in industry.
159:polymer electrolyte membrane
1102:10.1007/978-1-4684-8667-4_4
163:alkaline water electrolysis
1415:
1353:10.1007/s11426-010-0047-6
537:Technological Engineering
499:10.1134/S2075113317020095
460:American Chemical Society
26:
1256:10.1179/174591908X272924
1210:10.1179/174591908x345897
779:10.1103/PhysRev.65.287.2
372:improve micro hardness.
1341:Science China Chemistry
1307:10.3390/coatings8080260
1244:Transactions of the IMF
1198:Transactions of the IMF
1158:Transactions of the IMF
113:method that utilises a
97:Cell voltage efficiency
1071:de la Rive, A (1837).
557:10.2478/teen-2016-0011
289:
243:thermo-neutral voltage
221:
127:rectangular pulse wave
123:pulse width modulation
882:Technology in Society
402:electrolysis method.
287:
265:of 0.86T produced by
231:Electric Double Layer
219:
210:renewable fuel source
200:Experimental research
135:electrolysis of water
115:pulsed direct current
44:Type of Electrolysis:
452:Analytical Chemistry
308:Conflicting research
271:electrical potential
183:homopolar propellers
1020:Electrochimica Acta
970:2013NanoL..13.3903C
829:1985IJHE...10..101G
771:1944PhRv...65..287E
736:1969JElS..116..166A
693:1952JChPh..20..614B
623:1993JElS..140.2863S
549:2016TeEng..13...33P
468:10.1021/ac00085a042
259:homopolar generator
253:Use in Magnetolisis
225:Theoretical Concept
1389:Chemical processes
649:Surface Technology
290:
222:
206:non-renewable fuel
107:Pulse electrolysis
22:Pulse Electrolysis
16:Pulse Electrolysis
1053:(12): 4901–4934.
978:10.1021/nl4019879
926:(19): 7782–7791.
765:(9–10): 287–289.
744:10.1149/1.2411787
701:10.1063/1.1700503
631:10.1149/1.2220923
617:(10): 2863–2867.
267:permanent magnets
104:
103:
39:Typical Materials
1406:
1399:Electrochemistry
1373:
1372:
1336:
1330:
1329:
1319:
1309:
1285:
1276:
1275:
1239:
1230:
1229:
1193:
1182:
1181:
1153:
1142:
1124:
1115:
1114:
1089:
1080:
1069:
1063:
1062:
1042:
1036:
1035:
1015:
1006:
1005:
963:
954:(8): 3903–3908.
942:
936:
935:
911:
898:
897:
877:
871:
870:
850:
841:
840:
812:
791:
790:
754:
748:
747:
719:
713:
712:
676:
665:
664:
644:
635:
634:
606:
577:
576:
532:
511:
510:
478:
472:
471:
447:
441:
440:
438:
437:
423:
399:current polarity
169:Research history
139:produce hydrogen
109:is an alternate
73:Cell temperature
47:PDC Electrolysis
31:
19:
1414:
1413:
1409:
1408:
1407:
1405:
1404:
1403:
1379:
1378:
1377:
1376:
1338:
1337:
1333:
1287:
1286:
1279:
1241:
1240:
1233:
1195:
1194:
1185:
1155:
1154:
1145:
1125:
1118:
1112:
1091:
1090:
1083:
1070:
1066:
1044:
1043:
1039:
1017:
1016:
1009:
944:
943:
939:
913:
912:
901:
879:
878:
874:
852:
851:
844:
814:
813:
794:
759:Physical Review
756:
755:
751:
721:
720:
716:
678:
677:
668:
646:
645:
638:
608:
607:
580:
534:
533:
514:
480:
479:
475:
449:
448:
444:
435:
433:
425:
424:
420:
415:
407:over potentials
391:
382:
369:crystallisation
348:
346:Industrial Uses
310:
255:
235:diffusion layer
227:
202:
187:current density
171:
81:Current density
34:
17:
12:
11:
5:
1412:
1410:
1402:
1401:
1396:
1391:
1381:
1380:
1375:
1374:
1347:(3): 543–552.
1331:
1277:
1250:(2): 103–108.
1231:
1204:(5): 249–250.
1183:
1164:(1): 429–453.
1143:
1116:
1110:
1081:
1073:Comptes Rendus
1064:
1037:
1026:(4): 315–318.
1007:
937:
899:
888:(4): 455–476.
872:
842:
823:(2): 101–112.
792:
749:
714:
687:(4): 614–628.
666:
655:(4): 287–300.
636:
578:
512:
473:
442:
417:
416:
414:
411:
390:
387:
381:
378:
353:electroplating
347:
344:
319:weight percent
309:
306:
263:magnetic field
254:
251:
226:
223:
201:
198:
179:electromagnets
170:
167:
151:electroplating
146:electrolyser.
102:
101:
98:
94:
93:
90:
86:
85:
82:
78:
77:
74:
70:
69:
65:
64:
61:
57:
56:
53:
49:
48:
45:
41:
40:
36:
35:
32:
24:
23:
15:
13:
10:
9:
6:
4:
3:
2:
1411:
1400:
1397:
1395:
1392:
1390:
1387:
1386:
1384:
1370:
1366:
1362:
1358:
1354:
1350:
1346:
1342:
1335:
1332:
1327:
1323:
1318:
1317:11392/2436643
1313:
1308:
1303:
1299:
1295:
1291:
1284:
1282:
1278:
1273:
1269:
1265:
1261:
1257:
1253:
1249:
1245:
1238:
1236:
1232:
1227:
1223:
1219:
1215:
1211:
1207:
1203:
1199:
1192:
1190:
1188:
1184:
1179:
1175:
1171:
1167:
1163:
1159:
1152:
1150:
1148:
1144:
1140:
1136:
1132:
1131:German Patent
1128:
1123:
1121:
1117:
1113:
1111:9781468486698
1107:
1103:
1099:
1095:
1088:
1086:
1082:
1078:
1074:
1068:
1065:
1060:
1056:
1052:
1048:
1041:
1038:
1033:
1029:
1025:
1021:
1014:
1012:
1008:
1003:
999:
995:
991:
987:
983:
979:
975:
971:
967:
962:
957:
953:
949:
941:
938:
933:
929:
925:
921:
917:
910:
908:
906:
904:
900:
895:
891:
887:
883:
876:
873:
868:
864:
860:
856:
849:
847:
843:
838:
834:
830:
826:
822:
818:
811:
809:
807:
805:
803:
801:
799:
797:
793:
788:
784:
780:
776:
772:
768:
764:
760:
753:
750:
745:
741:
737:
733:
729:
725:
718:
715:
710:
706:
702:
698:
694:
690:
686:
682:
675:
673:
671:
667:
662:
658:
654:
650:
643:
641:
637:
632:
628:
624:
620:
616:
612:
605:
603:
601:
599:
597:
595:
593:
591:
589:
587:
585:
583:
579:
574:
570:
566:
562:
558:
554:
550:
546:
542:
538:
531:
529:
527:
525:
523:
521:
519:
517:
513:
508:
504:
500:
496:
492:
488:
484:
477:
474:
469:
465:
462:: 2197–2199.
461:
457:
453:
446:
443:
432:
428:
422:
419:
412:
410:
408:
403:
400:
395:
389:Disadvantages
388:
386:
379:
377:
373:
370:
366:
360:
358:
354:
345:
343:
341:
336:
332:
329:
323:
320:
314:
307:
305:
301:
297:
293:
286:
282:
279:
278:viscous force
274:
272:
268:
264:
260:
252:
250:
246:
244:
240:
236:
232:
224:
218:
214:
211:
207:
199:
197:
195:
190:
188:
184:
180:
175:
168:
166:
164:
160:
154:
152:
147:
144:
140:
136:
132:
128:
124:
120:
116:
112:
108:
99:
95:
91:
87:
83:
79:
75:
71:
66:
62:
58:
54:
50:
46:
42:
37:
30:
25:
20:
1394:Electrolysis
1344:
1340:
1334:
1297:
1293:
1247:
1243:
1201:
1197:
1161:
1157:
1138:
1134:
1130:
1126:
1093:
1076:
1072:
1067:
1050:
1046:
1040:
1023:
1019:
951:
948:Nano Letters
947:
940:
923:
919:
885:
881:
875:
858:
854:
820:
816:
762:
758:
752:
727:
723:
717:
684:
680:
652:
648:
614:
610:
543:(1): 33–34.
540:
536:
486:
482:
476:
458:(13). U.S.:
455:
451:
445:
434:. Retrieved
431:ResearchGate
430:
421:
404:
396:
392:
383:
374:
361:
349:
339:
337:
333:
327:
324:
315:
311:
302:
298:
294:
291:
275:
256:
247:
233:(EDL), or a
228:
220:Double Layer
203:
191:
176:
172:
155:
148:
117:to initiate
111:electrolysis
106:
105:
89:Cell voltage
861:: 205–213.
493:: 203–210.
239:capacitance
1383:Categories
1300:(8): 260.
1079:: 835–840.
730:(2): 166.
436:2019-05-31
413:References
380:Advantages
365:nucleation
300:achieved.
194:fuel cells
131:duty cycle
1361:1674-7291
1326:2079-6412
1264:0020-2967
1226:109143443
1218:0020-2967
1178:0020-2967
986:1530-6984
961:1307.6723
787:0031-899X
709:0021-9606
565:2451-3156
63:Aluminium
55:Aluminium
1369:96201556
1294:Coatings
1272:96146510
1127:A. Coehn
1002:20925098
994:23879333
573:99125043
507:99479894
92:+18 V DC
76:25 ± 2°C
966:Bibcode
825:Bibcode
767:Bibcode
732:Bibcode
689:Bibcode
619:Bibcode
545:Bibcode
357:plating
1367:
1359:
1324:
1270:
1262:
1224:
1216:
1176:
1108:
1000:
992:
984:
785:
707:
571:
563:
505:
340:et al.
328:et al.
129:, the
100:60-70%
84:400 mA
1365:S2CID
1268:S2CID
1222:S2CID
1135:75482
998:S2CID
956:arXiv
569:S2CID
503:S2CID
489:(2).
1357:ISSN
1322:ISSN
1260:ISSN
1214:ISSN
1174:ISSN
1139:1893
1106:ISBN
990:PMID
982:ISSN
783:ISSN
705:ISSN
561:ISSN
367:and
161:and
1349:doi
1312:hdl
1302:doi
1252:doi
1206:doi
1166:doi
1098:doi
1055:doi
1028:doi
974:doi
928:doi
890:doi
863:doi
833:doi
775:doi
740:doi
728:116
697:doi
657:doi
627:doi
615:140
553:doi
495:doi
464:doi
137:to
1385::
1363:.
1355:.
1345:53
1343:.
1320:.
1310:.
1296:.
1292:.
1280:^
1266:.
1258:.
1248:86
1246:.
1234:^
1220:.
1212:.
1202:86
1200:.
1186:^
1172:.
1162:31
1160:.
1146:^
1133:,
1129:,
1119:^
1104:,
1084:^
1075:.
1051:38
1049:.
1024:21
1022:.
1010:^
996:.
988:.
980:.
972:.
964:.
952:13
950:.
924:41
922:.
918:.
902:^
886:25
884:.
859:89
857:.
845:^
831:.
821:10
819:.
795:^
781:.
773:.
763:65
761:.
738:.
726:.
703:.
695:.
685:20
683:.
669:^
651:.
639:^
625:.
613:.
581:^
567:.
559:.
551:.
541:13
539:.
515:^
501:.
485:.
456:66
454:.
429:.
1371:.
1351::
1328:.
1314::
1304::
1298:8
1274:.
1254::
1228:.
1208::
1180:.
1168::
1141:)
1137:(
1100::
1077:4
1061:.
1057::
1034:.
1030::
1004:.
976::
968::
958::
934:.
930::
896:.
892::
869:.
865::
839:.
835::
827::
789:.
777::
769::
746:.
742::
734::
711:.
699::
691::
663:.
659::
653:6
633:.
629::
621::
575:.
555::
547::
509:.
497::
487:8
470:.
466::
439:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.