351:
413:
268:
481:
324:(preferably hydrochloric acid) and mixed with phosphoric acid as condensing agent. The resulting homogeneous solution is evaporated at 120 °C and the resulting glassy mass is then polycondensed at 180 °C to 200 °C for at least one hour. The phosphoric acid is washed out and the dried polysuccinimide is converted by mild alkaline hydrolysis into water-soluble polyaspartic acid; the molar mass of which can be determined by
24:
409:), hydrolysis takes place in α- and β-position of the succinimide (2,5-pyrrolidinedione) ring structures and racemization follows at the chiral center of the aspartic acid, yielding the water-soluble sodium salt of the poly(α, β)-DL-aspartic acid. The α form is formed to approx. 30%, the β form to approx. 70% in random arrangement along the polymer chain.
956:
858:
975:
880:
441:
under the brand name
Baypure® DSP with an average molecular weight of 4,400 g/mol is partially hydrolyzed even at slightly elevated pH values and is thus swellable in highly crosslinked form or water-soluble in linear form. The copoly-(succinimide-aspartic acid) formed by partial hydrolysis and
323:
A recent patent describes the simple preparation of high molecular weight, virtually colorless and linear, unbranched polysuccinimide. For this purpose, aspartic acid, which is present as crystalline zwitterion and practically water-insoluble, is firstly dissolved with an aqueous, volatile acid
1149:
937:
279:
upon water elimination. This is generally the case in the absence of strong acids, which suppress the thermal decomposition of free amino end groups and thus chain interruption reactions. The formation of the polyimide polysuccinimide can be followed by the intensive absorption band in the
918:
303:
in a thin layer is heated to 200 °C for 2 to 4 hours, polysuccinimide is produced with molar masses in the range of 30,000 g/mol and cream white shade. The implementation of the polycondensation in several steps (precondensation, comminution, postcondensation), with other
899:
424:
in the polymer chain are attacked upon degradation of the molar mass. The presence of amide bonds makes the polyaspartic acid obtained in the hydrolysis relatively biodegradable (about 70% in wastewater), even of initially highly crosslinked polysuccinimides.
1130:
1088:
Eberhard W. Neuse, Axel G. Perlwitz, Siegfried
Schmitt (1991-11-01), "Water-soluble polyamides as potential drug carriers. III. Relative main-chain stabilities of side chain-functionalized aspartamide polymers on aqueous-phase dialysis",
584:
M. Tomida, T. Nakato, M. Kuramochi, M. Shibata, S. Matsunami, T. Kakuchi (1996), "Novel method of synthesizig poly(succinimide) and its copolymeric derivatives by acid-catalysed polycondensation of L-aspartic acid",
263:
as early as 1897. When dry aspartic acid was heated for about 20 hours at 190 °C to 200 °C, a colorless product was obtained. Above 200 °C, a weak yellowing occurs, the yield was almost quantitative.
320:) provides higher molecular weight products with molar masses in the range of 10,000 to 200,000 g/mol. However, the patent literature does not address the polymer morphology, in particular the degree of branching.
450:, and as a setting retarder for cement in the construction industry. Patent literature mentions polysuccinimide applications as chelating agents, inhibitors against scale formation, dispersant, humectants, and
723:
Kenneth Doll, Randal
Shogren, Ronald Holser, J. Willett, Graham Swift (2005-12-01), "Polymerization of L-Aspartic Acid to Polysuccinimide and Copoly(Succinimide-Aspartate) in Supercritical Carbon Dioxide",
477:
with good biocompatibility and biodegradability, high water solubility at low manufacturing costs and was investigated more intensive as a potential drug carrier) in medical applications.
284:
at 1714 cm. Many process variants described in the patent literature yield besides a relatively low degree of polymerization often branched and yellow to brown discolored products.
954:, L.P. Koskan, A.R.Y. Meah, "Production of polysuccinimide and polyaspartic acid acid from maleic anhydride and ammonia", issued 1994-03-22, assigned to Donlar Corp.
339:
or based on the intermediately formed maleic acid monoamide achieved only low molar masses of a few 1,000 g/mol and yielded colored products. The same was the case for "
1111:
839:
792:
746:
609:
570:
856:, J. Knebel, K. Lehmann, "Method for increasing the molecular weight in the manufacture of polysuccinimide", issued 1992-08-25, assigned to Röhm GmbH
973:, M. B. Freeman et al., "Production of polysuccinimide by thermal polymerization of maleamic acid", issued 1995-02-28, assigned to Rohm and Haas Co.
878:, M. Uenaka et al., "Process for producing polysuccinimide and use of said compound", issued 1997-8-27, assigned to Mitsubishi Chemical Corp.
100:
291:
and achieving a linear chain structure while avoiding decomposition reactions. With a simple "oven process" in which a mixture or paste of crystalline
643:"Poly(aspartic acid) in Biomedical Applications: From Polymerization, Modification, Properties, Degradation, and Biocompatibility to Applications"
370:
Polysuccinimide is produced as an odourless, non-hygroscopic, cream-white to brown powder which is soluble in aprotic dipolar solvents such as
1147:, Y. Irizato et al., "Production process of cross-linked polyaspartic acid", issued 2000-06-06, assigned to Mitsui Chemicals
1067:
1001:
777:
555:
492:, has been extensively tested for its suitability as a biodegradable superabsorbent compared to the non-biodegradable standard cross-linked
809:
Paolo Neri, Guido Antoni, Franco
Benvenuti, Francesco Cocola, Guido Gazzei (1973-08-01), "Synthesis of α, β-poly , a new plasma expander",
496:. The results obtained have not yet led to the use of crosslinked polyaspartic acid in large-volume applications for superabsorbents (e.g.
935:, T. Groth et al., "Process for preparing polysuccinimide and polyaspartic acid", issued 1994-08-31, assigned to Bayer AG
442:
especially polyaspartic acid (trade name
Baypure® DS 100) produced by partial hydrolysis is suitable as a long-lasting inhibitor against
350:
358:
Due to the lower cost of maleic anhydride and ammonia, starting materials produced from fossil raw materials, no L-aspartic acid (of
412:
916:, C.S. Sikes, "Preparation of high molecular weight polysuccinimides", issued 2006-05-30, assigned to Aquero Co.
548:
Ullmann's
Polymers and Plastics, Products and Processes, Volume 1, Part 2: Organic Polymers, Polyaspartates and Polysuccinimide
267:
897:, G.Y. Mazo et al., "Catalytically polymerizing aspartic acid", issued 1998-05-26, assigned to Donlar Corp.
480:
516:
E. Jalalvandi, A. Shavandi (2018), "Polysuccinimide and its derivatives: Degradable and water soluble polymers (review)",
325:
344:
86:
205:
488:
Cross-linked poly(α, β)-DL aspartic acid sodium salt, which is the commercially most interesting polysuccinimide
406:
275:
In the experiments by Hugo Schiff, oligomers and low-molecular polymers were formed in a solid state reaction by
240:. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from
184:
soluble in
Dimethylformamid, Dimethylacetamid, Dimethylsulfoxid, N-Methylpyrrolidone, und Mesitylen+Sulfolan
587:
489:
383:
1128:, Y. Chou, "Forming superabsorbent polymer", issued 1999-01-19, assigned to Solutia Inc.
451:
1173:
237:
36:
1144:
1125:
970:
951:
932:
913:
894:
875:
853:
625:
188:
305:
493:
317:
313:
300:
52:
1060:
Biologically-responsive hybrid biomaterials: a reference for material scientists and bioengineers.
680:
529:
390:
379:
359:
241:
641:
Adelnia, Hossein; Tran, Huong D.N.; Little, Peter J.; Blakey, Idriss; Ta, Hang T. (2021-06-14).
1005:
1168:
1105:
1063:
997:
833:
822:
786:
773:
760:
Thomas Klein, Ralf-Johann Moritz, René Graupner (2008), "Polyaspartates and
Polysuccinimide",
740:
672:
603:
564:
551:
375:
371:
281:
362:
origin) is used in the production of the commercial product
Baypure® polysuccinimide either.
1094:
1071:
1056:
Design and synthesis of endosomolytic conjugated polyaspartamide for cytosolic drug delivery
1022:
814:
765:
729:
703:
662:
654:
592:
521:
332:
276:
175:
123:
328:. The process provides reproducible polysuccinimide with molar masses above 100,000 g/mol.
62:
474:
447:
340:
296:
199:
1162:
684:
596:
533:
421:
292:
248:
229:
658:
525:
497:
470:
642:
1026:
1098:
260:
158:
1075:
733:
458:
438:
402:
394:
331:
Synthetic routes for polysuccinimides based on maleic acid monoammonium salt,
288:
152:
1043:
Baypure®, An innovate product family for household and technical applications
769:
707:
466:
443:
398:
233:
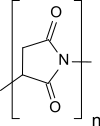
676:
457:
The opening of the pyrrolidinedione ring structures in polysuccinimide via
826:
23:
469:
poly-(α, β)-DL-aspartylhydrazide (PAHy) and with functional amines, e.g.
434:
818:
667:
336:
309:
198:
Except where otherwise noted, data are given for materials in their
473:
poly-(α), β)-DL-2-hydroxyethylaspartate (PHEA). PHEA can be used a
85:
75:
405:
in water only very slowly. In diluted alkaline media (e.g. 1M
236:. Polysuccinimide is insoluble in water, but soluble in some
479:
411:
349:
266:
702:(in German), vol. 30, no. 3, pp. 2449–2459,
420:
In more basic solutions or with longer reaction times, the
698:
446:in water treatment and applications in the oil and
228:, is formed during the thermal polycondensation of
1021:. 2nd ed. Springer Netherlands, 2002, S. 379–412,
718:
716:
259:The production of polysuccinimide was reported by
1045:. 5th Green Chemistry Conference 2003, Barcelona.
591:, vol. 37, no. 16, pp. 4435–4437,
1062:World Scientific Publishing Co., Singapur 2010,
347:and while avoiding mineral acids as catalysts.
61:
990:6. Commercial Poly(aspartic acid) and Its Uses
813:, vol. 16, no. 8, pp. 893–897,
762:Ullmann's Encyclopedia of Industrial Chemistry
700:Berichte der Deutschen Chemischen Gesellschaft
316:) or in the presence of solvents (for example
1093:, vol. 192, no. 1, pp. 35–50,
1058:. In: E. Jabbari, A. Khademhosseini (Hrsg.):
728:, vol. 2, no. 8, pp. 687–689,
621:
619:
465:OH) produces poly-(α, β)-DL-asparagine, with
8:
1110:: CS1 maint: multiple names: authors list (
994:Hydrophilic Polymers, Advances in Chemistry.
838:: CS1 maint: multiple names: authors list (
791:: CS1 maint: multiple names: authors list (
745:: CS1 maint: multiple names: authors list (
608:: CS1 maint: multiple names: authors list (
569:: CS1 maint: multiple names: authors list (
546:T. Klein, R.-J. Moritz, R. Graupner (2016),
1037:
1035:
647:ACS Biomaterials Science & Engineering
287:Recent work has focused on increasing the
15:
870:
868:
804:
802:
666:
550:, Weinheim: Wiley-VCH, pp. 742–743,
764:, Wiley-VCH Verlag GmbH & Co. KGaA,
508:
105:
1103:
831:
784:
738:
601:
562:
251:, the structurally related succinate.
187:30 to 35 at 20 °C in g·100 ml in
1091:Die Angewandte Makromolekulare Chemie
247:The name is derived from the salt of
7:
636:
634:
626:Baypure® General Product Information
40:Poly(2,5-dioxopyrrolidine-1,3-diyl)
14:
520:, vol. 109, pp. 43–54,
461:with ammonia water (containgin NH
433:The polysuccinimide developed by
271:polysuccinimide-Polykondensation
22:
659:10.1021/acsbiomaterials.1c00150
526:10.1016/j.eurpolymj.2018.08.056
484:polysuccinimide Derivatisierung
202:(at 25 °C , 100 kPa).
1006:doi:10.1021/ba-1996-0248.ch006
811:Journal of Medicinal Chemistry
1:
326:gel permeation chromatography
1027:10.1007/978-94-017-1217-0_11
726:Letters in Organic Chemistry
597:10.1016/0032-3861(96)00267-4
345:supercritical carbon dioxide
1099:10.1002/apmc.1991.051920103
354:PSI via Maleinsäureanhydrid
1190:
1076:10.1142/9789814295680_0009
734:10.2174/157017805774717553
401:mixtures. Polysuccinimide
416:PSI zu Polyasparaginsäure
407:sodium hydroxide solution
196:
116:
96:
45:
35:
30:
21:
770:10.1002/14356007.l21_l01
708:10.1002/cber.18970300316
238:aprotic dipolar solvents
222:polyanhydroaspartic acid
485:
417:
355:
343:" process variants in
306:dehydrating substances
272:
483:
415:
353:
270:
220:(PSI), also known as
181:* insoluble in water
452:fertilizer additives
444:limescale deposition
232:and is the simplest
1019:Degradable Polymers
819:10.1021/jm00266a006
494:sodium polyacrylate
318:propylene carbonate
314:triphenyl phosphite
301:polyphosphoric acid
242:renewable resources
176:Solubility in water
18:
992:. In: J.E. Glass:
486:
418:
391:triethylene glycol
387:-methylpyrrolidone
356:
273:
206:Infobox references
16:
1068:978-981-4295-67-3
1002:978-0-8412-3133-7
988:K.C. Low et al.:
779:978-3-527-30673-2
557:978-3-527-33823-8
448:mining industries
380:dimethylsulfoxide
376:dimethylacetamide
372:dimethylformamide
295:and concentrated
282:infrared spectrum
214:Chemical compound
212:
211:
108:*C1CC(=O)N(C1=O)*
87:Interactive image
1181:
1154:
1153:
1152:
1148:
1141:
1135:
1134:
1133:
1129:
1122:
1116:
1115:
1109:
1101:
1085:
1079:
1054:K. Seo, D. Kim:
1052:
1046:
1039:
1030:
1015:
1009:
986:
980:
979:
978:
974:
967:
961:
960:
959:
955:
948:
942:
941:
940:
936:
929:
923:
922:
921:
917:
910:
904:
903:
902:
898:
891:
885:
884:
883:
879:
872:
863:
862:
861:
857:
850:
844:
843:
837:
829:
806:
797:
796:
790:
782:
757:
751:
750:
744:
736:
720:
711:
710:
695:
689:
688:
670:
653:(6): 2083–2105.
638:
629:
628:(PDF) Lanxess AG
623:
614:
613:
607:
599:
581:
575:
574:
568:
560:
543:
537:
536:
513:
437:and marketed by
333:maleic anhydride
277:polycondensation
189:Triethylenglycol
124:Chemical formula
89:
65:
26:
19:
17:Polysuccinimide
1189:
1188:
1184:
1183:
1182:
1180:
1179:
1178:
1159:
1158:
1157:
1150:
1143:
1142:
1138:
1131:
1124:
1123:
1119:
1102:
1087:
1086:
1082:
1053:
1049:
1040:
1033:
1016:
1012:
987:
983:
976:
969:
968:
964:
957:
950:
949:
945:
938:
931:
930:
926:
919:
912:
911:
907:
900:
893:
892:
888:
881:
874:
873:
866:
859:
852:
851:
847:
830:
808:
807:
800:
783:
780:
759:
758:
754:
737:
722:
721:
714:
697:
696:
692:
640:
639:
632:
624:
617:
600:
583:
582:
578:
561:
558:
545:
544:
540:
515:
514:
510:
506:
475:plasma expander
464:
431:
368:
297:phosphoric acid
257:
226:polyaspartimide
218:Polysuccinimide
215:
208:
203:
178:
146:
140:
136:
132:
126:
112:
109:
104:
103:
92:
79:
68:
55:
41:
12:
11:
5:
1187:
1185:
1177:
1176:
1171:
1161:
1160:
1156:
1155:
1136:
1117:
1080:
1070:, S. 191–212,
1047:
1031:
1010:
981:
962:
943:
924:
905:
886:
864:
845:
798:
778:
752:
712:
690:
630:
615:
576:
556:
538:
518:Eur. Polym. J.
507:
505:
502:
462:
430:
427:
422:amide linkages
367:
364:
256:
253:
213:
210:
209:
204:
200:standard state
197:
194:
193:
192:
191:
185:
179:
174:
171:
170:
167:
163:
162:
161:
155:
149:
148:
142:
138:
134:
130:
127:
122:
119:
118:
114:
113:
111:
110:
107:
99:
98:
97:
94:
93:
91:
90:
82:
80:
73:
70:
69:
67:
66:
58:
56:
51:
48:
47:
43:
42:
39:
33:
32:
28:
27:
13:
10:
9:
6:
4:
3:
2:
1186:
1175:
1172:
1170:
1167:
1166:
1164:
1146:
1140:
1137:
1127:
1121:
1118:
1113:
1107:
1100:
1096:
1092:
1084:
1081:
1077:
1073:
1069:
1065:
1061:
1057:
1051:
1048:
1044:
1038:
1036:
1032:
1028:
1024:
1020:
1014:
1011:
1007:
1004:, S. 99–111,
1003:
999:
995:
991:
985:
982:
972:
966:
963:
953:
947:
944:
934:
928:
925:
915:
909:
906:
896:
890:
887:
877:
871:
869:
865:
855:
849:
846:
841:
835:
828:
824:
820:
816:
812:
805:
803:
799:
794:
788:
781:
775:
771:
767:
763:
756:
753:
748:
742:
735:
731:
727:
719:
717:
713:
709:
705:
701:
694:
691:
686:
682:
678:
674:
669:
664:
660:
656:
652:
648:
644:
637:
635:
631:
627:
622:
620:
616:
611:
605:
598:
594:
590:
589:
580:
577:
572:
566:
559:
553:
549:
542:
539:
535:
531:
527:
523:
519:
512:
509:
503:
501:
499:
495:
491:
482:
478:
476:
472:
468:
460:
455:
453:
449:
445:
440:
436:
428:
426:
423:
414:
410:
408:
404:
400:
396:
392:
388:
386:
381:
377:
373:
365:
363:
361:
352:
348:
346:
342:
338:
334:
329:
327:
321:
319:
315:
311:
308:(for example
307:
302:
298:
294:
293:aspartic acid
290:
285:
283:
278:
269:
265:
262:
254:
252:
250:
249:succinic acid
245:
243:
239:
235:
231:
230:aspartic acid
227:
223:
219:
207:
201:
195:
190:
186:
183:
182:
180:
177:
173:
172:
168:
165:
164:
160:
157:97.07 g·
156:
154:
151:
150:
145:
128:
125:
121:
120:
115:
106:
102:
95:
88:
84:
83:
81:
77:
72:
71:
64:
60:
59:
57:
54:
50:
49:
44:
38:
34:
29:
25:
20:
1174:Succinimides
1139:
1120:
1090:
1083:
1059:
1055:
1050:
1042:
1018:
1013:
993:
989:
984:
965:
946:
927:
908:
889:
848:
810:
761:
755:
725:
699:
693:
668:10072/404497
650:
646:
586:
579:
547:
541:
517:
511:
498:baby diapers
487:
471:ethanolamine
456:
432:
419:
384:
369:
357:
330:
322:
286:
274:
258:
246:
225:
221:
217:
216:
143:
46:Identifiers
996:248, 1996,
261:Hugo Schiff
166:Appearance
117:Properties
1163:Categories
1145:US 6072024
1126:US 5859179
1041:T. Klein:
1017:G. Swift:
971:US 5393868
952:US 5296578
933:EU 0612784
914:US 7053170
895:US 5756595
876:EU 0791616
854:US 5142062
504:References
490:derivative
459:aminolysis
439:Lanxess AG
403:hydrolyses
395:mesitylene
366:Properties
289:molar mass
255:Production
153:Molar mass
74:3D model (
63:31586-29-5
53:CAS Number
37:IUPAC name
685:232761877
534:106107591
467:hydrazine
399:sulfolane
234:polyimide
1169:Polymers
1106:citation
834:citation
787:citation
741:citation
677:33797239
604:citation
565:citation
435:Bayer AG
360:biogenic
310:zeolites
827:4745831
588:Polymer
337:ammonia
147:
1151:
1132:
1066:
1000:
977:
958:
939:
920:
901:
882:
860:
825:
776:
683:
675:
554:
532:
169:solid
101:SMILES
31:Names
681:S2CID
530:S2CID
341:green
76:JSmol
1112:link
1064:ISBN
998:ISBN
840:link
823:PMID
793:link
774:ISBN
747:link
673:PMID
610:link
571:link
552:ISBN
335:and
159:mole
1095:doi
1072:doi
1023:doi
815:doi
766:doi
730:doi
704:doi
663:hdl
655:doi
593:doi
522:doi
500:).
429:Use
393:or
299:or
224:or
1165::
1108:}}
1104:{{
1034:^
867:^
836:}}
832:{{
821:,
801:^
789:}}
785:{{
772:,
743:}}
739:{{
715:^
679:.
671:.
661:.
649:.
645:.
633:^
618:^
606:}}
602:{{
567:}}
563:{{
528:,
454:.
389:,
382:,
378:,
374:,
312:,
244:.
137:NO
129:(C
1114:)
1097::
1078:.
1074::
1029:.
1025::
1008:.
842:)
817::
795:)
768::
749:)
732::
706::
687:.
665::
657::
651:7
612:)
595::
573:)
524::
463:4
397:/
385:N
144:n
141:)
139:2
135:3
133:H
131:4
78:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.