397:
168:
31:
360:
transmembrane signaling. The palmitoyl group can be removed by palmitoyl thioesterases. It is believed that this reverse palmitoylation may regulate the interaction of the protein with the membrane and thus have a role in signaling processes. Furthermore, this allows for the regulation of protein subcellular localization, stability and trafficking. An example in which palmitoylation of a protein plays a role in cell signaling pathways is in the clustering of proteins in the
263:
344:
1636:
109:
492:. Releasing the IZUMO1R (JUNO) GPI protein from the egg plasma membrane does not allow for sperm to fuse with the egg and it is suggested that this mechanism may contribute to the polyspermy block at the plasma membrane in eggs. Other roles that GPI modification allows for is in the association with membrane microdomains, transient
135:
379:
and facilitates the clustering of proteins. The clustering can increase the proximity of two molecules. Alternatively, clustering can sequester a protein away from a substrate. For example, palmitoylation of phospholipase D (PLD) sequesters the enzyme away from its substrate phosphatidylcholine. When
455:
group of the respective protein. The GPI attachment occurs through the action of GPI-transamidase complex. The fatty acid chains of the phosphatidylinositol are inserted into the membrane and thus are what anchor the protein to the membrane. These proteins are only located on the exterior surface of
179:
Prenylated proteins are particularly important for eukaryotic cell growth, differentiation and morphology. Furthermore, protein prenylation is a reversible post-translational modification to the cell membrane. This dynamic interaction of prenylated proteins with the cell membrane is important for
72:
The lipid groups play a role in protein interaction and can contribute to the function of the protein to which it is attached. Furthermore, the lipid serves as a mediator of membrane associations or as a determinant for specific protein-protein interactions. For example, lipid groups can play an
188:
and when it is switched on it can turn on genes involved in cell growth and differentiation. Thus overactiving Ras signalling can lead to cancer. An understanding of these prenylated proteins and their mechanisms have been important for the drug development efforts in combating cancer. Other
359:
can also be used when other medium and long fatty acids chains are also attached to palmitoylated proteins. No consensus sequence for protein palmitoylation has been identified. Palmitoylated proteins are mainly found on the cytoplasmic side of the plasma membrane where they play a role in
146:
motif “CaaX box” is the most common prenylation site in proteins, that is, the site where farnesyl or geranylgeranyl covalently attach. In the CaaX box sequence, the C represents the cysteine that is prenylated, the A represents any
250:
are proteins that have been post-translationally modified to include the covalent attachment of fatty acids at certain amino acid residues. The most common fatty acids that are covalently attached to the protein are the saturated
131:(20-carbon) are attached to the protein via thioether linkages at cysteine residues near the C terminal of the protein. This prenylation of lipid chains to proteins facilitate their interaction with the cell membrane.
281:-myristoylation (i.e. attachment of myristic acid) is generally an irreversible protein modification that typically occurs during protein synthesis in which the myrisitc acid is attached to the α-amino group of an
480:
and complement regulatory proteins. Furthermore, GPI proteins play an important in embryogenesis, development, neurogenesis, the immune system and fertilization. More specifically, the GPI protein
1592:"Treatment of mouse oocytes with PI-PLC releases 70-kDa (pI 5) and 35- to 45-kDa (pI 5.5) protein clusters from the egg surface and inhibits sperm-oolemma binding and fusion"
65:
tails. The lipid-anchored protein can be located on either side of the cell membrane. Thus, the lipid serves to anchor the protein to the cell membrane. They are a type of
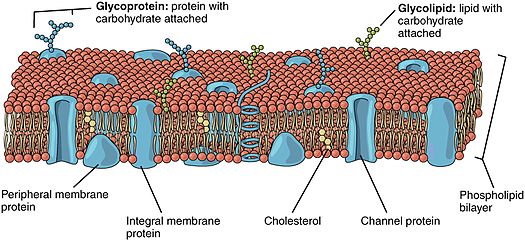
312:, protein-protein interactions and in mechanisms that regulate protein targeting and function. An example in which the myristoylation of a protein is important is in
100:. A protein can have multiple lipid groups covalently attached to it, but the site where the lipids bind to the protein depends both on the lipid group and protein.
351:
S-palmitoylation (i.e. attachment of palmitic acid) is a reversible protein modification in which a palmitic acid is attached to a specific cysteine residue via
895:
Reuter CW, Morgan MA, Bergmann L (September 2000). "Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies?".
756:"Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I"
151:
amino acid and the X determines the type of prenylation that will occur. If the X is an Ala, Met, Ser or Gln the protein will be farnesylated via the
1852:
1668:
468:
group vary depending on the protein. This great diversity is what allows the GPI proteins to have a wide range of functions including acting as
529:
586:
317:
61:
embedded within the cell membrane. These proteins insert and assume a place in the bilayer structure of the membrane alongside the similar
123:
polymers (i.e. branched five-carbon hydrocarbon) at cysteine residues of the protein. More specifically, these isoprenoid groups, usually
989:"Proteomic analysis of fatty-acylated proteins in mammalian cells with chemical reporters reveals S-acylation of histone H3 variants"
1082:
Martin DD, Beauchamp E, Berthiaume LG (January 2011). "Post-translational myristoylation: Fat matters in cellular life and death".
324:, which then ultimately leads to cell death. Other proteins that are myristoylated and involved in the regulation of apoptosis are
81:. In a dynamic role, lipidation can sequester a protein away from its substrate to inactivate the protein and then activate it by
1640:
156:
381:
1661:
477:
1333:"Disruption of palmitate-mediated localization; a shared pathway of force and anesthetic activation of TREK-1 channels"
1746:
309:
1117:
Aicart-Ramos C, Valero RA, Rodriguez-Crespo I (December 2011). "Protein palmitoylation and subcellular trafficking".
1842:
30:
1837:
1737:
797:"Towards the systematic mapping and engineering of the protein prenylation machinery in Saccharomyces cerevisiae"
290:
1654:
368:
is palmitoylated, it is restricted to the membrane and allows it to bind to and cluster ion channels in the
180:
their signalling functions and is often deregulated in disease processes such as cancer. More specifically,
1732:
385:
224:
82:
1847:
1786:
1741:
220:
172:
167:
320:(Bid) has been myristoylated, it targets the protein to move to the mitochondrial membrane to release
1546:
1238:
1180:
808:
465:
432:
428:
415:
carboxyl group. This GPI complex consists of several main components that are all interconnected: a
396:
448:
440:
416:
185:
152:
128:
966:
682:
148:
941:
Resh MD (November 2006). "Trafficking and signaling by fatty-acylated and prenylated proteins".
1791:
1613:
1572:
1515:
1464:
1420:
1364:
1313:
1264:
1208:
1134:
1099:
1064:
1020:
958:
912:
877:
836:
777:
729:"Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health, Seventh Ed"
674:
634:
582:
525:
519:
473:
469:
444:
384:
is disrupted, the enzyme trafficks to PIP2 where it encounters its substrate and is active by
372:
membrane. Thus, palmitoylation can play a role in the regulation of neurotransmitter release.
259:
acid (16-carbon). Proteins can be modified to contain either one or both of these fatty acids.
228:
204:
201:
400:
Structure of the glycophosphatidylinositol anchor in the plasma membrane of a eukaryotic cell
1806:
1724:
1603:
1562:
1554:
1505:
1495:
1454:
1410:
1400:
1354:
1344:
1303:
1295:
1254:
1246:
1198:
1188:
1126:
1091:
1054:
1010:
1000:
950:
904:
867:
826:
816:
767:
709:
666:
624:
493:
485:
1590:
Coonrod SA, Naaby-Hansen S, Shetty J, Shibahara H, Chen M, White JM, Herr JC (March 1999).
728:
1801:
1483:
452:
436:
420:
208:
190:
1550:
1242:
1184:
812:
1686:
1567:
1534:
1510:
1415:
1388:
1359:
1332:
1308:
1283:
1259:
1227:
1203:
1168:
1015:
988:
831:
796:
78:
713:
1831:
1811:
1771:
1694:
1678:
489:
408:
286:
256:
252:
181:
124:
54:
50:
686:
17:
1709:
1699:
1169:"Palmitoylation regulates raft affinity for the majority of integral raft proteins"
1167:
Levental, I.; Lingwood, D.; Grzybek, M.; Coskun, U.; Simons, K. (3 December 2010).
970:
369:
321:
1646:
872:
855:
1484:"Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling"
1389:"Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling"
1349:
1130:
1095:
821:
155:
enzyme and if the X is a Leu then the protein will be geranylgeranylated via the
1704:
376:
356:
232:
143:
116:
66:
1299:
772:
755:
1781:
1776:
670:
412:
282:
74:
62:
908:
657:
Novelli G, D'Apice MR (September 2012). "Protein farnesylation and disease".
159:
enzyme. Both of these enzymes are similar with each containing two subunits.
88:
Overall, there are three main types of lipid-anchored proteins which include
1539:
Proceedings of the Japan
Academy. Series B, Physical and Biological Sciences
1193:
1005:
629:
612:
464:
The sugar residues in the tetrasaccaride and the fatty acid residues in the
352:
343:
313:
305:
262:
245:
1617:
1608:
1591:
1576:
1519:
1468:
1424:
1368:
1317:
1268:
1228:"Kinetic disruption of lipid rafts is a mechanosensor for phospholipase D."
1212:
1138:
1103:
1068:
1059:
1042:
1024:
962:
916:
881:
840:
781:
678:
1635:
638:
77:. This allows for the interaction of proteins with cellular membranes and
1816:
1766:
329:
216:
212:
120:
1558:
1500:
1405:
1250:
1226:
Petersen, EN; Chung, HW; Nayebosadri, A; Hansen, SB (15 December 2016).
954:
451:. The phosphoethanolamine is then amide linked to the C-terminal of the
1459:
1442:
481:
424:
361:
46:
405:
Glycosylphosphatidylinositol-anchored proteins (GPI-anchored proteins)
1796:
1714:
795:
Stein V, Kubala MH, Steen J, Grimmond SM, Alexandrov K (2015-01-01).
439:
linked to the non-N-acetylated glucosamine of the tetrasaccharide. A
365:
308:
at position 5. Proteins that have been myristoylated are involved in
1284:"Tools for Understanding Nanoscale Lipid Regulation of Ion Channels"
108:
134:
987:
Wilson JP, Raghavan AS, Yang YY, Charron G, Hang HC (March 2011).
325:
194:
166:
133:
58:
700:
Ferguson MA (August 1991). "Lipid anchors on membrane proteins".
1331:
Petersen, EN; Pavel, MA; Wang, H; Hansen, SB (28 October 2019).
1650:
1535:"Biosynthesis and deficiencies of glycosylphosphatidylinositol"
200:
Some important prenylation chains that are involved in the
119:
proteins are proteins with covalently attached hydrophobic
581:(4th ed.). John Wiley & Sons, Inc. p. 263.
579:
1043:"The biology and enzymology of protein N-myristoylation"
380:
cholesterol levels decrease or PIP2 levels increase the
227:) are involved in the condensations via enzymes such as
1443:"Glycosylphosphatidylinositol (GPI)-Anchored Proteins"
1282:
Robinson, CV; Rohacs, T; Hansen, SB (September 2019).
375:
Palmitoylation mediates the affinity of a protein for
1152:
Dityatev, Alexander (2006). El-Husseini, Alaa (ed.).
407:
are attached to a GPI complex molecular group via an
521:
Cell and
Molecular Biology: Concepts and Experiments
1759:
1723:
1685:
1337:Biochimica et Biophysica Acta (BBA) - Biomembranes
1119:Biochimica et Biophysica Acta (BBA) - Biomembranes
98:glycosylphosphatidylinositol-linked proteins (GPI)
1041:Farazi TA, Waksman G, Gordon JI (October 2001).
1173:Proceedings of the National Academy of Sciences
184:is the protein that undergoes prenylation via
1662:
856:"The molecular perspective: the ras oncogene"
488:) on the egg plasma has an essential role in
8:
316:, programmed cell death. After the protein
189:prenylated proteins include members of the
1669:
1655:
1647:
1086:. Bioactive Lipids, Nutrition and Health.
513:
511:
509:
443:is then formed between the mannose at the
1607:
1566:
1509:
1499:
1458:
1414:
1404:
1358:
1348:
1307:
1258:
1202:
1192:
1058:
1014:
1004:
871:
830:
820:
771:
628:
496:or in apical sorting in polarized cells.
395:
366:postsynaptic density protein 95 (PSD-95)
342:
261:
107:
29:
505:
73:important role in increasing molecular
1482:Kinoshita T, Fujita M (January 2016).
1447:Biological and Pharmaceutical Bulletin
1387:Kinoshita T, Fujita M (January 2016).
1154:Molecular Mechanisms of Synaptogenesis
659:Journal of Inherited Metabolic Disease
524:. John Wiley and Sons. pp. 128–.
296:. These proteins usually begin with a
1436:
1434:
1382:
1380:
1378:
1156:. New York: Springer. pp. 72–75.
1036:
1034:
982:
980:
936:
934:
932:
930:
928:
926:
702:Current Opinion in Structural Biology
572:
570:
568:
304:sequence and with either a serine or
7:
749:
747:
745:
652:
650:
648:
606:
604:
602:
600:
598:
566:
564:
562:
560:
558:
556:
554:
552:
550:
548:
447:end (of the tetrasaccaride) and the
318:BH3 interacting-domain death agonist
34:Lipid membrane with various proteins
1047:The Journal of Biological Chemistry
993:Molecular & Cellular Proteomics
617:The Journal of Biological Chemistry
611:Casey PJ, Seabra MC (March 1996).
577:Voet D, Voet JG, Pratt CW (2013).
297:
289:. This reaction is facilitated by
25:
231:that eventually cyclizes to form
1634:
754:Lane KT, Beese LS (April 2006).
219:. These isoprene polymers (e.g.
1853:Post-translational modification
382:palmitate mediated localization
301:
1288:Trends in Biochemical Sciences
49:located on the surface of the
1:
1441:Ikezawa, Hiroh (2002-01-01).
873:10.1634/theoncologist.4-3-263
714:10.1016/s0959-440x(05)80072-7
1350:10.1016/j.bbamem.2019.183091
1131:10.1016/j.bbamem.2011.07.009
1096:10.1016/j.biochi.2010.10.018
822:10.1371/journal.pone.0120716
613:"Protein prenyltransferases"
193:and Rho families as well as
1747:Peripheral membrane protein
484:(also named JUNO after the
310:signal transduction cascade
285:glycine residue through an
157:geranylgeranyltransferase I
1869:
1738:Integral membrane proteins
1300:10.1016/j.tibs.2019.04.001
854:Goodsell DS (1999-01-01).
773:10.1194/jlr.R600002-JLR200
486:Roman goddess of fertility
427:and a glucosaminyl) and a
1488:Journal of Lipid Research
1393:Journal of Lipid Research
760:Journal of Lipid Research
671:10.1007/s10545-011-9445-y
171:Prenylation chains (e.g.
909:10.1182/blood.V96.5.1655
1782:Lipid raft/microdomains
1194:10.1073/pnas.1016184107
1006:10.1074/mcp.M110.001198
943:Nature Chemical Biology
630:10.1074/jbc.271.10.5289
239:Fatty acylated proteins
94:fatty acylated proteins
39:Lipid-anchored proteins
1787:Membrane contact sites
1751:Lipid-anchored protein
1733:Membrane glycoproteins
1641:Lipid-anchored protein
1609:10.1006/dbio.1998.9161
1060:10.1074/jbc.R100042200
401:
386:substrate presentation
348:
267:
225:farnesyl pyrophosphate
176:
139:
113:
83:substrate presentation
35:
1742:transmembrane protein
1596:Developmental Biology
1231:Nature Communications
456:the plasma membrane.
399:
346:
294:-myristoyltransferase
265:
255:(14-carbon) acid and
221:geranyl pyrophosphate
173:geranyl pyrophosphate
170:
137:
111:
43:lipid-linked proteins
33:
1767:Caveolae/Coated pits
1643:at Wikimedia Commons
1533:Kinoshita T (2014).
518:Gerald Karp (2009).
466:phosphatidylinositol
433:phosphatidylinositol
429:phosphatidylinositol
18:GPI-anchored protein
1559:10.2183/pjab.90.130
1551:2014PJAB...90..130K
1501:10.1194/jlr.R063313
1406:10.1194/jlr.R063313
1251:10.1038/ncomms13873
1243:2016NatCo...713873P
1185:2010PNAS..10722050L
1179:(51): 22050–22054.
955:10.1038/nchembio834
813:2015PLoSO..1020716S
449:phosphoethanolamine
441:phosphodiester bond
423:(composed of three
417:phosphoethanolamine
186:farnesyltransferase
153:farnesyltransferase
104:Prenylated proteins
90:prenylated proteins
1792:Membrane nanotubes
1677:Structures of the
1460:10.1248/bpb.25.409
999:(3): M110.001198.
478:protease inhibitor
470:hydrolytic enzymes
460:Roles and function
402:
355:linkage. The term
349:
268:
177:
163:Roles and function
140:
114:
36:
1843:Membrane proteins
1825:
1824:
1725:Membrane proteins
1639:Media related to
727:isoprene (2003).
531:978-0-470-48337-4
474:adhesion molecule
411:to the protein's
229:prenyltransferase
205:metabolic pathway
202:HMG-CoA reductase
16:(Redirected from
1860:
1838:Membrane biology
1807:Nuclear envelope
1802:Nodes of Ranvier
1671:
1664:
1657:
1648:
1638:
1622:
1621:
1611:
1587:
1581:
1580:
1570:
1530:
1524:
1523:
1513:
1503:
1479:
1473:
1472:
1462:
1438:
1429:
1428:
1418:
1408:
1384:
1373:
1372:
1362:
1352:
1328:
1322:
1321:
1311:
1279:
1273:
1272:
1262:
1223:
1217:
1216:
1206:
1196:
1164:
1158:
1157:
1149:
1143:
1142:
1114:
1108:
1107:
1079:
1073:
1072:
1062:
1038:
1029:
1028:
1018:
1008:
984:
975:
974:
938:
921:
920:
892:
886:
885:
875:
851:
845:
844:
834:
824:
792:
786:
785:
775:
751:
740:
739:
737:
735:
724:
718:
717:
697:
691:
690:
654:
643:
642:
632:
608:
593:
592:
588:978-0470-54784-7
574:
543:
542:
540:
538:
515:
494:homodimerization
490:sperm-egg fusion
303:
299:
127:(15-carbon) and
27:Membrane protein
21:
1868:
1867:
1863:
1862:
1861:
1859:
1858:
1857:
1828:
1827:
1826:
1821:
1755:
1719:
1687:Membrane lipids
1681:
1675:
1631:
1626:
1625:
1589:
1588:
1584:
1532:
1531:
1527:
1481:
1480:
1476:
1440:
1439:
1432:
1386:
1385:
1376:
1330:
1329:
1325:
1281:
1280:
1276:
1225:
1224:
1220:
1166:
1165:
1161:
1151:
1150:
1146:
1125:(12): 2981–94.
1116:
1115:
1111:
1081:
1080:
1076:
1053:(43): 39501–4.
1040:
1039:
1032:
986:
985:
978:
940:
939:
924:
894:
893:
889:
853:
852:
848:
807:(3): e0120716.
794:
793:
789:
753:
752:
743:
733:
731:
726:
725:
721:
699:
698:
694:
656:
655:
646:
623:(10): 5289–92.
610:
609:
596:
589:
576:
575:
546:
536:
534:
532:
517:
516:
507:
502:
462:
421:tetrasaccharide
394:
341:
339:-palmitoylation
276:
266:Myristoylation
241:
209:geranylgeraniol
165:
106:
79:protein domains
41:(also known as
28:
23:
22:
15:
12:
11:
5:
1866:
1864:
1856:
1855:
1850:
1845:
1840:
1830:
1829:
1823:
1822:
1820:
1819:
1814:
1812:Phycobilisomes
1809:
1804:
1799:
1794:
1789:
1784:
1779:
1774:
1772:Cell junctions
1769:
1763:
1761:
1757:
1756:
1754:
1753:
1744:
1735:
1729:
1727:
1721:
1720:
1718:
1717:
1712:
1707:
1702:
1697:
1691:
1689:
1683:
1682:
1676:
1674:
1673:
1666:
1659:
1651:
1645:
1644:
1630:
1629:External links
1627:
1624:
1623:
1582:
1525:
1474:
1453:(4): 409–417.
1430:
1374:
1323:
1294:(9): 795–806.
1274:
1218:
1159:
1144:
1109:
1074:
1030:
976:
949:(11): 584–90.
922:
903:(5): 1655–69.
887:
860:The Oncologist
846:
787:
741:
719:
692:
644:
594:
587:
544:
530:
504:
503:
501:
498:
461:
458:
437:glycosidically
393:
390:
347:Palmitoylation
340:
334:
275:
274:myristoylation
269:
240:
237:
164:
161:
129:geranylgeranyl
105:
102:
75:hydrophobicity
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1865:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1835:
1833:
1818:
1815:
1813:
1810:
1808:
1805:
1803:
1800:
1798:
1797:Myelin sheath
1795:
1793:
1790:
1788:
1785:
1783:
1780:
1778:
1775:
1773:
1770:
1768:
1765:
1764:
1762:
1758:
1752:
1748:
1745:
1743:
1739:
1736:
1734:
1731:
1730:
1728:
1726:
1722:
1716:
1713:
1711:
1710:Sphingolipids
1708:
1706:
1703:
1701:
1700:Phospholipids
1698:
1696:
1695:Lipid bilayer
1693:
1692:
1690:
1688:
1684:
1680:
1679:cell membrane
1672:
1667:
1665:
1660:
1658:
1653:
1652:
1649:
1642:
1637:
1633:
1632:
1628:
1619:
1615:
1610:
1605:
1602:(2): 334–49.
1601:
1597:
1593:
1586:
1583:
1578:
1574:
1569:
1564:
1560:
1556:
1552:
1548:
1545:(4): 130–43.
1544:
1540:
1536:
1529:
1526:
1521:
1517:
1512:
1507:
1502:
1497:
1493:
1489:
1485:
1478:
1475:
1470:
1466:
1461:
1456:
1452:
1448:
1444:
1437:
1435:
1431:
1426:
1422:
1417:
1412:
1407:
1402:
1398:
1394:
1390:
1383:
1381:
1379:
1375:
1370:
1366:
1361:
1356:
1351:
1346:
1343:(1): 183091.
1342:
1338:
1334:
1327:
1324:
1319:
1315:
1310:
1305:
1301:
1297:
1293:
1289:
1285:
1278:
1275:
1270:
1266:
1261:
1256:
1252:
1248:
1244:
1240:
1236:
1232:
1229:
1222:
1219:
1214:
1210:
1205:
1200:
1195:
1190:
1186:
1182:
1178:
1174:
1170:
1163:
1160:
1155:
1148:
1145:
1140:
1136:
1132:
1128:
1124:
1120:
1113:
1110:
1105:
1101:
1097:
1093:
1089:
1085:
1078:
1075:
1070:
1066:
1061:
1056:
1052:
1048:
1044:
1037:
1035:
1031:
1026:
1022:
1017:
1012:
1007:
1002:
998:
994:
990:
983:
981:
977:
972:
968:
964:
960:
956:
952:
948:
944:
937:
935:
933:
931:
929:
927:
923:
918:
914:
910:
906:
902:
898:
891:
888:
883:
879:
874:
869:
865:
861:
857:
850:
847:
842:
838:
833:
828:
823:
818:
814:
810:
806:
802:
798:
791:
788:
783:
779:
774:
769:
766:(4): 681–99.
765:
761:
757:
750:
748:
746:
742:
730:
723:
720:
715:
711:
707:
703:
696:
693:
688:
684:
680:
676:
672:
668:
665:(5): 917–26.
664:
660:
653:
651:
649:
645:
640:
636:
631:
626:
622:
618:
614:
607:
605:
603:
601:
599:
595:
590:
584:
580:
573:
571:
569:
567:
565:
563:
561:
559:
557:
555:
553:
551:
549:
545:
533:
527:
523:
522:
514:
512:
510:
506:
499:
497:
495:
491:
487:
483:
479:
476:, receptors,
475:
471:
467:
459:
457:
454:
450:
446:
442:
438:
434:
430:
426:
422:
418:
414:
410:
409:amide linkage
406:
398:
391:
389:
387:
383:
378:
373:
371:
367:
363:
358:
354:
345:
338:
335:
333:
331:
327:
323:
319:
315:
311:
307:
295:
293:
288:
287:amide linkage
284:
280:
273:
270:
264:
260:
258:
254:
249:
247:
238:
236:
234:
230:
226:
222:
218:
214:
210:
206:
203:
198:
196:
192:
187:
183:
174:
169:
162:
160:
158:
154:
150:
145:
136:
132:
130:
126:
122:
118:
112:Isoprene unit
110:
103:
101:
99:
95:
91:
86:
84:
80:
76:
70:
68:
64:
60:
56:
52:
51:cell membrane
48:
44:
40:
32:
19:
1848:Lipoproteins
1750:
1705:Lipoproteins
1599:
1595:
1585:
1542:
1538:
1528:
1491:
1487:
1477:
1450:
1446:
1396:
1392:
1340:
1336:
1326:
1291:
1287:
1277:
1234:
1230:
1221:
1176:
1172:
1162:
1153:
1147:
1122:
1118:
1112:
1090:(1): 18–31.
1087:
1083:
1077:
1050:
1046:
996:
992:
946:
942:
900:
896:
890:
866:(3): 263–4.
863:
859:
849:
804:
800:
790:
763:
759:
732:. Retrieved
722:
708:(4): 522–9.
705:
701:
695:
662:
658:
620:
616:
578:
535:. Retrieved
520:
463:
404:
403:
392:GPI proteins
374:
370:postsynaptic
350:
336:
322:cytochrome c
291:
278:
277:
271:
243:
242:
199:
178:
141:
115:
97:
93:
89:
87:
71:
67:proteolipids
57:attached to
42:
38:
37:
1494:(1): 6–24.
1399:(1): 6–24.
734:28 November
537:13 November
445:nonreducing
419:, a linear
377:lipid rafts
364:. When the
357:S-acylation
233:cholesterol
144:prenylation
1832:Categories
1777:Glycocalyx
500:References
413:C-terminal
283:N-terminal
117:Prenylated
63:fatty acid
55:covalently
1817:Porosomes
1237:: 13873.
1084:Biochimie
435:group is
353:thioester
314:apoptosis
306:threonine
149:aliphatic
53:that are
1618:10068467
1577:24727937
1520:26563290
1469:11995915
1425:26563290
1369:31672538
1318:31060927
1269:27976674
1213:21131568
1139:21819967
1104:21056615
1069:11527981
1025:21076176
963:17051234
917:10961860
882:10394594
841:25768003
801:PLOS ONE
782:16477080
687:11555502
679:22307208
453:carboxyl
330:gelsolin
257:palmitic
253:myristic
248:proteins
246:acylated
217:dolichol
213:farnesol
138:Caax Box
125:farnesyl
121:isoprene
47:proteins
1715:Sterols
1568:4055706
1547:Bibcode
1511:4689344
1416:4689344
1360:6907892
1309:6729126
1260:5171650
1239:Bibcode
1204:3009825
1181:Bibcode
1016:3047146
971:9734759
832:4358939
809:Bibcode
639:8621375
482:IZUMO1R
425:mannose
362:synapse
1616:
1575:
1565:
1518:
1508:
1467:
1423:
1413:
1367:
1357:
1316:
1306:
1267:
1257:
1211:
1201:
1137:
1102:
1067:
1023:
1013:
969:
961:
915:
880:
839:
829:
780:
685:
677:
637:
585:
528:
431:. The
244:Fatty
195:lamins
59:lipids
45:) are
1760:Other
967:S2CID
897:Blood
683:S2CID
326:actin
1614:PMID
1573:PMID
1516:PMID
1465:PMID
1421:PMID
1365:PMID
1341:1862
1314:PMID
1265:PMID
1209:PMID
1135:PMID
1123:1808
1100:PMID
1065:PMID
1021:PMID
959:PMID
913:PMID
878:PMID
837:PMID
778:PMID
736:2015
675:PMID
635:PMID
583:ISBN
539:2010
526:ISBN
328:and
223:and
215:and
207:are
142:The
96:and
1604:doi
1600:207
1563:PMC
1555:doi
1506:PMC
1496:doi
1455:doi
1411:PMC
1401:doi
1355:PMC
1345:doi
1304:PMC
1296:doi
1255:PMC
1247:doi
1199:PMC
1189:doi
1177:107
1127:doi
1092:doi
1055:doi
1051:276
1011:PMC
1001:doi
951:doi
905:doi
868:doi
827:PMC
817:doi
768:doi
710:doi
667:doi
625:doi
621:271
302:Gly
298:Met
191:Rab
182:Ras
1834::
1612:.
1598:.
1594:.
1571:.
1561:.
1553:.
1543:90
1541:.
1537:.
1514:.
1504:.
1492:57
1490:.
1486:.
1463:.
1451:25
1449:.
1445:.
1433:^
1419:.
1409:.
1397:57
1395:.
1391:.
1377:^
1363:.
1353:.
1339:.
1335:.
1312:.
1302:.
1292:44
1290:.
1286:.
1263:.
1253:.
1245:.
1233:.
1207:.
1197:.
1187:.
1175:.
1171:.
1133:.
1121:.
1098:.
1088:93
1063:.
1049:.
1045:.
1033:^
1019:.
1009:.
997:10
995:.
991:.
979:^
965:.
957:.
945:.
925:^
911:.
901:96
899:.
876:.
862:.
858:.
835:.
825:.
815:.
805:10
803:.
799:.
776:.
764:47
762:.
758:.
744:^
704:.
681:.
673:.
663:35
661:.
647:^
633:.
619:.
615:.
597:^
547:^
508:^
472:,
388:.
332:.
272:N-
235:.
211:,
197:.
92:,
85:.
69:.
1749:/
1740:/
1670:e
1663:t
1656:v
1620:.
1606::
1579:.
1557::
1549::
1522:.
1498::
1471:.
1457::
1427:.
1403::
1371:.
1347::
1320:.
1298::
1271:.
1249::
1241::
1235:7
1215:.
1191::
1183::
1141:.
1129::
1106:.
1094::
1071:.
1057::
1027:.
1003::
973:.
953::
947:2
919:.
907::
884:.
870::
864:4
843:.
819::
811::
784:.
770::
738:.
716:.
712::
706:1
689:.
669::
641:.
627::
591:.
541:.
337:S
300:-
292:N
279:N
175:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.