155:
144:
238:
313:
361:
47:
372:
concentration is the same as that of another solution. In biology, the solutions on either side of a cell membrane are isotonic if the concentration of solutes outside the cell is equal to the concentration of solutes inside the cell. In this case the cell neither swells nor shrinks because there is
209:
Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the
257:
inside the cell. When a cell is immersed in a hypertonic solution, osmotic pressure tends to force water to flow out of the cell in order to balance the concentrations of the solutes on either side of the cell membrane. The cytosol is conversely categorized as hypotonic, opposite of the outer
373:
no concentration gradient to induce the diffusion of large amounts of water across the cell membrane. Water molecules freely diffuse through the plasma membrane in both directions, and as the rate of water diffusion is the same in each direction, the cell will neither gain nor lose water.
494:
596:
518:
Argyropoulos, Christos; Rondon-Berrios, Helbert; Raj, Dominic S; Malhotra, Deepak; Agaba, Emmanuel I; Rohrscheib, Mark; Khitan, Zeid; Murata, Glen H; Shapiro, Joseph I.; Tzamaloukas, Antonios H (2 May 2016).
320:
A hypotonic solution has a lower concentration of solutes than another solution. In biology, a solution outside of a cell is called hypotonic if it has a lower concentration of solutes relative to the
277:. In plant cells the terms isotonic, hypotonic and hypertonic cannot strictly be used accurately because the pressure exerted by the cell wall significantly affects the osmotic equilibrium point.
348:
takes on extra water and pushes the cell membrane against the cell wall. Due to the rigidity of the cell wall, it pushes back, preventing the cell from bursting. This is called
570:
Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James (2000). "Osmosis, Water
Channels, and the Regulation of Cell Volume".
249:
than another solution. In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of a
400:). Thus, normal saline is almost isotonic to blood plasma. Neither sodium nor chloride ions can freely pass through the plasma membrane, unlike
452:
296:, they lose water osmotically to the sea from gill cells. They respond to the loss by drinking large amounts of saltwater, and actively
130:
154:
68:
376:
An iso-osmolar solution can be hypotonic if the solute is able to penetrate the cell membrane. For example, an iso-osmolar
392:
dissolved in water to a total volume of one liter, is a close approximation to the osmolarity of NaCl in blood (about 290
336:
such as animal cells, if the gradient is large enough, the uptake of excess water can produce enough pressure to induce
111:
83:
57:
64:
90:
384:. This is due to urea entering the cell down its concentration gradient, followed by water. The osmolarity of
143:
253:; a solution outside of a cell is called hypertonic if it has a greater concentration of solutes than the
183:
97:
689:
413:
237:
312:
273:, and the plasmodesmata almost cease to function because they become constricted, a condition known as
360:
179:
79:
261:
When plant cells are in a hypertonic solution, the flexible cell membrane pulls away from the rigid
643:
552:
448:
385:
661:
633:
542:
532:
325:
217:
There are three classifications of tonicity that one solution can have relative to another:
171:
163:
280:
Some organisms have evolved intricate methods of circumventing hypertonicity. For example,
389:
349:
175:
468:
Buckley, Gabe (20 January 2017). "Hypertonic
Solution". In Biologydictionary.net (ed.).
595:
Soult, Allison (2020). "8.4 - Osmosis and
Diffusion". In University of Kentucky (ed.).
547:
520:
301:
203:
148:
104:
683:
469:
393:
369:
266:
250:
191:
187:
293:
241:
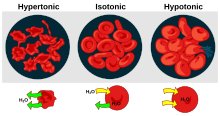
A red blood cell in a hypertonic solution, causing water to move out of the cell.
274:
202:. It is commonly used when describing the swelling-versus-shrinking response of
46:
571:
316:
A red blood cell in a hypotonic solution, causing water to move into the cell.
270:
211:
35:
638:
621:
337:
333:
297:
262:
31:
17:
647:
556:
198:
across a cell membrane which determine the direction and extent of osmotic
30:"Hypotonic" and "Hypertonic" redirect here. For the physical diseases, see
423:
281:
537:
418:
345:
344:
of the cell. When plant cells are in a hypotonic solution, the central
321:
254:
195:
341:
329:
288:
that live in it. Because the fish need a large surface area in their
246:
210:
membrane without net solvent movement. It is also a factor affecting
245:
A hypertonic solution has a greater concentration of non-permeating
521:"Hypertonicity: Pathophysiologic Concept and Experimental Studies"
397:
381:
153:
142:
493:
LibreTexts
Project: Medicine (18 July 2018). "3.3C - Tonicity".
401:
377:
289:
285:
199:
27:
Measure of water potential across a semi-permeable cell membrane
445:
Cell
Physiology Source Book: Essentials of Membrane Biophysics
40:
328:, water diffuses into the cell, and the cell often appears
380:
solution is hypotonic to red blood cells, causing their
265:, but remains joined to the cell wall at points called
364:
Depiction of a red blood cell in an isotonic solution.
576:(4th ed.). New York: W. H. Freeman and Company
229:. A hypotonic solution example is distilled water.
71:. Unsourced material may be challenged and removed.
601:. Open Education Resource (OER) LibreTexts Project
158:Micrographs of osmotic pressure on red blood cells
269:. The cells often take on the appearance of a
8:
368:A solution is isotonic when its effective
637:
546:
536:
131:Learn how and when to remove this message
474:(Online ed.). Biologydictionary.net
359:
311:
300:the excess salt. This process is called
236:
435:
499:(Online ed.). med.libretexts.org/
7:
69:adding citations to reliable sources
626:The Journal of Experimental Biology
190:. Tonicity depends on the relative
622:"Osmoregulation in marine mammals"
496:Anatomy and Physiology (Boundless)
332:, or bloated. For cells without a
206:immersed in an external solution.
194:of selective membrane-impermeable
25:
147:Effect of different solutions on
45:
447:. Academic Press. p. 288.
56:needs additional citations for
170:is a measure of the effective
1:
662:"Definition — hypotonic"
443:Sperelakis, Nicholas (2011).
292:in contact with seawater for
598:Chemistry for Allied Health
706:
29:
639:10.1242/jeb.204.11.1831
620:Ortiz, RM (June 2001).
573:Molecular Cell Biology
365:
317:
242:
159:
151:
664:. The Free Dictionary
414:Osmotic concentration
363:
315:
284:is hypertonic to the
240:
157:
146:
65:improve this article
233:Hypertonic solution
184:partially-permeable
632:(Pt 11): 1831–44.
538:10.7759/cureus.596
471:Biology Dictionary
366:
318:
308:Hypotonic solution
243:
160:
152:
454:978-0-12-387738-3
141:
140:
133:
115:
16:(Redirected from
697:
674:
673:
671:
669:
658:
652:
651:
641:
617:
611:
610:
608:
606:
592:
586:
585:
583:
581:
567:
561:
560:
550:
540:
515:
509:
508:
506:
504:
490:
484:
483:
481:
479:
465:
459:
458:
440:
326:osmotic pressure
172:osmotic pressure
164:chemical biology
136:
129:
125:
122:
116:
114:
73:
49:
41:
21:
705:
704:
700:
699:
698:
696:
695:
694:
680:
679:
678:
677:
667:
665:
660:
659:
655:
619:
618:
614:
604:
602:
594:
593:
589:
579:
577:
569:
568:
564:
517:
516:
512:
502:
500:
492:
491:
487:
477:
475:
467:
466:
462:
455:
442:
441:
437:
432:
410:
358:
350:turgor pressure
310:
235:
182:separated by a
176:water potential
149:red blood cells
137:
126:
120:
117:
74:
72:
62:
50:
39:
28:
23:
22:
15:
12:
11:
5:
703:
701:
693:
692:
682:
681:
676:
675:
653:
612:
587:
562:
510:
485:
460:
453:
434:
433:
431:
428:
427:
426:
421:
416:
409:
406:
357:
354:
309:
306:
302:osmoregulation
234:
231:
174:gradient; the
139:
138:
53:
51:
44:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
702:
691:
688:
687:
685:
663:
657:
654:
649:
645:
640:
635:
631:
627:
623:
616:
613:
600:
599:
591:
588:
575:
574:
566:
563:
558:
554:
549:
544:
539:
534:
530:
526:
522:
514:
511:
498:
497:
489:
486:
473:
472:
464:
461:
456:
450:
446:
439:
436:
429:
425:
422:
420:
417:
415:
412:
411:
407:
405:
403:
399:
395:
391:
387:
386:normal saline
383:
379:
374:
371:
362:
355:
353:
351:
347:
343:
339:
335:
331:
327:
323:
314:
307:
305:
303:
299:
295:
291:
287:
283:
278:
276:
272:
268:
267:plasmodesmata
264:
259:
256:
252:
251:cell membrane
248:
239:
232:
230:
228:
224:
220:
215:
213:
207:
205:
201:
197:
193:
192:concentration
189:
185:
181:
177:
173:
169:
165:
156:
150:
145:
135:
132:
124:
121:February 2018
113:
110:
106:
103:
99:
96:
92:
89:
85:
82: –
81:
77:
76:Find sources:
70:
66:
60:
59:
54:This article
52:
48:
43:
42:
37:
33:
19:
690:Cell biology
666:. Retrieved
656:
629:
625:
615:
603:. Retrieved
597:
590:
578:. Retrieved
572:
565:
528:
524:
513:
501:. Retrieved
495:
488:
476:. Retrieved
470:
463:
444:
438:
375:
367:
319:
294:gas exchange
279:
260:
244:
226:
222:
218:
216:
208:
167:
161:
127:
118:
108:
101:
94:
87:
75:
63:Please help
58:verification
55:
18:Hyperosmotic
531:(5): e596.
356:Isotonicity
275:plasmolysis
430:References
388:, 9 grams
271:pincushion
258:solution.
219:hypertonic
212:imbibition
91:newspapers
80:"Tonicity"
36:Hypertonia
668:23 August
605:19 August
580:19 August
503:19 August
478:19 August
342:rupturing
338:cytolysis
334:cell wall
324:. Due to
298:excreting
282:saltwater
263:cell wall
223:hypotonic
180:solutions
32:Hypotonia
684:Category
648:11441026
557:27382523
424:Salinity
408:See also
227:isotonic
188:membrane
168:tonicity
548:4895078
419:Osmosis
346:vacuole
322:cytosol
255:cytosol
247:solutes
196:solutes
178:of two
105:scholar
646:
555:
545:
525:Cureus
451:
370:osmole
330:turgid
225:, and
107:
100:
93:
86:
78:
382:lysis
340:, or
290:gills
204:cells
186:cell
112:JSTOR
98:books
670:2012
644:PMID
607:2021
582:2021
553:PMID
505:2021
480:2021
449:ISBN
402:urea
394:mOsm
390:NaCl
378:urea
286:fish
200:flux
84:news
34:and
634:doi
630:204
543:PMC
533:doi
162:In
67:by
686::
642:.
628:.
624:.
551:.
541:.
527:.
523:.
404:.
352:.
304:.
221:,
214:.
166:,
672:.
650:.
636::
609:.
584:.
559:.
535::
529:8
507:.
482:.
457:.
398:L
396:/
134:)
128:(
123:)
119:(
109:·
102:·
95:·
88:·
61:.
38:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.