31:
502:
The Tolman cone angle method assumes empirical bond data and defines the perimeter as the maximum possible circumscription of an idealized free-spinning substituent. The metal-ligand bond length in the Tolman model was determined empirically from crystal structures of tetrahedral nickel complexes. In
678:
Manz, T. A.; Phomphrai, K.; Medvedev, G.; Krishnamurthy, B. B.; Sharma, S.; Haq, J.; Novstrup, K. A.; Thomson, K. T.; Delgass, W. N.; Caruthers, J. M.; Abu-Omar, M. M. (2007). "Structure−Activity
Correlation in Titanium Single-Site Olefin Polymerization Catalysts Containing Mixed
901:
Newman-Stonebraker, Samuel H.; Smith, Sleight R.; Borowski, Julia E.; Peters, Ellyn; Gensch, Tobias; Johnson, Heather C.; Sigman, Matthew S.; Doyle, Abigail G. (2021). "Univariate classification of phosphine ligation state and reactivity in cross-coupling catalysis".
534:
of a metal center. Recent research has found that other descriptors—such as percent buried volume—are more accurate than cone angle at capturing the relevant steric effects of the phosphine ligand(s) when bound to the metal center.
492:
148:, assuming a bite angle of 74°, 85°, and 90° for diphosphines with methylene, ethylene, and propylene backbones, respectively. The Manz cone angle is often easier to compute than the Tolman cone angle:
98:. But the approach has been refined to include less symmetrical ligands of the type PRR′R″ as well as diphosphines. In such asymmetric cases, the substituent angles' half angles,
503:
contrast, the solid-angle concept derives both bond length and the perimeter from empirical solid state crystal structures. There are advantages to each system.
619:
Tolman, C. A.; Seidel, W. C.; Gosser, L. W. (1974-01-01). "Formation of three-coordinate nickel(0) complexes by phosphorus ligand dissociation from NiL
86:. Tolman originally developed the method for phosphine ligands in nickel complexes, determining them from measurements of accurate physical models.
437:
770:
969:
591:
Tolman, Chadwick A. (1970-05-01). "Phosphorus ligand exchange equilibriums on zerovalent nickel. Dominant role for steric effects".
267:
247:
257:
212:
974:
874:
741:
Niksch, Tobias; Görls, Helmar; Weigand, Wolfgang (2009). "The
Extension of the Solid-Angle Concept to Bidentate Ligands".
959:
550:
522:
because the size of the ligand affects the reactivity of the attached metal center. In an example, the selectivity of
650:
Tolman, C. A. (1977). "Steric
Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous Catalysis".
872:
Evans, D.; Osborn, J. A.; Wilkinson, G. (1968). "Hydroformylation of
Alkenes by Use of Rhodium Complex Catalyst".
839:"Analytical Algorithms for Ligand Cone Angles Calculations. Application to Triphenylphosphine Palladium Complexes"
964:
714:
Immirzi, A.; Musco, A. (1977). "A method to measure the size of phosphorus ligands in coordination complexes".
71:
506:
If the geometry of a ligand is known, either through crystallography or computations, an exact cone angle (
382:
327:
182:
39:
843:
768:
Bilbrey, Jenna A.; Kazez, Arianna H.; Locklin, J.; Allen, Wesley D. (2013). "Exact ligand cone angles".
519:
911:
67:
55:
74:
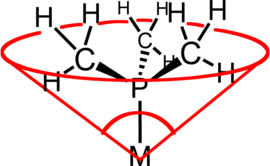
are commonly classified using this parameter, but the method can be applied to any ligand. The term
531:
195:
935:
813:
795:
306:
230:
79:
927:
787:
696:
593:
277:
34:
Ligand cone angle shows how much space is taken up by a ligand coordinated to a metal center.
919:
883:
852:
779:
750:
723:
688:
660:
632:
601:
523:
510:) can be calculated. No assumptions about the geometry are made, unlike the Tolman method.
915:
544:
527:
727:
953:
939:
94:
The concept of cone angle is most easily visualized with symmetrical ligands, e.g. PR
799:
17:
59:
47:
857:
838:
652:
145:
526:
catalysts is strongly influenced by the size of the coligands. Despite being
923:
169:
153:
931:
791:
754:
700:
887:
664:
636:
605:
487:{\displaystyle \theta ={\frac {2}{3}}\sum _{i}{\frac {\theta _{i}}{2}}}
408:
70:
of the ligand atoms at the perimeter of the base of the cone. Tertiary
783:
692:
83:
51:
530:, some phosphines are large enough to occupy more than half of the
30:
29:
63:
27:
Measure of the steric bulk of a ligand in a coordination complex
119:, are averaged and then doubled to find the total cone angle,
518:
The concept of cone angle is of practical importance in
440:
144:
of the backbone is approximated as half the chelate
486:
586:
8:
584:
582:
580:
578:
576:
574:
572:
570:
568:
566:
150:
856:
473:
467:
461:
447:
439:
62:formed with the metal at the vertex of a
562:
679:Cyclopentadienyl/Aryloxide Ligation".
7:
123:. In the case of diphosphines, the
771:Journal of Computational Chemistry
25:
875:Journal of the Chemical Society
66:and the outermost edge of the
1:
728:10.1016/S0020-1693(00)95635-4
551:Tolman electronic parameter
547:(versus electronic effects)
991:
858:10.1016/j.crci.2015.04.004
837:Petitjean, Michel (2015).
818:aarontools.readthedocs.io
970:Organometallic chemistry
82:, a research chemist at
78:was first introduced by
46:(θ) is a measure of the
924:10.1126/science.abj4213
58:. It is defined as the
975:Coordination chemistry
755:10.1002/ejic.200900825
488:
152:Cone angles of common
54:in a transition metal
40:coordination chemistry
35:
844:Comptes Rendus Chimie
520:homogeneous catalysis
489:
68:van der Waals spheres
33:
888:10.1039/J19680003133
438:
56:coordination complex
960:Tertiary phosphines
916:2021Sci...374..301N
743:Eur. J. Inorg. Chem
665:10.1021/cr60307a002
637:10.1021/ja00808a009
606:10.1021/ja00713a007
532:coordination sphere
157:
484:
466:
151:
80:Chadwick A. Tolman
36:
910:(6565): 301–308.
882:(21): 3133–3142.
784:10.1002/jcc.23217
778:(14): 1189–1197.
716:Inorg. Chim. Acta
693:10.1021/ja0640849
687:(13): 3776–3777.
600:(10): 2956–2965.
594:J. Am. Chem. Soc.
482:
457:
455:
432:
431:
72:phosphine ligands
44:ligand cone angle
18:Tolman cone angle
16:(Redirected from
982:
944:
943:
898:
892:
891:
869:
863:
862:
860:
834:
828:
827:
825:
824:
810:
804:
803:
765:
759:
758:
738:
732:
731:
711:
705:
704:
681:J. Am. Chem. Soc
675:
669:
668:
647:
641:
640:
625:J. Am. Chem. Soc
616:
610:
609:
588:
524:hydroformylation
493:
491:
490:
485:
483:
478:
477:
468:
465:
456:
448:
158:
143:
141:
140:
137:
134:
118:
116:
115:
112:
109:
90:Asymmetric cases
21:
990:
989:
985:
984:
983:
981:
980:
979:
965:Stereochemistry
950:
949:
948:
947:
900:
899:
895:
871:
870:
866:
836:
835:
831:
822:
820:
812:
811:
807:
767:
766:
762:
740:
739:
735:
713:
712:
708:
677:
676:
672:
649:
648:
644:
622:
618:
617:
613:
590:
589:
564:
559:
541:
516:
500:
469:
436:
435:
425:
420:
416:
412:
398:
394:
390:
386:
374:
370:
366:
355:
339:
335:
331:
318:
314:
310:
289:
285:
281:
238:
234:
203:
199:
186:
173:
138:
135:
132:
127:
126:
124:
113:
110:
107:
102:
101:
99:
97:
92:
28:
23:
22:
15:
12:
11:
5:
988:
986:
978:
977:
972:
967:
962:
952:
951:
946:
945:
893:
864:
851:(6): 678–684.
829:
805:
760:
733:
706:
670:
642:
620:
611:
561:
560:
558:
555:
554:
553:
548:
545:Steric effects
540:
537:
515:
512:
499:
496:
495:
494:
481:
476:
472:
464:
460:
454:
451:
446:
443:
430:
429:
426:
423:
418:
414:
410:
404:
403:
400:
396:
392:
388:
384:
379:
378:
375:
372:
368:
364:
360:
359:
356:
353:
345:
344:
341:
337:
333:
329:
324:
323:
320:
316:
312:
308:
303:
302:
299:
295:
294:
291:
287:
283:
279:
274:
273:
270:
264:
263:
260:
254:
253:
250:
244:
243:
240:
236:
232:
227:
226:
223:
219:
218:
215:
209:
208:
205:
201:
197:
192:
191:
188:
184:
179:
178:
175:
171:
166:
165:
162:
130:
105:
95:
91:
88:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
987:
976:
973:
971:
968:
966:
963:
961:
958:
957:
955:
941:
937:
933:
929:
925:
921:
917:
913:
909:
905:
897:
894:
889:
885:
881:
877:
876:
868:
865:
859:
854:
850:
846:
845:
840:
833:
830:
819:
815:
809:
806:
801:
797:
793:
789:
785:
781:
777:
773:
772:
764:
761:
756:
752:
749:(1): 95–105.
748:
744:
737:
734:
729:
725:
721:
717:
710:
707:
702:
698:
694:
690:
686:
682:
674:
671:
666:
662:
659:(3): 313–48.
658:
655:
654:
646:
643:
638:
634:
630:
626:
615:
612:
607:
603:
599:
596:
595:
587:
585:
583:
581:
579:
577:
575:
573:
571:
569:
567:
563:
556:
552:
549:
546:
543:
542:
538:
536:
533:
529:
525:
521:
513:
511:
509:
504:
497:
479:
474:
470:
462:
458:
452:
449:
444:
441:
434:
433:
427:
421:
406:
405:
401:
399:
381:
380:
376:
362:
361:
357:
351:
347:
346:
342:
340:
326:
325:
321:
319:
305:
304:
300:
297:
296:
292:
290:
276:
275:
271:
269:
266:
265:
261:
259:
256:
255:
251:
249:
246:
245:
241:
239:
229:
228:
224:
221:
220:
216:
214:
211:
210:
206:
204:
194:
193:
189:
187:
181:
180:
176:
174:
168:
167:
163:
160:
159:
155:
149:
147:
133:
122:
108:
89:
87:
85:
81:
77:
73:
69:
65:
61:
57:
53:
49:
45:
41:
32:
19:
907:
903:
896:
879:
873:
867:
848:
842:
832:
821:. Retrieved
817:
814:"AaronTools"
808:
775:
769:
763:
746:
742:
736:
719:
715:
709:
684:
680:
673:
656:
651:
645:
631:(1): 53–60.
628:
624:
614:
597:
592:
517:
507:
505:
501:
349:
128:
120:
103:
93:
75:
43:
37:
722:: L41–L42.
514:Application
60:solid angle
48:steric bulk
954:Categories
823:2023-05-30
653:Chem. Rev.
557:References
528:monovalent
498:Variations
164:Angle (°)
146:bite angle
76:cone angle
940:238991361
471:θ
459:∑
442:θ
328:P(cyclo-C
154:phosphine
932:34648340
800:23864226
792:23408559
701:17348648
539:See also
407:P(2,4,6-
156:ligands
912:Bibcode
904:Science
142:
125:
117:
100:
938:
930:
798:
790:
699:
161:Ligand
84:DuPont
52:ligand
42:, the
936:S2CID
796:S2CID
391:-2-CH
196:P(OCH
50:of a
928:PMID
788:PMID
747:2010
697:PMID
428:212
402:194
377:184
358:182
352:-Bu)
343:179
322:145
301:142
298:dcpe
293:132
278:P(CH
272:127
268:dppp
262:125
258:dppe
252:121
248:dppm
242:118
231:P(CH
225:115
222:depe
217:107
213:dmpe
207:107
190:104
64:cone
920:doi
908:374
884:doi
853:doi
780:doi
751:doi
724:doi
689:doi
685:129
661:doi
633:doi
623:".
602:doi
383:P(C
363:P(C
307:P(C
177:87
38:In
956::
934:.
926:.
918:.
906:.
880:33
878:.
849:18
847:.
841:.
816:.
794:.
786:.
776:34
774:.
745:.
720:25
718:.
695:.
683:.
657:77
629:96
627:.
598:92
565:^
409:Me
348:P(
334:11
282:CH
183:PF
170:PH
942:.
922::
914::
890:.
886::
861:.
855::
826:.
802:.
782::
757:.
753::
730:.
726::
703:.
691::
667:.
663::
639:.
635::
621:4
608:.
604::
508:θ
480:2
475:i
463:i
453:3
450:2
445:=
424:3
422:)
419:2
417:H
415:6
413:C
411:3
397:3
395:)
393:3
389:4
387:H
385:6
373:3
371:)
369:5
367:F
365:6
354:3
350:t
338:3
336:)
332:H
330:6
317:3
315:)
313:5
311:H
309:6
288:3
286:)
284:3
280:2
237:3
235:)
233:3
202:3
200:)
198:3
185:3
172:3
139:2
136:/
131:i
129:θ
121:θ
114:2
111:/
106:i
104:θ
96:3
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.