28:
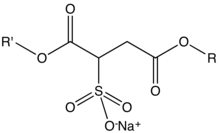
74:, pharmaceuticals, and cleaning agents. They are colorless salts. These materials can be further classified into monoesters (R' = H, R = alkyl) and diesters (R and R' = alkyl).
192:
122:
without the use of co-surfactants, and it has a rich variety of aqueous-phase behavior including multiple liquid crystalline phases.
247:
106:
A high volume example is sodium bis(2-ethylhexyl) sulfosuccinate. This is perhaps best known as the laxative
252:
87:
214:
114:
for which it finds common use in personal-care and household-care products, often under the name
188:
223:
212:; Penfold, Jeff (November 2000). "What Is So Special about Aerosol-OT? 1. Aqueous Systems".
180:
155:
83:
39:
119:
95:
241:
209:
27:
184:
59:
111:
63:
17:
71:
107:
67:
160:
143:
91:
227:
26:
98:, which, concomitant with protonation, adds to the C=C bond.
177:Ullmann's Encyclopedia of Industrial Chemistry
8:
118:. It is unusual in that it is able to form
31:Structure of sodium sulfosuccinate esters.
175:Holmberg, Krister (2019). "Surfactants".
159:
131:
137:
135:
144:"Sulfosuccinates as Mild surfactants"
7:
82:They are produced by treatment of
62:. They comprise a large class of
25:
110:, however its main use is as a
1:
185:10.1002/14356007.a25_747.pub2
142:Deepika; Tyagi, V. K (2006).
58:R where R and R' can be H or
36:Sodium sulfosuccinate esters
269:
90:. The resulting mono or
148:Journal of Oleo Science
94:are then treated with
32:
30:
42:with the formula NaO
248:Anionic surfactants
161:10.5650/jos.55.429
33:
228:10.1021/la000341q
222:(23): 8733–8740.
194:978-3-527-30673-2
179:. pp. 1–56.
40:organic compounds
16:(Redirected from
260:
232:
231:
208:Nave, Sandrine;
205:
199:
198:
172:
166:
165:
163:
139:
84:maleic anhydride
21:
268:
267:
263:
262:
261:
259:
258:
257:
238:
237:
236:
235:
207:
206:
202:
195:
174:
173:
169:
141:
140:
133:
128:
104:
80:
57:
53:
49:
45:
23:
22:
15:
12:
11:
5:
266:
264:
256:
255:
253:Sulfonic acids
250:
240:
239:
234:
233:
210:Eastoe, Julian
200:
193:
167:
154:(9): 429–439.
130:
129:
127:
124:
120:microemulsions
103:
100:
96:sodium sulfite
79:
76:
55:
51:
47:
43:
24:
18:Sulfosuccinate
14:
13:
10:
9:
6:
4:
3:
2:
265:
254:
251:
249:
246:
245:
243:
229:
225:
221:
217:
216:
211:
204:
201:
196:
190:
186:
182:
178:
171:
168:
162:
157:
153:
149:
145:
138:
136:
132:
125:
123:
121:
117:
113:
109:
101:
99:
97:
93:
89:
85:
77:
75:
73:
69:
65:
61:
41:
37:
29:
19:
219:
213:
203:
176:
170:
151:
147:
115:
105:
81:
60:alkyl groups
35:
34:
116:Aerosol-OTs
102:Application
68:emulsifiers
64:surfactants
242:Categories
126:References
112:surfactant
78:Synthesis
72:cosmetics
215:Langmuir
108:docusate
92:diesters
88:alcohols
70:used in
191:
46:SCH(CO
86:with
50:R')CH
189:ISBN
66:and
38:are
224:doi
181:doi
156:doi
244::
220:16
218:.
187:.
152:55
150:.
146:.
134:^
54:CO
230:.
226::
197:.
183::
164:.
158::
56:2
52:2
48:2
44:3
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.