38:
330:
46:
419:
288:, alkyl alcohols can also be converted to alkyl tosylates, often through addition of tosyl chloride. In this reaction, the lone pair of the alcohol oxygen attacks the sulfur of the tosyl chloride, displacing the chloride and forming the tosylate with retention of reactant stereochemistry. This is useful because alcohols are poor leaving groups in S
357:
617:
De Castro, Kathlia A.; Ko, Jungnam; Park, Daejong; Park, Sungdae; Rhee, Hakjune (2007). "Reduction of Ethyl
Benzoylacetate and Selective Protection of 2-(3-Hydroxy-1-phenylpropyl)-4-methylphenol: A New and Facile Synthesis of Tolterodine".
303:
in organic synthesis. Alcohols can be converted to tosylate groups so that they do not react. The tosylate group may later be converted back into an alcohol. The use of these functional groups is exemplified in
450:
620:
721:
Haskell, Betty E.; Bowlus, Stephen B. (1976-01-01). "New synthesis of L-2-amino-3-oxalylaminopropionic acid, the
Lathyrus sativus neurotoxin".
764:
Sabitha, Gowravaram; Reddy, B. V. Subba; Abraham, Sunny; Yadav, J. S. (1999-02-19). "Deprotection of sulfonamides using iodotrimethylsilane".
601:
697:
664:
292:
2 reactions, in contrast to the tosylate group. It is the transformation of alkyl alcohols to alkyl tosylates that allows an S
240:(–OTs) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term
191:
329:
135:
885:
556:
536:
257:
875:
880:
427:
341:
167:
441:
317:
37:
703:
547:
to a phenol. The tosyl and nosyl groups are introduced as their respective chlorides with either
544:
532:
49:
Tosylate group with a generic "R" group attached. Note the extra oxygen, compared to plain tosyl.
851:
812:
746:
738:
693:
660:
597:
564:
524:
377:
305:
54:
682:
Greene's
Protective Groups in Organic Synthesis, Fourth Edition - Wuts - Wiley Online Library
843:
804:
773:
730:
685:
652:
629:
560:
548:
409:
369:
321:
300:
78:
74:
397:
495:
389:
777:
869:
832:"Reductive cleavage of sulfonamides with sodium bis(2-methoxyethoxy)aluminum hydride"
568:
552:
431:
269:
707:
792:
490:
435:
281:
413:
309:
344:
structure is extremely stable. It can be deprotected to reveal the amine using
45:
31:
855:
816:
742:
563:, the remaining tosyl group is removed by another round of NaOH. Not shown:
514:
In this article, "Ts", unless otherwise stated, means tosyl, not tennessine.
466:
750:
418:
540:
393:
119:
847:
808:
734:
17:
689:
656:
100:
831:
633:
373:
313:
131:
356:
320:
as its nosylate. The latter is a leaving group for displacement by
417:
355:
345:
337:
261:
245:
163:
159:
296:
2 reaction to occur in the presence of a good nucleophile.
336:
The tosyl group is also useful as a protecting group for
793:"Deprotection of Arenesulfonamides with Samarium iodide"
596:(6th ed.). John Wiley & Sons. p. 497.
198:-toluenesulfonyl) is most common, and by convention
41:
Tosyl group (blue) with a generic "R" group attached
316:group is protected as its tosylate and the primary
134:. This group is usually derived from the compound
680:Wuts, Peter G. M.; Greene, Theodora W. (2006).
649:Greene's Protective Groups in Organic Synthesis
830:Gold, Elijah H.; Babad, Esther. (1972-06-01).
8:
791:Vedejs, Edwin; Lin, Shouzhong (April 1994).
621:Organic Process Research & Development
461:Closely related to the tosylates are the
592:Smith, Michael B.; March, Jerry (2007).
236:
232:
228:
224:
185:
181:
177:
173:
153:
149:
145:
141:
125:
114:
110:
106:
95:
91:
87:
83:
44:
36:
584:
507:
481:-bromobenzenesulfonates, respectively.
469:, which are the abbreviated names for
404:Most common amine deprotection methods
647:Wuts, P. G. M.; Greene, T.W. (2006).
7:
384:Most common amine protection methods
594:March's Advanced Organic Chemistry
25:
360:A tosylamide (toluenesulfonamide)
268:-toluenesulfonic acid, TsOR (R =
836:The Journal of Organic Chemistry
797:The Journal of Organic Chemistry
723:The Journal of Organic Chemistry
328:
299:A tosyl group can function as a
158:(abbreviated TsCl), which forms
348:or strongly acidic conditions.
202:without a prefix refers to the
368:) group is commonly used as a
27:Chemical group (–SO₂–C₆H₄–CH₃)
1:
778:10.1016/S0040-4039(98)02646-X
352:Amine protection – tosyl (Ts)
312:, wherein one of the steps a
571:to optically pure (R)-isomer
555:as a base. The next step is
477:nitrobenzenesulfonates and
130:, with the open valence on
902:
29:
557:nucleophilic displacement
537:Friedel-Crafts alkylation
258:sodium p-toluenesulfonate
248:containing the anion of
244:may either refer to the
206:-toluenesulfonyl group.
190:(abbreviated TsOH). The
30:Not to be confused with
535:to a diol, followed by
252:-toluenesulfonic acid,
559:of the nosyl group by
422:
361:
260:), or it may refer to
50:
42:
421:
359:
59:toluenesulfonyl group
48:
40:
529:ethyl benzoylacetate
168:toluenesulfonic acid
848:10.1021/jo00978a034
809:10.1021/jo00086a005
766:Tetrahedron Letters
735:10.1021/jo00863a042
523:Reaction sequence:
99:. It consists of a
690:10.1002/0470053488
657:10.1002/0470053488
565:optical resolution
545:iron(III) chloride
533:sodium borohydride
423:
362:
51:
43:
842:(13): 2208–2210.
634:10.1021/op7001134
603:978-0-471-72091-1
525:organic reduction
457:Related compounds
378:organic synthesis
306:organic synthesis
73:) is a univalent
55:organic chemistry
16:(Redirected from
893:
886:Sulfonate esters
860:
859:
827:
821:
820:
803:(7): 1602–1603.
788:
782:
781:
772:(8): 1569–1570.
761:
755:
754:
718:
712:
711:
677:
671:
670:
651:. NY: J. Wiley.
644:
638:
637:
614:
608:
607:
589:
572:
561:diisopropylamine
549:sodium hydroxide
521:
515:
512:
370:protecting group
340:. The resulting
332:
322:diisopropylamine
301:protecting group
255:
239:
211:toluenesulfonate
192:para orientation
189:
157:
129:
117:
98:
79:chemical formula
75:functional group
21:
901:
900:
896:
895:
894:
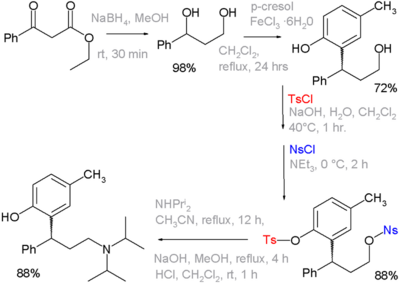
892:
891:
890:
876:Sulfonyl groups
866:
865:
864:
863:
829:
828:
824:
790:
789:
785:
763:
762:
758:
720:
719:
715:
700:
679:
678:
674:
667:
646:
645:
641:
616:
615:
611:
604:
591:
590:
586:
581:
576:
575:
569:L-tartaric acid
522:
518:
513:
509:
504:
487:
459:
449:Reduction with
445:
440:Reduction with
426:Refluxing with
406:
398:dichloromethane
386:
354:
295:
291:
285:
278:
253:
238:
234:
230:
226:
222:
187:
183:
179:
175:
171:
155:
151:
147:
143:
139:
127:
123:
116:
112:
108:
104:
97:
93:
89:
85:
81:
35:
28:
23:
22:
15:
12:
11:
5:
899:
897:
889:
888:
883:
881:Leaving groups
878:
868:
867:
862:
861:
822:
783:
756:
729:(1): 159–160.
713:
698:
672:
665:
639:
628:(5): 918–921.
609:
602:
583:
582:
580:
577:
574:
573:
516:
506:
505:
503:
500:
499:
498:
496:Sulfonyl group
493:
486:
483:
458:
455:
454:
453:
447:
443:
438:
424:
405:
402:
401:
400:
390:Tosyl chloride
385:
382:
353:
350:
334:
333:
293:
289:
283:
277:
274:
221:refers to the
136:tosyl chloride
118:, joined to a
65:, abbreviated
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
898:
887:
884:
882:
879:
877:
874:
873:
871:
857:
853:
849:
845:
841:
837:
833:
826:
823:
818:
814:
810:
806:
802:
798:
794:
787:
784:
779:
775:
771:
767:
760:
757:
752:
748:
744:
740:
736:
732:
728:
724:
717:
714:
709:
705:
701:
699:9780470053485
695:
691:
687:
683:
676:
673:
668:
666:9780470053485
662:
658:
654:
650:
643:
640:
635:
631:
627:
623:
622:
613:
610:
605:
599:
595:
588:
585:
578:
570:
566:
562:
558:
554:
553:triethylamine
550:
546:
542:
538:
534:
530:
526:
520:
517:
511:
508:
501:
497:
494:
492:
489:
488:
484:
482:
480:
476:
472:
468:
464:
456:
452:
448:
446:
439:
437:
433:
432:sodium iodide
429:
425:
420:
416:at 70 °C
415:
411:
408:
407:
403:
399:
395:
391:
388:
387:
383:
381:
379:
375:
371:
367:
358:
351:
349:
347:
343:
339:
331:
327:
326:
325:
323:
319:
315:
311:
307:
302:
297:
287:
275:
273:
271:
270:organyl group
267:
263:
259:
251:
247:
243:
220:
216:
212:
207:
205:
201:
197:
194:illustrated (
193:
169:
165:
161:
137:
133:
121:
102:
80:
76:
72:
68:
64:
60:
56:
47:
39:
33:
19:
839:
835:
825:
800:
796:
786:
769:
765:
759:
726:
722:
716:
681:
675:
648:
642:
625:
619:
612:
593:
587:
528:
519:
510:
491:Tosylic acid
478:
474:
470:
462:
460:
436:acetonitrile
365:
363:
335:
308:of the drug
298:
279:
276:Applications
265:
249:
241:
218:
214:
210:
208:
203:
199:
195:
70:
66:
62:
58:
52:
414:acetic acid
342:sulfonamide
310:tolterodine
286:2 reactions
101:tolyl group
63:tosyl group
870:Categories
579:References
467:brosylates
32:Tennessine
856:0022-3263
817:0022-3263
743:0022-3263
463:nosylates
346:reductive
77:with the
708:83393227
541:p-cresol
485:See also
394:pyridine
242:tosylate
215:tosylate
120:sulfonyl
18:Tosylate
751:1244456
364:Tosyl (
318:alcohol
256:(e.g.,
122:group,
854:
815:
749:
741:
706:
696:
663:
600:
451:Red-Al
374:amines
338:amines
314:phenol
262:esters
164:amides
160:esters
132:sulfur
704:S2CID
539:with
502:Notes
473:- or
428:TMSCl
246:salts
223:−O−SO
219:group
200:tosyl
852:ISSN
813:ISSN
747:PMID
739:ISSN
694:ISBN
661:ISBN
598:ISBN
543:and
465:and
434:and
412:and
392:and
372:for
280:For
254:TsOM
213:(or
209:The
162:and
57:, a
844:doi
805:doi
774:doi
731:doi
686:doi
653:doi
630:doi
567:by
551:or
531:by
527:of
442:SmI
410:HBr
396:in
376:in
324::
272:).
264:of
166:of
124:−SO
113:−CH
94:−CH
82:−SO
71:Tos
69:or
53:In
872::
850:.
840:37
838:.
834:.
811:.
801:59
799:.
795:.
770:40
768:.
745:.
737:.
727:41
725:.
702:.
692:.
684:.
659:.
626:11
624:.
475:p-
430:,
380:.
366:Ts
235:CH
217:)
188:OH
184:SO
172:CH
170:,
156:Cl
152:SO
140:CH
138:,
105:−C
103:,
86:−C
67:Ts
858:.
846::
819:.
807::
780:.
776::
753:.
733::
710:.
688::
669:.
655::
636:.
632::
606:.
479:p
471:o
444:2
294:N
290:N
284:N
282:S
266:p
250:p
237:3
233:4
231:H
229:6
227:C
225:2
204:p
196:p
186:2
182:4
180:H
178:6
176:C
174:3
154:2
150:4
148:H
146:6
144:C
142:3
128:−
126:2
115:3
111:4
109:H
107:6
96:3
92:4
90:H
88:6
84:2
61:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.