1616:
1745:
3716:
3567:
3635:
3472:
1515:
31:
149:
718:
1540:
1419:
2881:
Deerenberg, Sirik; Schakel, Marius; de
Keijzer, Adrianus H. J. F.; Kranenburg, Mirko; Lutz, Martin; Spek, Anthony L.; Lammertsma, Koop (2002). "Tetraalkylammonium pentaorganosilicates: the first highly stable silicates with five hydrocarbon ligands".
1688:
In general, almost any silicon-heteroatom bond is water-sensitive, and will spontaneously hydrolyze. Unstrained silicon-carbon bonds, however, are very strong, and cleave only in a small number of extreme conditions. Strong acids will
2853:
Aubouy, Laurent; Gerbier, Philippe; Huby, Nolwenn; Wantz, Guillaume; Vignau, Laurence; Hirsch, Lionel; Jano, Jean-Marc (2004). "Synthesis of new dipyridylphenylaminosiloles for highly emissive organic electroluminescent devices".
1348:. More specialized derivatives that find commercial applications include dichloromethylphenylsilane, trichloro(chloromethyl)silane, trichloro(dichlorophenyl)silane, trichloroethylsilane, and phenyltrichlorosilane.
2973:
Schmidtmann, Eric S.; Oestreich, Martin (2006). "Mechanistic insight into copper-catalysed allylic substitutions with bis(triorganosilyl) zincs. Enantiospecific preparation of -chiral silanes".
545:
The Si–C bond (1.89 Å) is significantly longer than a typical C–C bond (1.54 Å), suggesting that silyl substitutents have less steric demand than their organyl analogues. When geometry allows,
2702:
Gusel'Nikov, L.E.; Flowers, M.C. (1967). "The thermal decomposition of 1,1-dimethyl-1-silacyclobutane and some reactions of an unstable intermediate containing a silicon–carbon double bond".
845:
Silicon is a component of many functional groups. Most of these are analogous to organic compounds. The overarching exception is the rarity of multiple bonds to silicon, as reflected in the
522:(C 2.55 vs Si 1.90), and single bonds from Si to electronegative elements are very strong. Silicon is thus susceptible to nucleophilic attack by O, Cl, or F; the energy of an
1767:
3150:
2783:
Gau, D.; Kato, T.; Saffon-Merceron, N.; De Cózar, A.; Cossío, F.; Baceiredo, A. (2010). "Synthesis and
Structure of a Base-Stabilized C-Phosphino-Si-Amino Silyne".
2725:
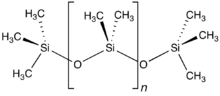
Brook, Adrian G.; Abdesaken, Fereydon; Gutekunst, Brigitte; Gutekunst, Gerhard; Kallury, R. Krishna (1981). "A solid silaethene: isolation and characterization".
2232:
Kinrade, Stephen D.; Gillson, Ashley-M. E.; Knight, Christopher T. G. (2002). "Silicon-29 NMR evidence of a transient hexavalent silicon complex in the diatom
1693:
arylsilanes and, in the presence of a Lewis acid catalyst, alkylsilanes. Most nucleophiles are too weak to displace carbon from silicon: the exceptions are
2130:
2461:
2102:
Janeta, Mateusz; Szafert, Sławomir (2017). "Synthesis, characterization and thermal properties of T8 type amido-POSS with p-halophenyl end-group".
113:
had established an award in 1960s that is given for significant contributions into the silicon chemistry. In his works
Kipping was noted for using
690:
Another major method for the formation of Si-C bonds is hydrosilylation (also called hydrosilation). In this process, compounds with Si-H bonds (
2957:
2444:
2388:
2216:
1998:
676:. About 1 million tons of organosilicon compounds are prepared annually by this route. The method can also be used for phenyl chlorosilanes.
1433:
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more
546:
63:. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air.
1626:, a coupling reaction used in certain specialized organic synthetic applications. The reaction begins with the activation of Si-C bond by
1744:
3143:
99:
and methyl-o-silicic acid. Extensive research in the field of organosilicon compounds was pioneered in the beginning of 20th century by
2667:
Ottosson, Henrik; Steel, Patrick G. (2006). "Silylenes, Silenes, and
Disilenes: Novel Silicon-Based Reagents for Organic Synthesis?".
2505:
2576:
2342:
3848:
95:
made the first organochlorosilane compound. The same year they also described a «polysilicic acid ether» in the preparation of
2036:
3136:
1186:
791:
512:
2460:
Ramírez-Oliva, E.; Hernández, A.; Martínez-Rosales, J.M.; Aguilar-Elguezabal, A.; Herrera-Pérez, G.; Cervantes, J. (2006).
2156:
1778:
Organosilicon compounds affect bee (and other insect) immune expression, making them more susceptible to viral infection.
1602:
Unlike carbon, silicon compounds can be coordinated to five atoms as well in a group of compounds ranging from so-called
1713:
535:
2620:
Chatgilialoglu, Chryssostomos; Ferreri, Carla; Landais, Yannick; Timokhin, Vitaliy I. (2018). "Thirty Years of (TMS)
1441:. Commonly the presence of the hydride is not mentioned in the name of the compound. Triethylsilane has the formula
3705:
3700:
3695:
3690:
3685:
3680:
3675:
3670:
3665:
3660:
3655:
3650:
3640:
3583:
3477:
3398:
3393:
3250:
2022:
1514:
1422:
3111:
3741:
3443:
3413:
3403:
3383:
3371:
3339:
3304:
3272:
3240:
3235:
3195:
1796:
109:, this is erroneous though) in relation to these materials in 1904. In recognition of Kipping's achievements the
3210:
3174:
3128:
3786:
3781:
3776:
3771:
3766:
3761:
3756:
3751:
3746:
3731:
3721:
3572:
3547:
3542:
3527:
3512:
3492:
3487:
3438:
3366:
3349:
3299:
3294:
3289:
3284:
3260:
3220:
1810:
1615:
82:
2289:
523:
137:
903:
Less frequently silanols are prepared by oxidation of silyl hydrides, a reaction that uses a metal catalyst:
3736:
3726:
3537:
3522:
3507:
3497:
3482:
3423:
3408:
3388:
3378:
3359:
3354:
3344:
3334:
3277:
2306:
1792:
1356:
1328:
1123:
have the connectivity Si-O-C. They are typically prepared by the reaction of alcohols with silyl chlorides:
669:
45:
3215:
3205:
3645:
3560:
3502:
3465:
3460:
3448:
3428:
3418:
3324:
3319:
3314:
3255:
3230:
1287:
615:
2259:
Bains, W.; Tacke, R. (2003). "Silicon chemistry as a novel source of chemical diversity in drug design".
3453:
3265:
3200:
3190:
1800:
1312:
673:
152:
Silicone caulk, commercial sealants, are mainly composed of organosilicon compounds mixed with hardener.
1690:
1566:
and other electronic properties. Siloles are efficient in electron transport. They owe their low lying
1950:
3532:
3517:
3329:
3309:
3063:
3016:) it can be demonstrated that the reaction proceeds not through the symmetrical π-allyl intermediate
1843:
1756:
1597:
726:
539:
110:
34:
156:
Organosilicon compounds are widely encountered in commercial products. Most common are antifoamers,
3433:
1818:
1563:
1179:
531:
69:
2952:. Translated by Oliveira, José; Elschenbroich, Christoph (3rd ed.). Wiley. pp. 240–244.
3037:
2918:
2649:
2187:
2016:
1814:
188:, however enzymes have been used to artificially create carbon-silicon bonds in living microbes.
1951:"CCXXII.—Organic derivatives of silicon. Part XV. The nomenclature of organic silicon compounds"
3089:
3052:"An Inert Pesticide Adjuvant Synergizes Viral Pathogenicity and Mortality in Honey Bee Larvae"
2988:
2953:
2910:
2826:
2800:
2765:
2684:
2641:
2594:
2572:
2501:
2440:
2384:
2338:
2268:
2212:
2150:
2081:
2004:
1994:
1970:
1931:
1887:
1434:
1352:
566:
519:
1506:. In 1967, Gusel'nikov and Flowers provided the first evidence for silenes from pyrolysis of
3079:
3071:
3013:
3009:
3006:
2980:
2900:
2892:
2863:
2835:
2792:
2757:
2730:
2707:
2676:
2633:
2602:
2564:
2530:
2493:
2488:
Linderman, Russell J.; Stiasni, Nikola; Hiersemann, Martin (2009). "Trimethylsilyllithium".
2432:
2409:
2378:
2241:
2206:
2179:
2111:
2073:
1962:
1921:
1879:
1788:
1709:
1175:
950:
846:
527:
133:
114:
100:
526:
in particular is strikingly high. This feature is exploited in many reactions such as the
3116:
1623:
1607:
1559:
733:
685:
611:
558:
136:
also made a significant contribution into the organosilicon chemistry by first describing
88:
64:
1282:
Organosilyl chlorides are important commodity chemicals. They are mainly used to produce
860:
are analogues of alcohols. They are generally prepared by hydrolysis of silyl chlorides:
3067:
3084:
3051:
2552:
1345:
745:
607:
126:
2568:
3842:
3805:
2975:
2884:
2355:
2191:
2115:
1702:
1397:
56:
2922:
2653:
2462:"Effect of the synthetic method of Pt/MgO in the hydrosilylation of phenylacetylene"
1351:
Although proportionately a minor outlet, organosilicon compounds are widely used in
3122:
2818:
2061:
1806:
1277:
562:
185:
92:
2497:
2637:
2436:
1867:
1578:
1571:
1510:. The first stable (kinetically shielded) silene was reported in 1981 by Brook.
1503:
1491:
1426:
1413:
1120:
691:
570:
515:. Compared to carbon–carbon bonds, carbon–silicon bonds are longer and weaker.
508:
204:
197:
2183:
1926:
1910:"Frederic Stanley Kipping—Pioneer in Silicon Chemistry: His Life & Legacy"
1909:
1833:
1581:
1574:
1490:
Organosilicon compounds, unlike their carbon counterparts, do not have a rich
201:
2839:
2534:
2413:
2085:
2008:
1974:
1935:
1891:
207:. Several organosilicon compounds have been investigated as pharmaceuticals.
17:
1837:
1751:
In this reaction type, silicon polarity is reversed in a chemical bond with
1603:
1585:
1555:
1375:
for the synthesis of this compound class is by heating hexaalkyldisiloxanes
714: — also participate, but these reactions are of little economic value.
173:
169:
3093:
3050:
Fine, Julia D.; Cox-Foster, Diana L.; Mullin, Christopher A. (2017-01-16).
2992:
2914:
2804:
2796:
2769:
2688:
2680:
2645:
2272:
2170:
Frampton, Mark B.; Zelisko, Paul M. (2009). "Organosilicon
Biotechnology".
518:
The C–Si bond is somewhat polarised towards carbon due to carbon's greater
30:
3125:
Thematic Series in the Open Access
Beilstein Journal of Organic Chemistry.
1988:
2734:
2711:
1966:
1823:
1698:
1694:
1627:
1524:
1520:
1283:
1109:
1105:
1050:
1004:
711:
189:
165:
161:
2606:
2404:
Röshe, L.; John, P.; Reitmeier, R. (2003). "Organic
Silicon Compounds".
2077:
576:
The bulk of organosilicon compounds derive from organosilicon chlorides
2761:
2469:
1827:
1760:
857:
694:) add to unsaturated substrates. Commercially, the main substrates are
614:
with a silicon-copper alloy. The main and most sought-after product is
96:
53:
3075:
2905:
2748:
Baines, Kim M. (2013). "Brook silenes: inspiration for a generation".
2521:
Dickhaut, Joachim; Giese, Bernd (1992). "Tris(trimethylsilyl)silane".
1286:
polymers as described above. Especially important silyl chlorides are
3160:
2984:
2896:
2867:
2245:
1883:
1479:
1401:
707:
699:
695:
464:
193:
105:
49:
725:
Hydrosilylation requires metal catalysts, especially those based on
721:
Idealized mechanism for metal-catalysed hydrosilylation of an alkene
557:
The first organosilicon compound, tetraethylsilane, was prepared by
148:
1185:
Exploiting the strength of the Si-F bond, fluoride sources such as
1003:. They are about 500x more acidic than the corresponding alcohols.
717:
1724:
1720:
1539:
1538:
1502:) are laboratory curiosities such as the silicon benzene analogue
1417:
1227:
1093:
1038:
878:
716:
703:
157:
147:
118:
29:
2362:
2313:
2290:"Common crop chemical leaves bees susceptible to deadly viruses"
1752:
1567:
1418:
481:
122:
3132:
2592:
1701:, although the latter often deprotonate the organosilane to a
164:. Other important uses include agricultural and plant control
1531:(with a silicon to carbon triple bond) was reported in 2010.
790:
Similarly, tris(trimethylsilyl)silyl lithium is derived from
1371:
is the main silylating agent. One classic method called the
1993:. Uri Tsoler, Paul Sosis. Boca Raton, FL: CRC Press. 2009.
2551:
Lickiss, Paul D. (1995). "The
Synthesis and Structure of
1622:
The stability of hypervalent silicon is the basis of the
549:, reversing the usual polarization on neighboring atoms.
2624:
SiH: A Milestone in
Radical-Based Synthetic Chemistry".
507:
In the great majority of organosilicon compounds, Si is
216:
Electronegativities Relevant to Organosilicon Chemistry
27:
Organometallic compound containing carbon–silicon bonds
2037:"Frederic Stanley Kipping Award in Silicon Chemistry"
1104:
Polymers with repeating siloxane linkages are called
103:. He also had coined the term "silicone" (resembling
1598:
Hypervalent molecule § Pentacoordinated silicon
1554:, are members of a larger class of compounds called
668:
A variety of other products are obtained, including
3797:
2097:
2095:
1562:and are of current academic interest due to their
1437:than silicon hence the naming convention of silyl
2062:"The Direct Synthesis of Organosilicon Compounds"
1189:(TBAF) are used in deprotection of silyl ethers:
200:is an organosilicon compound that functions as a
1731:and monosilylcopper compounds, which are formed
251:Properties Relevant to Organosilicon Chemistry
37:(PDMS) is the principal component of silicones.
2490:Encyclopedia of Reagents for Organic Synthesis
2427:Marciniec, B., ed. (2009). "Hydrosilylation".
2406:Ullmann's Encyclopedia of Industrial Chemistry
1868:"One hundred years of organosilicon chemistry"
1527:are silicon analogues of an alkyne. The first
1007:are the deprotonated derivatives of silanols:
3144:
1955:Journal of the Chemical Society, Transactions
1768:are susceptible to electrophilic substitution
698:. Other unsaturated functional groups —
192:, on the other hand, have known existence in
160:(sealant), adhesives, and coatings made from
8:
1735:by the reaction of the disilylzinc compound
1610:, to a uniquely stable pentaorganosilicate:
1108:. Compounds with an Si=O double bond called
3112:Selected Aspects of Organosilicon Chemistry
3038:oxidative addition or reductive elimination
2284:
2282:
948:Many silanols have been isolated including
3151:
3137:
3129:
2546:
2544:
2307:"Properties of atoms, radicals, and bonds"
2301:
2299:
2137:. Archived from the original on 2017-08-21
1990:Handbook of detergents. Part F, Production
547:silicon exhibits negative hyperconjugation
348:Dissociation energies of bonds to silicon
346:
249:
214:
3163:with other elements in the periodic table
3083:
2904:
2131:"Possibility Of Silicon Based Life Grows"
1925:
3012:on the substrate (replacing hydrogen by
2935:
2431:. Vol. 1. Springer. pp. 3–51.
2066:Journal of the American Chemical Society
1949:Kipping, Frederic Stanley (1912-01-01).
1229:
1095:
1040:
880:
832:
828:
824:
820:
816:
812:
808:
804:
800:
782:
778:
774:
770:
766:
762:
758:
754:
2819:"Direct synthesis of 2,5-dihalosiloles"
2785:Angewandte Chemie International Edition
2337:(81st ed.). CRC Press. June 2000.
1855:
1570:to a favorable interaction between the
606:. These chlorides are produced by the "
3180:
2148:
2014:
736:, a metal replaces the hydrogen atom.
3123:Contemporary organosilicon chemistry.
3020:which would give an equal mixture of
1723:silanes can be prepared from allylic
1174:Silyl ethers are extensively used as
7:
3822:Academic research, no widespread use
1903:
1901:
1861:
1859:
2104:Journal of Organometallic Chemistry
1766:Unsaturated silanes like the above
1049:Silanols tend to dehydrate to give
184:Carbon–silicon bonds are absent in
2948:Elschenbroich, Christoph (2006) .
1558:. They are the silicon analogs of
853:Silanols, siloxides, and siloxanes
168:commonly used in conjunction with
25:
3028:but through the Π-δ intermediate
2335:Handbook of Chemistry and Physics
610:", which entails the reaction of
129:and polymers for the first time.
3714:
3633:
3565:
3470:
3170:
2116:10.1016/j.jorganchem.2017.05.044
1908:Thomas, Neil R. (October 2010).
1866:Muller, Richard (January 1965).
1743:
1614:
1513:
748:to give trimethylsilyl lithium:
2557:Advances in Inorganic Chemistry
2060:Rochow, Eugene G. (June 1945).
1805:Compounds of carbon with other
744:Hexamethyldisilane reacts with
125:silanes and the preparation of
1187:tetra-n-butylammonium fluoride
792:tetrakis(trimethylsilyl)silane
513:tetrahedral molecular geometry
1:
2569:10.1016/S0898-8838(08)60053-7
2498:10.1002/047084289X.rt312.pub2
1872:Journal of Chemical Education
3117:Silicon in organic synthesis
2727:J. Chem. Soc., Chem. Commun.
2380:Silicon in Organic Synthesis
2261:Curr. Opin. Drug Discov. Dev
2238:J. Chem. Soc., Dalton Trans.
1543:Chemical structure of silole
1523:have Si=Si double bonds and
2638:10.1021/acs.chemrev.8b00109
2437:10.1007/978-1-4020-8172-9_1
2429:Advances in Silicon Science
144:Occurrence and applications
59:, to which they are called
3865:
1797:organophosphorus compounds
1739:, with Copper Iodide, in:
1595:
1498:Si=C bonds (also known as
1494:chemistry. Compounds with
1423:Tris(trimethylsilyl)silane
1411:
1275:
683:
80:
3711:
3630:
3182:
3178:
3168:
2211:. de Gruyter. p. 7.
2184:10.1007/s12633-009-9021-3
2155:: CS1 maint: unfit URL (
2041:American Chemical Society
1927:10.1007/s12633-010-9051-x
1811:organogermanium compounds
1787:Compounds of carbon with
2840:10.15227/orgsyn.085.0053
2535:10.15227/orgsyn.070.0164
2414:10.1002/14356007.a24_021
1793:organoaluminum compounds
1592:Pentacoordinated silicon
1577:silicon orbital with an
1112:are extremely unstable.
83:Organometallic chemistry
46:organometallic compounds
3849:Organosilicon compounds
2208:Organosilicon Chemistry
1508:dimethylsilacyclobutane
1425:is a well-investigated
1357:trimethylsilyl chloride
1329:trimethylsilyl chloride
740:Cleavage of Si-Si bonds
670:trimethylsilyl chloride
565:in 1863 by reaction of
536:Fleming–Tamao oxidation
61:organosilicon compounds
42:Organosilicon chemistry
3817:Many uses in chemistry
3812:Core organic chemistry
2797:10.1002/anie.201003616
2704:Chem. Commun. (London)
2681:10.1002/chem.200500429
2205:Pawlenko, S. (2011) .
2021:: CS1 maint: others (
1801:organosulfur compounds
1544:
1466:. The parent compound
1430:
1344:) are all produced by
1288:dimethyldichlorosilane
722:
616:dimethyldichlorosilane
153:
38:
3121:S. Marsden (Editor):
2135:Astrobiology Magazine
1774:Environmental effects
1712:source, hydrosilanes
1542:
1421:
1313:methyltrichlorosilane
727:platinum group metals
720:
674:methyltrichlorosilane
151:
138:Müller-Rochow process
33:
2735:10.1039/C39810000191
2712:10.1039/C19670000864
2377:Colvin, E. (2014) .
2234:Navicula pelliculosa
1967:10.1039/CT9120102106
1844:Decamethylsilicocene
1819:organolead compounds
1757:allylic substitution
1552:silacyclopentadienes
540:Peterson olefination
180:Biology and medicine
111:Dow Chemical Company
35:Polydimethylsiloxane
3068:2017NatSR...740499F
2607:10.1021/ja01331a504
2351:Parameter error in
2078:10.1021/ja01222a026
1815:organotin compounds
1763:group takes place.
1714:are good reductants
1564:electroluminescence
532:Brook rearrangement
349:
252:
217:
101:Frederic S. Kipping
3056:Scientific Reports
2762:10.1039/C3CC42595A
1545:
1454:. Phenylsilane is
1431:
1396:with concentrated
723:
347:
264:strength (kJ/mol)
250:
215:
154:
127:silicone oligomers
39:
3836:
3835:
3792:
3791:
3076:10.1038/srep40499
3036:only, through an
2959:978-3-527-29390-2
2827:Organic Syntheses
2595:J. Am. Chem. Soc.
2446:978-1-4020-8172-9
2390:978-1-4831-4223-4
2292:. Phys.org. 2017.
2218:978-3-11-086238-6
2000:978-1-4200-1465-5
1789:period 3 elements
1684:Various reactions
1500:alkylidenesilanes
1353:organic synthesis
1176:protective groups
841:Functional groups
567:tetrachlorosilane
520:electronegativity
505:
504:
345:
344:
248:
247:
115:Grignard reagents
16:(Redirected from
3856:
3828:
3823:
3818:
3813:
3718:
3717:
3637:
3636:
3569:
3568:
3474:
3473:
3171:
3153:
3146:
3139:
3130:
3110:Magnus Walter's
3098:
3097:
3087:
3047:
3041:
3010:desymmetrisation
3003:
2997:
2996:
2985:10.1039/b606589a
2970:
2964:
2963:
2945:
2939:
2933:
2927:
2926:
2908:
2897:10.1039/b109816k
2878:
2872:
2871:
2868:10.1039/b405238b
2850:
2844:
2843:
2823:
2815:
2809:
2808:
2780:
2774:
2773:
2745:
2739:
2738:
2722:
2716:
2715:
2699:
2693:
2692:
2664:
2658:
2657:
2626:Chemical Reviews
2617:
2611:
2610:
2589:
2583:
2582:
2548:
2539:
2538:
2518:
2512:
2511:
2485:
2479:
2478:
2466:
2457:
2451:
2450:
2424:
2418:
2417:
2401:
2395:
2394:
2374:
2368:
2367:
2366:
2360:
2354:
2348:
2331:
2325:
2324:
2322:
2320:
2312:. Zakarian lab,
2311:
2303:
2294:
2293:
2286:
2277:
2276:
2256:
2250:
2249:
2246:10.1039/b105379p
2229:
2223:
2222:
2202:
2196:
2195:
2167:
2161:
2160:
2154:
2146:
2144:
2142:
2126:
2120:
2119:
2099:
2090:
2089:
2057:
2051:
2050:
2048:
2047:
2033:
2027:
2026:
2020:
2012:
1985:
1979:
1978:
1946:
1940:
1939:
1929:
1905:
1896:
1895:
1884:10.1021/ed042p41
1863:
1747:
1710:covalent hydride
1679:
1678:
1677:
1668:
1666:
1665:
1655:
1654:
1653:
1644:
1643:
1642:
1618:
1560:cyclopentadienes
1517:
1477:
1476:
1475:
1465:
1464:
1463:
1453:
1451:
1450:
1395:
1394:
1393:
1385:
1384:
1370:
1368:
1367:
1343:
1341:
1340:
1326:
1325:
1324:
1310:
1309:
1308:
1300:
1299:
1267:
1266:
1265:
1256:
1254:
1253:
1245:
1244:
1234:
1224:
1223:
1222:
1213:
1211:
1210:
1202:
1201:
1169:
1167:
1166:
1158:
1157:
1147:
1145:
1144:
1136:
1135:
1100:
1090:
1089:
1088:
1080:
1079:
1069:
1067:
1066:
1045:
1035:
1033:
1032:
1022:
1020:
1019:
1002:
1000:
999:
991:
990:
982:
981:
971:
968:
967:
959:
958:
944:
942:
941:
931:
930:
929:
919:
917:
916:
898:
896:
895:
885:
875:
873:
872:
847:double bond rule
836:
786:
664:
663:
662:
654:
653:
645:
644:
634:
632:
631:
605:
604:
603:
595:
594:
586:
585:
528:Sakurai reaction
356:Energy (kJ/mol)
350:
259:Bond length (pm)
253:
218:
134:Eugene G. Rochow
44:is the study of
21:
3864:
3863:
3859:
3858:
3857:
3855:
3854:
3853:
3839:
3838:
3837:
3832:
3831:
3826:
3821:
3816:
3811:
3793:
3715:
3634:
3566:
3471:
3164:
3157:
3107:
3102:
3101:
3049:
3048:
3044:
3004:
3000:
2972:
2971:
2967:
2960:
2950:Organometallics
2947:
2946:
2942:
2934:
2930:
2880:
2879:
2875:
2852:
2851:
2847:
2834:: 53–63. 2008.
2821:
2817:
2816:
2812:
2782:
2781:
2777:
2747:
2746:
2742:
2724:
2723:
2719:
2701:
2700:
2696:
2666:
2665:
2661:
2632:(14): 6516–72.
2623:
2619:
2618:
2614:
2591:
2590:
2586:
2579:
2550:
2549:
2542:
2520:
2519:
2515:
2508:
2487:
2486:
2482:
2464:
2459:
2458:
2454:
2447:
2426:
2425:
2421:
2403:
2402:
2398:
2391:
2383:. Butterworth.
2376:
2375:
2371:
2358:
2352:
2350:
2349:
2345:
2333:
2332:
2328:
2318:
2316:
2309:
2305:
2304:
2297:
2288:
2287:
2280:
2258:
2257:
2253:
2231:
2230:
2226:
2219:
2204:
2203:
2199:
2169:
2168:
2164:
2147:
2140:
2138:
2129:Choi, Charles.
2128:
2127:
2123:
2101:
2100:
2093:
2059:
2058:
2054:
2045:
2043:
2035:
2034:
2030:
2013:
2001:
1987:
1986:
1982:
1948:
1947:
1943:
1907:
1906:
1899:
1865:
1864:
1857:
1852:
1784:
1776:
1686:
1676:
1674:
1673:
1672:
1670:
1664:
1661:
1660:
1659:
1657:
1652:
1650:
1649:
1648:
1646:
1641:
1638:
1637:
1636:
1634:
1624:Hiyama coupling
1608:phenylsilatrane
1600:
1594:
1537:
1488:
1474:
1471:
1470:
1469:
1467:
1462:
1459:
1458:
1457:
1455:
1449:
1446:
1445:
1444:
1442:
1435:electronegative
1416:
1410:
1392:
1389:
1388:
1387:
1383:
1380:
1379:
1378:
1376:
1366:
1363:
1362:
1361:
1359:
1339:
1336:
1335:
1334:
1332:
1323:
1320:
1319:
1318:
1316:
1307:
1304:
1303:
1302:
1298:
1295:
1294:
1293:
1291:
1280:
1274:
1272:Silyl chlorides
1264:
1262:
1261:
1260:
1258:
1252:
1249:
1248:
1247:
1243:
1240:
1239:
1238:
1236:
1231:
1226:
1221:
1219:
1218:
1217:
1215:
1209:
1206:
1205:
1204:
1200:
1197:
1196:
1195:
1193:
1165:
1162:
1161:
1160:
1156:
1153:
1152:
1151:
1149:
1143:
1140:
1139:
1138:
1134:
1131:
1130:
1129:
1127:
1118:
1097:
1092:
1087:
1084:
1083:
1082:
1078:
1075:
1074:
1073:
1071:
1065:
1062:
1061:
1060:
1058:
1042:
1037:
1031:
1028:
1027:
1026:
1024:
1018:
1015:
1014:
1013:
1011:
998:
995:
994:
993:
989:
986:
985:
984:
980:
977:
976:
975:
973:
966:
963:
962:
961:
957:
954:
953:
952:
949:
940:
937:
936:
935:
933:
928:
925:
924:
923:
921:
915:
912:
911:
910:
908:
894:
891:
890:
889:
887:
882:
877:
871:
868:
867:
866:
864:
855:
843:
834:
830:
826:
822:
818:
814:
810:
806:
802:
798:
784:
780:
776:
772:
768:
764:
760:
756:
752:
742:
734:silylmetalation
732:In the related
688:
686:Hydrosilylation
682:
680:Hydrosilylation
661:
658:
657:
656:
652:
649:
648:
647:
643:
640:
639:
638:
636:
630:
627:
626:
625:
623:
612:methyl chloride
602:
599:
598:
597:
593:
590:
589:
588:
584:
581:
580:
579:
577:
559:Charles Friedel
555:
524:Si–O bond
490:
486:
473:
469:
456:
452:
263:
213:
182:
146:
89:Charles Friedel
85:
79:
65:Silicon carbide
28:
23:
22:
15:
12:
11:
5:
3862:
3860:
3852:
3851:
3841:
3840:
3834:
3833:
3830:
3829:
3824:
3819:
3814:
3809:
3806:Chemical bonds
3802:
3801:
3799:
3795:
3794:
3790:
3789:
3784:
3779:
3774:
3769:
3764:
3759:
3754:
3749:
3744:
3739:
3734:
3729:
3724:
3719:
3712:
3709:
3708:
3703:
3698:
3693:
3688:
3683:
3678:
3673:
3668:
3663:
3658:
3653:
3648:
3643:
3638:
3631:
3628:
3627:
3623:
3622:
3619:
3616:
3613:
3610:
3607:
3604:
3601:
3598:
3595:
3592:
3589:
3586:
3581:
3578:
3575:
3570:
3563:
3558:
3554:
3553:
3550:
3545:
3540:
3535:
3530:
3525:
3520:
3515:
3510:
3505:
3500:
3495:
3490:
3485:
3480:
3475:
3468:
3463:
3457:
3456:
3451:
3446:
3441:
3436:
3431:
3426:
3421:
3416:
3411:
3406:
3401:
3396:
3391:
3386:
3381:
3376:
3374:
3369:
3363:
3362:
3357:
3352:
3347:
3342:
3337:
3332:
3327:
3322:
3317:
3312:
3307:
3302:
3297:
3292:
3287:
3282:
3280:
3275:
3269:
3268:
3263:
3258:
3253:
3248:
3243:
3238:
3233:
3227:
3226:
3223:
3218:
3213:
3208:
3203:
3198:
3193:
3187:
3186:
3183:
3181:
3179:
3177:
3169:
3166:
3165:
3158:
3156:
3155:
3148:
3141:
3133:
3127:
3126:
3119:
3114:
3106:
3105:External links
3103:
3100:
3099:
3042:
2998:
2979:(34): 3643–5.
2965:
2958:
2940:
2928:
2873:
2845:
2810:
2791:(37): 6585–8.
2775:
2756:(57): 6366–9.
2740:
2717:
2694:
2675:(6): 1576–85.
2659:
2621:
2612:
2584:
2577:
2553:Organosilanols
2540:
2513:
2507:978-0471936237
2506:
2480:
2452:
2445:
2419:
2396:
2389:
2369:
2343:
2326:
2295:
2278:
2267:(4): 526–543.
2251:
2224:
2217:
2197:
2178:(3): 147–163.
2162:
2121:
2091:
2072:(6): 963–965.
2052:
2028:
1999:
1980:
1941:
1920:(4): 187–193.
1897:
1854:
1853:
1851:
1848:
1847:
1846:
1841:
1831:
1821:
1803:
1783:
1780:
1775:
1772:
1749:
1748:
1685:
1682:
1681:
1680:
1675:
1662:
1651:
1639:
1620:
1619:
1593:
1590:
1550:, also called
1536:
1533:
1487:
1484:
1472:
1460:
1447:
1412:Main article:
1409:
1408:Silyl hydrides
1406:
1390:
1381:
1373:Flood reaction
1364:
1346:direct process
1337:
1321:
1305:
1296:
1276:Main article:
1273:
1270:
1269:
1268:
1263:
1250:
1241:
1220:
1207:
1198:
1172:
1171:
1163:
1154:
1141:
1132:
1117:
1114:
1102:
1101:
1085:
1076:
1063:
1047:
1046:
1029:
1016:
996:
987:
978:
964:
955:
946:
945:
938:
926:
913:
901:
900:
892:
869:
854:
851:
842:
839:
838:
837:
788:
787:
746:methyl lithium
741:
738:
684:Main article:
681:
678:
666:
665:
659:
650:
641:
628:
608:Direct process
600:
591:
582:
554:
551:
503:
502:
499:
495:
494:
491:
488:
484:
478:
477:
474:
471:
467:
461:
460:
457:
454:
450:
446:
445:
442:
438:
437:
434:
430:
429:
426:
422:
421:
418:
414:
413:
410:
406:
405:
402:
398:
397:
394:
390:
389:
386:
382:
381:
378:
374:
373:
370:
366:
365:
362:
358:
357:
354:
343:
342:
339:
336:
332:
331:
328:
325:
321:
320:
317:
314:
310:
309:
306:
303:
299:
298:
295:
292:
288:
287:
284:
281:
277:
276:
273:
270:
266:
265:
260:
257:
246:
245:
242:
239:
236:
232:
231:
228:
225:
222:
212:
209:
181:
178:
145:
142:
78:
75:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
3861:
3850:
3847:
3846:
3844:
3825:
3820:
3815:
3810:
3807:
3804:
3803:
3800:
3796:
3788:
3785:
3783:
3780:
3778:
3775:
3773:
3770:
3768:
3765:
3763:
3760:
3758:
3755:
3753:
3750:
3748:
3745:
3743:
3740:
3738:
3735:
3733:
3730:
3728:
3725:
3723:
3720:
3713:
3710:
3707:
3704:
3702:
3699:
3697:
3694:
3692:
3689:
3687:
3684:
3682:
3679:
3677:
3674:
3672:
3669:
3667:
3664:
3662:
3659:
3657:
3654:
3652:
3649:
3647:
3644:
3642:
3639:
3632:
3629:
3625:
3624:
3620:
3617:
3614:
3611:
3608:
3605:
3602:
3599:
3596:
3593:
3590:
3587:
3585:
3582:
3579:
3576:
3574:
3571:
3564:
3562:
3559:
3556:
3555:
3551:
3549:
3546:
3544:
3541:
3539:
3536:
3534:
3531:
3529:
3526:
3524:
3521:
3519:
3516:
3514:
3511:
3509:
3506:
3504:
3501:
3499:
3496:
3494:
3491:
3489:
3486:
3484:
3481:
3479:
3476:
3469:
3467:
3464:
3462:
3459:
3458:
3455:
3452:
3450:
3447:
3445:
3442:
3440:
3437:
3435:
3432:
3430:
3427:
3425:
3422:
3420:
3417:
3415:
3412:
3410:
3407:
3405:
3402:
3400:
3397:
3395:
3392:
3390:
3387:
3385:
3382:
3380:
3377:
3375:
3373:
3370:
3368:
3365:
3364:
3361:
3358:
3356:
3353:
3351:
3348:
3346:
3343:
3341:
3338:
3336:
3333:
3331:
3328:
3326:
3323:
3321:
3318:
3316:
3313:
3311:
3308:
3306:
3303:
3301:
3298:
3296:
3293:
3291:
3288:
3286:
3283:
3281:
3279:
3276:
3274:
3271:
3270:
3267:
3264:
3262:
3259:
3257:
3254:
3252:
3249:
3247:
3244:
3242:
3239:
3237:
3234:
3232:
3229:
3228:
3224:
3222:
3219:
3217:
3214:
3212:
3209:
3207:
3204:
3202:
3199:
3197:
3194:
3192:
3189:
3188:
3184:
3176:
3173:
3172:
3167:
3162:
3159:Compounds of
3154:
3149:
3147:
3142:
3140:
3135:
3134:
3131:
3124:
3120:
3118:
3115:
3113:
3109:
3108:
3104:
3095:
3091:
3086:
3081:
3077:
3073:
3069:
3065:
3061:
3057:
3053:
3046:
3043:
3039:
3035:
3032:resulting in
3031:
3027:
3023:
3019:
3015:
3011:
3008:
3002:
2999:
2994:
2990:
2986:
2982:
2978:
2977:
2976:Chem. Commun.
2969:
2966:
2961:
2955:
2951:
2944:
2941:
2937:
2936:Pawlenko 2011
2932:
2929:
2924:
2920:
2916:
2912:
2907:
2902:
2898:
2894:
2890:
2887:
2886:
2885:Chem. Commun.
2877:
2874:
2869:
2865:
2861:
2857:
2849:
2846:
2841:
2837:
2833:
2829:
2828:
2820:
2814:
2811:
2806:
2802:
2798:
2794:
2790:
2786:
2779:
2776:
2771:
2767:
2763:
2759:
2755:
2751:
2744:
2741:
2736:
2732:
2728:
2721:
2718:
2713:
2709:
2706:(17): 864–5.
2705:
2698:
2695:
2690:
2686:
2682:
2678:
2674:
2670:
2663:
2660:
2655:
2651:
2647:
2643:
2639:
2635:
2631:
2627:
2616:
2613:
2608:
2604:
2601:(4): 1735–6.
2600:
2597:
2596:
2588:
2585:
2580:
2578:9780120236428
2574:
2570:
2566:
2562:
2558:
2554:
2547:
2545:
2541:
2536:
2532:
2528:
2524:
2517:
2514:
2509:
2503:
2499:
2495:
2491:
2484:
2481:
2476:
2472:
2471:
2463:
2456:
2453:
2448:
2442:
2438:
2434:
2430:
2423:
2420:
2415:
2411:
2407:
2400:
2397:
2392:
2386:
2382:
2381:
2373:
2370:
2364:
2357:
2346:
2344:0-8493-0481-4
2340:
2336:
2330:
2327:
2315:
2308:
2302:
2300:
2296:
2291:
2285:
2283:
2279:
2274:
2270:
2266:
2262:
2255:
2252:
2247:
2243:
2239:
2235:
2228:
2225:
2220:
2214:
2210:
2209:
2201:
2198:
2193:
2189:
2185:
2181:
2177:
2173:
2166:
2163:
2158:
2152:
2136:
2132:
2125:
2122:
2117:
2113:
2109:
2105:
2098:
2096:
2092:
2087:
2083:
2079:
2075:
2071:
2067:
2063:
2056:
2053:
2042:
2038:
2032:
2029:
2024:
2018:
2010:
2006:
2002:
1996:
1992:
1991:
1984:
1981:
1976:
1972:
1968:
1964:
1961:: 2106–2107.
1960:
1956:
1952:
1945:
1942:
1937:
1933:
1928:
1923:
1919:
1915:
1911:
1904:
1902:
1898:
1893:
1889:
1885:
1881:
1877:
1873:
1869:
1862:
1860:
1856:
1849:
1845:
1842:
1839:
1835:
1832:
1829:
1825:
1822:
1820:
1816:
1812:
1808:
1804:
1802:
1798:
1794:
1790:
1786:
1785:
1781:
1779:
1773:
1771:
1769:
1764:
1762:
1758:
1755:and a formal
1754:
1746:
1742:
1741:
1740:
1738:
1734:
1730:
1726:
1722:
1717:
1715:
1711:
1706:
1704:
1703:silicon ylide
1700:
1696:
1692:
1691:protodesilate
1683:
1633:
1632:
1631:
1629:
1625:
1617:
1613:
1612:
1611:
1609:
1605:
1599:
1591:
1589:
1587:
1583:
1580:
1576:
1573:
1569:
1565:
1561:
1557:
1553:
1549:
1541:
1534:
1532:
1530:
1526:
1522:
1518:
1516:
1511:
1509:
1505:
1501:
1497:
1493:
1485:
1483:
1481:
1440:
1436:
1428:
1424:
1420:
1415:
1407:
1405:
1403:
1400:and a sodium
1399:
1398:sulfuric acid
1374:
1358:
1354:
1349:
1347:
1330:
1314:
1289:
1285:
1279:
1271:
1233:
1192:
1191:
1190:
1188:
1183:
1181:
1177:
1126:
1125:
1124:
1122:
1115:
1113:
1111:
1107:
1099:
1056:
1055:
1054:
1052:
1044:
1010:
1009:
1008:
1006:
970:
906:
905:
904:
884:
863:
862:
861:
859:
852:
850:
848:
840:
797:
796:
795:
793:
751:
750:
749:
747:
739:
737:
735:
730:
728:
719:
715:
713:
709:
705:
701:
697:
693:
687:
679:
677:
675:
671:
621:
620:
619:
617:
613:
609:
574:
572:
568:
564:
560:
552:
550:
548:
543:
541:
537:
533:
529:
525:
521:
516:
514:
510:
500:
497:
496:
492:
487:Si–SiAr
483:
480:
479:
475:
470:Si–SiMe
466:
463:
462:
458:
448:
447:
443:
440:
439:
435:
432:
431:
427:
424:
423:
419:
416:
415:
411:
408:
407:
403:
400:
399:
395:
392:
391:
387:
384:
383:
379:
376:
375:
371:
368:
367:
363:
360:
359:
355:
352:
351:
340:
337:
334:
333:
329:
326:
323:
322:
318:
315:
312:
311:
307:
304:
301:
300:
296:
293:
290:
289:
285:
282:
279:
278:
274:
271:
268:
267:
261:
258:
255:
254:
243:
240:
237:
234:
233:
229:
226:
223:
220:
219:
210:
208:
206:
203:
199:
195:
191:
187:
179:
177:
175:
171:
167:
163:
159:
150:
143:
141:
139:
135:
130:
128:
124:
120:
116:
112:
108:
107:
102:
98:
94:
90:
84:
76:
74:
72:
71:
66:
62:
58:
55:
51:
47:
43:
36:
32:
19:
18:Organosilicon
3827:Bond unknown
3245:
3059:
3055:
3045:
3033:
3029:
3025:
3021:
3017:
3001:
2974:
2968:
2949:
2943:
2938:, p. 3.
2931:
2891:(4): 348–9.
2888:
2883:
2876:
2859:
2855:
2848:
2831:
2825:
2813:
2788:
2784:
2778:
2753:
2750:Chem. Commun
2749:
2743:
2729:(4): 191–2.
2726:
2720:
2703:
2697:
2672:
2669:Chem. Eur. J
2668:
2662:
2629:
2625:
2615:
2598:
2593:
2587:
2560:
2556:
2526:
2522:
2516:
2489:
2483:
2474:
2468:
2455:
2428:
2422:
2405:
2399:
2379:
2372:
2359:}}
2353:{{
2334:
2329:
2317:. Retrieved
2264:
2260:
2254:
2240:(3): 307–9.
2237:
2233:
2227:
2207:
2200:
2175:
2171:
2165:
2139:. Retrieved
2134:
2124:
2107:
2103:
2069:
2065:
2055:
2044:. Retrieved
2040:
2031:
1989:
1983:
1958:
1954:
1944:
1917:
1913:
1875:
1871:
1840:counterparts
1830:counterparts
1777:
1765:
1750:
1736:
1732:
1728:
1718:
1707:
1687:
1621:
1601:
1551:
1547:
1546:
1528:
1519:
1512:
1507:
1499:
1495:
1489:
1438:
1432:
1372:
1350:
1281:
1278:Chlorosilane
1184:
1173:
1121:Silyl ethers
1119:
1116:Silyl ethers
1103:
1048:
947:
902:
856:
844:
827:SiLi + (CH
789:
777:SiLi + (CH
769:Li → (CH
743:
731:
724:
692:hydrosilanes
689:
667:
575:
563:James Crafts
556:
544:
517:
506:
453:Si–SiH
262:Approx. bond
183:
155:
131:
121:silanes and
104:
93:James Crafts
86:
68:
60:
41:
40:
2862:: 1086–90.
2856:New J. Chem
2563:: 147–262.
2110:: 173–183.
1834:Silylenoids
1705:instead.
1579:antibonding
1572:antibonding
1504:silabenzene
1492:double bond
1427:hydrosilane
1414:Hydrosilane
815:Li → ((CH
571:diethylzinc
553:Preparation
509:tetravalent
498:Si–Te
441:Si–Se
404:298.49(46)
385:Si–Cl
369:Si–Br
361:Si–Si
205:insecticide
198:Silafluofen
48:containing
2906:1874/14327
2523:Org. Synth
2361:: Missing
2141:28 October
2046:2022-12-22
1850:References
1809:elements:
1761:benzoyloxy
1606:, such as
1604:silatranes
1596:See also:
1588:fragment.
1582:pi orbital
1556:metalloles
1478:is called
1355:. Notably
1257:+ H-O-R +
538:, and the
433:Si–S
425:Si–O
417:Si–N
409:Si–I
401:Si–H
393:Si–F
377:Si–C
202:pyrethroid
174:fungicides
170:herbicides
81:See also:
73:compound.
3808:to carbon
3062:: 40499.
3014:deuterium
2408:. Wiley.
2192:195219283
2086:0002-7863
2017:cite book
2009:319710487
1975:0368-1645
1936:1876-990X
1892:0021-9584
1878:(1): 41.
1838:carbenoid
1824:Silylenes
1699:alkoxides
1697:ions and
1656:→ R-R" +
1645:+ R"-X +
1586:butadiene
1525:disilynes
1521:Disilenes
1110:silanones
1106:silicones
1051:siloxanes
1023:+ NaOH →
1005:Siloxides
811:Si + CH
712:aldehydes
190:Silicates
166:adjuvants
162:silicones
70:inorganic
3843:Category
3094:28091574
3007:isotopic
2993:17047792
2923:20937906
2915:12120068
2805:20677192
2770:23752786
2689:16138382
2654:49413857
2646:29938502
2273:12951816
2151:cite web
1807:group 14
1782:See also
1727:such as
1719:Certain
1695:fluoride
1628:fluoride
1439:hydrides
1284:silicone
1180:alcohols
1148:+ ROH →
1081:Si-O-SiR
858:Silanols
501:506(38)
493:368(31)
459:339(17)
444:531(25)
436:619(13)
420:439(38)
412:339(84)
396:540(13)
388:456(42)
380:435(21)
372:343(50)
364:327(10)
132:In 1945
117:to make
87:In 1863
3626:
3085:5238421
3064:Bibcode
2529:: 164.
2470:Arkivoc
2172:Silicon
1914:Silicon
1828:carbene
1759:on the
1733:in situ
1584:of the
1548:Siloles
1535:Siloles
1486:Silenes
1327:), and
708:ketones
700:alkynes
696:alkenes
635:+ Si →
428:798(8)
211:Bonding
194:diatoms
186:biology
106:ketones
77:History
54:silicon
3798:Legend
3161:carbon
3092:
3082:
2991:
2956:
2921:
2913:
2803:
2768:
2687:
2652:
2644:
2575:
2504:
2477:: 136.
2443:
2387:
2341:
2319:28 Nov
2271:
2215:
2190:
2084:
2007:
1997:
1973:
1934:
1890:
1836:, the
1826:, the
1725:esters
1635:R-SiR'
1529:Silyne
1496:silene
1480:silane
1402:halide
1386:SiOSiR
1317:MeSiCl
1212:Si-O-R
1168:Si-O-R
710:, and
704:imines
534:, the
530:, the
158:caulks
97:ethyl-
67:is an
50:carbon
2919:S2CID
2822:(PDF)
2650:S2CID
2465:(PDF)
2310:(PDF)
2188:S2CID
1721:allyl
1708:As a
1575:sigma
1456:PhSiH
1170:+ HCl
1034:SiONa
899:+ HCl
765:+ CH
569:with
511:with
280:Si-Si
119:alkyl
57:bonds
3090:PMID
3040:step
3024:and
2989:PMID
2954:ISBN
2911:PMID
2801:PMID
2766:PMID
2685:PMID
2642:PMID
2573:ISBN
2502:ISBN
2441:ISBN
2385:ISBN
2363:ISBN
2356:ISBN
2339:ISBN
2321:2022
2314:UCSB
2269:PMID
2213:ISBN
2157:link
2143:2019
2082:ISSN
2023:link
2005:OCLC
1995:ISBN
1971:ISSN
1932:ISSN
1888:ISSN
1753:zinc
1716:.
1568:LUMO
1369:SiCl
1342:SiCl
1301:SiCl
1255:Si-F
1178:for
1146:SiCl
1068:SiOH
1021:SiOH
1001:SiOH
972:and
969:SiOH
943:SiOH
932:→ 2
897:SiOH
874:SiCl
799:((CH
672:and
655:SiCl
596:SiCl
561:and
542:.
476:339
353:Bond
341:460
335:Si-O
330:355
319:314
313:Si-H
308:414
297:314
291:C-Si
286:196
275:334
256:Bond
244:3.4
172:and
123:aryl
91:and
3772:CEs
3767:CCf
3762:CBk
3757:CCm
3752:CAm
3747:CPu
3742:CNp
3732:CPa
3727:CTh
3706:CYb
3701:CTm
3696:CEr
3691:CHo
3686:CDy
3681:CTb
3676:CGd
3671:CEu
3666:CSm
3661:CPm
3656:CNd
3651:CPr
3646:CCe
3641:CLa
3621:Og
3618:Ts
3615:Lv
3612:Mc
3609:Fl
3606:Nh
3603:Cn
3600:Rg
3597:Ds
3594:Mt
3591:Hs
3588:Bh
3584:CSg
3580:Db
3577:Rf
3561:CRa
3557:Fr
3552:Rn
3548:CAt
3543:CPo
3538:CBi
3533:CPb
3528:CTl
3523:CHg
3518:CAu
3513:CPt
3508:CIr
3503:COs
3498:CRe
3488:CTa
3483:CHf
3478:CLu
3466:CBa
3461:CCs
3454:CXe
3444:CTe
3439:CSb
3434:CSn
3429:CIn
3424:CCd
3419:CAg
3414:CPd
3409:CRh
3404:CRu
3399:CTc
3394:CMo
3389:CNb
3384:CZr
3372:CSr
3367:CRb
3360:CKr
3355:CBr
3350:CSe
3345:CAs
3340:CGe
3335:CGa
3330:CZn
3325:CCu
3320:CNi
3315:CCo
3310:CFe
3305:CMn
3300:CCr
3290:CTi
3285:CSc
3278:CCa
3266:CAr
3261:CCl
3246:CSi
3241:CAl
3236:CMg
3231:CNa
3225:Ne
3196:CBe
3191:CLi
3185:He
3080:PMC
3072:doi
3005:By
2981:doi
2901:hdl
2893:doi
2864:doi
2836:doi
2793:doi
2758:doi
2731:doi
2708:doi
2677:doi
2634:doi
2630:118
2603:doi
2565:doi
2555:".
2531:doi
2494:doi
2475:126
2433:doi
2410:doi
2242:doi
2236:".
2180:doi
2112:doi
2108:847
2074:doi
1963:doi
1959:101
1922:doi
1880:doi
1667:SiF
1468:SiH
1452:SiH
1311:),
1237:(CH
1194:(CH
1150:(CH
1128:(CH
951:(CH
918:SiH
823:Si)
807:Si)
753:(CH
637:(CH
592:4-x
578:(CH
338:159
327:145
324:C-O
316:146
305:110
302:C-H
294:186
283:234
272:154
269:C-C
241:2.1
238:1.8
235:2.5
3845::
3787:No
3782:Md
3777:Fm
3737:CU
3722:Ac
3573:Lr
3493:CW
3449:CI
3379:CY
3295:CV
3273:CK
3256:CS
3251:CP
3221:CF
3216:CO
3211:CN
3206:CC
3201:CB
3175:CH
3088:.
3078:.
3070:.
3058:.
3054:.
3034:3a
3026:3b
3022:3a
2987:.
2917:.
2909:.
2899:.
2860:28
2858:.
2832:85
2830:.
2824:.
2799:.
2789:49
2787:.
2764:.
2754:49
2752:.
2683:.
2673:12
2671:.
2648:.
2640:.
2628:.
2599:55
2571:.
2561:42
2559:.
2543:^
2527:70
2525:.
2500:.
2492:.
2473:.
2467:.
2439:.
2298:^
2281:^
2263:.
2186:.
2174:.
2153:}}
2149:{{
2133:.
2106:.
2094:^
2080:.
2070:67
2068:.
2064:.
2039:.
2019:}}
2015:{{
2003:.
1969:.
1957:.
1953:.
1930:.
1916:.
1912:.
1900:^
1886:.
1876:42
1874:.
1870:.
1858:^
1817:,
1813:,
1799:,
1795:,
1791::
1770:.
1669:+
1658:R'
1630::
1482:.
1443:Et
1404:.
1360:Me
1333:Me
1292:Me
1259:OH
1235:→
1225:+
1214:+
1182:.
1091:+
1070:→
1057:2
1053::
1036:+
974:(C
920:+
907:2
886:→
876:+
849:.
835:Si
794::
785:Si
761:Si
729:.
706:,
702:,
633:Cl
624:CH
622:2
618::
573:.
482:Ar
465:Me
230:O
224:Si
196:.
176:.
140:.
3152:e
3145:t
3138:v
3096:.
3074::
3066::
3060:7
3030:4
3018:5
2995:.
2983::
2962:.
2925:.
2903::
2895::
2889:4
2870:.
2866::
2842:.
2838::
2807:.
2795::
2772:.
2760::
2737:.
2733::
2714:.
2710::
2691:.
2679::
2656:.
2636::
2622:3
2609:.
2605::
2581:.
2567::
2537:.
2533::
2510:.
2496::
2449:.
2435::
2416:.
2412::
2393:.
2365:.
2347:.
2323:.
2275:.
2265:6
2248:.
2244::
2221:.
2194:.
2182::
2176:1
2159:)
2145:.
2118:.
2114::
2088:.
2076::
2049:.
2025:)
2011:.
1977:.
1965::
1938:.
1924::
1918:2
1894:.
1882::
1737:2
1729:1
1671:X
1663:3
1647:F
1640:3
1473:4
1461:3
1448:3
1429:.
1391:3
1382:3
1377:R
1365:3
1338:3
1331:(
1322:3
1315:(
1306:2
1297:2
1290:(
1251:3
1246:)
1242:3
1232:O
1230:2
1228:H
1216:F
1208:3
1203:)
1199:3
1164:3
1159:)
1155:3
1142:3
1137:)
1133:3
1098:O
1096:2
1094:H
1086:3
1077:3
1072:R
1064:3
1059:R
1043:O
1041:2
1039:H
1030:3
1025:R
1017:3
1012:R
997:3
992:)
988:5
983:H
979:6
965:3
960:)
956:3
939:3
934:R
927:2
922:O
914:3
909:R
893:3
888:R
883:O
881:2
879:H
870:3
865:R
833:4
831:)
829:3
825:3
821:3
819:)
817:3
813:3
809:4
805:3
803:)
801:3
783:4
781:)
779:3
775:3
773:)
771:3
767:3
763:2
759:6
757:)
755:3
660:2
651:2
646:)
642:3
629:3
601:x
587:)
583:3
489:3
485:3
472:3
468:3
455:3
451:3
449:H
227:H
221:C
52:–
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.