107:
spiked. This leads to a linear relationship between the analyte signal and the amount of analyte added, allowing for the determination of the unknown's concentration by extrapolating the zero analyte signal. One disadvantage of this approach is that it requires sufficient amount of the unknown. When working with limiting amount of sample, an analyst might need to make a single addition, but it is generally considered a best practice to make at least two additions whenever possible.
156:
1266:
1290:
71:, the standard addition method involves creating two samples – one sample without any spikes, and another one with spikes. By comparing the current measured from two samples, the amount of analyte in the unknown is determined. This approach was the first reported use of standard addition, and was introduced by a German mining chemist, Hans Hohn, in 1937. In his polarography practical book, titled
1302:
1278:
106:
To apply this method, analysts prepare multiple solutions containing equal amounts of unknown and spike them with varying concentrations of the analyte. The amount of unknown and the total volume are the same across the standards and the only difference between the standards is the amount of analyte
171:
For standard additions, equal volumes of the sample solutions are taken, and all are separately spiked with varying amounts of the analyte – 0, 1, 2, 3, 4, 5 mL, where 0 mL addition is a pure test sample solution. All solutions are then diluted to the same volume of 25 mL, by using the same solvent
193:
matrix effects. These effects are caused by other substances present in the unknown sample that are often independent of the analyte concentration. They are commonly referred to as 'background' and can impact the intercept of the regression line without affecting the slope. This results in bias
159:
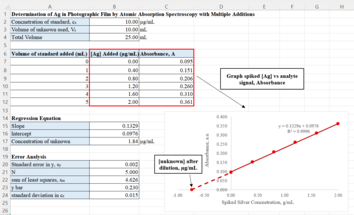
Spreadsheet for standard addition example on determining the silver concentration of photographic film waste. Please refer to the
Limitation and Uncertainty of Standard Addition section for more information on how to calculate for the Error Analysis portion. The silver concentration in the test
172:
as the one used to prepare the spiking solutions. Each prepared solution is then analyzed using an atomic absorption spectrometer. The resulting signals and corresponding spiked silver concentrations are plotted, with concentration on the x-axis and the signal on the y-axis. A
152:, and use the calibration graph to determine the amount of silver present in the waste samples. This method, however, assumes the pure aqueous solution of silver and a photographic waste sample have the same matrix and therefore the waste samples are free of matrix effect.
433:
180:
analysis and the x-intercept of the line is determined by the ratio of the y-intercept and the slope of the regression line. This x-intercept represents the silver concentration of the test sample where there is no standard solution added.
86:
Modern polarography typically involves using three solutions: the standard solution, the unknown solution, and a mixture of the standard and unknown solution. By measuring any two of these solutions, the unknown concentration is calculated.
59:. By increasing the number of spikes, the analyst can extrapolate for the analyte concentration in the unknown that has not been spiked. There are multiple approaches to the standard addition. The following section summarize each approach.
46:
method, the standard addition method has the advantage of the matrices of the unknown and standards being nearly identical. This minimizes the potential bias arising from the matrix effect when determining the concentration.
102:
was the next earliest reference book to mention standard addition. Harvey's approach, which involves the successive addition of standards, closely resembles the most commonly used method of standard addition today.
555:
284:
121:, background signal cannot be resolved by standard addition. Thus, background signal must be subtracted from the unknown and standard intensities prior to extrapolating for the zero signal.
90:
As polarographic standard addition involves using only one solution with the standard added – the two-level design, polarographers always refer to the method as singular, standard addition.
712:
652:
681:
467:
276:
249:
222:
984:
621:
599:
577:
165:
1213:
189:
While the standard addition method is effective in reducing the interference of most matrix effects on the analyte signal, it cannot correct for the
1236:
1024:
160:
sample is the x-intercept of the plot. The dilution factor is multiplied by this initial concentration to determine the original concentration.
808:
194:
towards the unknown concentration. In other words, standard addition will not correct for these backgrounds or other spectral interferences.
471:
977:
1306:
873:
168:, which have a reputation for being relatively free from interferences. As such, analyst would use standard additions in this case.
197:
Analysts also needs to evaluate the precision of the determined unknown concentration by calculating for the standard deviation,
428:{\displaystyle s_{x}={\frac {s_{y}}{|m|}}{\sqrt {{\frac {1}{n}}+{\frac {{\bar {y}}^{2}}{m^{2}\sum {(x_{i}-{\bar {x}})^{2}}}}}}}
1333:
1282:
1243:
1009:
141:
111:
1328:
1270:
970:
118:
1014:
1187:
79:, which translates to "calibration addition" in English. Later in the German literature, this method was called as
1090:
1080:
1161:
1121:
1250:
1131:
1039:
38:, quantifies the analyte present in an unknown. This method is useful for analyzing complex samples where a
1229:
1029:
124:
As this approach involves varying amount of standards added, it is often referred in the plural form as
891:"A standard addition method to quantify serum lithium by inductively coupled plasma mass spectrometry"
1085:
1049:
1001:
993:
35:
55:
Standard addition involves adding known amounts of analyte to an unknown sample, a process known as
1126:
1289:
1156:
1151:
1146:
1019:
688:
628:
1222:
1192:
1182:
1095:
1054:
1034:
949:
910:
869:
804:
778:
733:
173:
137:
136:
Suppose an analyst is determining the concentration of silver in samples of waste solution in
43:
1294:
1202:
941:
902:
770:
728:
149:
659:
445:
254:
227:
200:
155:
17:
1075:
723:
606:
584:
562:
1322:
1141:
177:
39:
1177:
1100:
145:
68:
758:
1070:
1136:
1044:
906:
889:
Fan, Xiaoyu; Li, Qing; Lin, Ping; Jin, Zhonggan; Chen, Meizi; Yi, Zu (2022).
782:
759:"A systematic approach to standard addition methods in instrumental analysis"
1105:
929:
890:
953:
914:
114:, for example, standard additions are often used with solid as the sample.
864:
Robinson, James W.; Skelly Frame, Eileen M.; Frame II, George M. (2005).
962:
774:
945:
144:. Using the calibration curve method, the analyst can calibrate the
94:
Successive addition of standards in constant sample and total volume
803:(6th ed.). Boston, MA, USA: Cengage Learning. pp. 13–14.
550:{\displaystyle ={\sqrt {\frac {\sum {(y_{i}-mx_{i}-b)}^{2}}{n-2}}}}
799:
Skoog, Douglas A.; Holler, James F.; Crouch, Stanley R. (2016).
966:
251:
indicates greater precision of the measurements. The value of
868:(6th ed.). New York: Marcel Dekker. pp. 84–87.
42:
interferes with the analyte signal. In comparison to the
848:(1st ed.). Glendale: Applied Research Laboratories.
438:
where the calculation involves the following variables:
559:
absolute value of the slope of the least-squares line,
691:
662:
631:
609:
587:
565:
474:
448:
287:
257:
230:
203:
110:
Note that this is not limited to liquid samples. In
1211:
1170:
1114:
1063:
1000:
706:
675:
646:
615:
593:
571:
549:
461:
427:
270:
243:
216:
98:Outside the field of polarography, Harvey's book
928:Ellison, Stephen L.; Thompson, Michael (2008).
164:Matrix effects occur even with methods such as
828:. Berlin, Germany: SpringerVerlag. p. 51.
978:
63:Single standard addition used in polarography
8:
985:
971:
963:
83:, meaning "standard addition" in English.
693:
692:
690:
667:
661:
633:
632:
630:
608:
586:
564:
526:
510:
494:
486:
478:
473:
453:
447:
413:
398:
397:
388:
380:
371:
360:
349:
348:
345:
332:
330:
322:
314:
307:
301:
292:
286:
262:
256:
235:
229:
208:
202:
826:Chemische Analysen mit dem Polarographen
685:average concentration of the standards,
154:
73:Chemische Analysen mit dem Polargraphen,
744:
1237:Analytical and Bioanalytical Chemistry
930:"Standard additions: myth and reality"
625:average measurement of the standards,
1025:High-performance liquid chromatograph
442:standard deviation of the residuals,
7:
1277:
859:
857:
855:
839:
837:
835:
794:
792:
752:
750:
748:
1301:
801:Principles of Instrumental Analysis
25:
656:concentrations of the standards,
581:y-intercept of the linear curve,
1300:
1288:
1276:
1265:
1264:
1010:Atomic absorption spectrometer
698:
638:
522:
487:
410:
403:
381:
354:
323:
315:
142:atomic absorption spectroscopy
112:atomic absorption spectroscopy
27:Method in analytical chemistry
1:
763:Journal of Chemical Education
75:Hohn referred this method as
866:Introduction to Spectroscopy
119:atomic emission spectroscopy
1015:Flame emission spectrometer
844:Harvey, Charles E. (1950).
1350:
846:Spectrochemical Procedures
707:{\displaystyle {\bar {x}}}
647:{\displaystyle {\bar {y}}}
100:Spectrochemical Procedures
1260:
1091:Ion mobility spectrometry
1081:Electroanalytical methods
907:10.1177/00045632211054745
18:Standard addition method
1251:Analytical Biochemistry
1040:Melting point apparatus
1230:Analytica Chimica Acta
757:Bader, Morris (1980).
708:
677:
648:
617:
595:
573:
551:
463:
429:
272:
245:
218:
176:is calculated through
161:
34:method, often used in
1334:Laboratory techniques
1122:Coning and quartering
1030:Infrared spectrometer
709:
678:
676:{\displaystyle x_{i}}
649:
618:
603:number of standards,
596:
574:
552:
464:
462:{\displaystyle s_{y}}
430:
273:
271:{\displaystyle s_{x}}
246:
244:{\displaystyle s_{x}}
219:
217:{\displaystyle s_{x}}
158:
1329:Analytical chemistry
1244:Analytical Chemistry
1086:Gravimetric analysis
1050:Optical spectrometer
994:Analytical chemistry
689:
660:
629:
607:
585:
563:
472:
446:
285:
255:
228:
201:
36:analytical chemistry
824:Hohn, Hans (1937).
166:plasma spectrometry
148:with a pure silver
1157:Separation process
1152:Sample preparation
704:
673:
644:
613:
591:
569:
547:
459:
425:
268:
241:
214:
162:
126:standard additions
1316:
1315:
1198:Standard addition
1193:Internal standard
1183:Calibration curve
1096:Mass spectrometry
1055:Spectrophotometer
1035:Mass spectrometer
1020:Gas chromatograph
810:978-1-305-57721-3
775:10.1021/ed057p703
734:Internal standard
701:
641:
616:{\displaystyle n}
594:{\displaystyle b}
572:{\displaystyle m}
545:
544:
423:
421:
406:
357:
340:
328:
150:aqueous solutions
138:photographic film
44:calibration curve
32:Standard addition
16:(Redirected from
1341:
1304:
1303:
1292:
1280:
1279:
1268:
1267:
1203:Isotope dilution
987:
980:
973:
964:
958:
957:
946:10.1039/b717660k
925:
919:
918:
895:Ann Clin Biochem
886:
880:
879:
861:
850:
849:
841:
830:
829:
821:
815:
814:
796:
787:
786:
754:
729:Isotope dilution
713:
711:
710:
705:
703:
702:
694:
682:
680:
679:
674:
672:
671:
653:
651:
650:
645:
643:
642:
634:
622:
620:
619:
614:
600:
598:
597:
592:
578:
576:
575:
570:
556:
554:
553:
548:
546:
543:
532:
531:
530:
525:
515:
514:
499:
498:
480:
479:
468:
466:
465:
460:
458:
457:
434:
432:
431:
426:
424:
422:
420:
419:
418:
417:
408:
407:
399:
393:
392:
376:
375:
365:
364:
359:
358:
350:
346:
341:
333:
331:
329:
327:
326:
318:
312:
311:
302:
297:
296:
277:
275:
274:
269:
267:
266:
250:
248:
247:
242:
240:
239:
223:
221:
220:
215:
213:
212:
21:
1349:
1348:
1344:
1343:
1342:
1340:
1339:
1338:
1319:
1318:
1317:
1312:
1256:
1207:
1166:
1110:
1059:
1002:Instrumentation
996:
991:
961:
927:
926:
922:
888:
887:
883:
876:
863:
862:
853:
843:
842:
833:
823:
822:
818:
811:
798:
797:
790:
756:
755:
746:
742:
720:
687:
686:
663:
658:
657:
627:
626:
605:
604:
583:
582:
561:
560:
533:
506:
490:
485:
481:
470:
469:
449:
444:
443:
409:
384:
367:
366:
347:
313:
303:
288:
283:
282:
258:
253:
252:
231:
226:
225:
204:
199:
198:
187:
174:regression line
134:
96:
65:
53:
28:
23:
22:
15:
12:
11:
5:
1347:
1345:
1337:
1336:
1331:
1321:
1320:
1314:
1313:
1311:
1310:
1298:
1286:
1274:
1261:
1258:
1257:
1255:
1254:
1247:
1240:
1233:
1226:
1218:
1216:
1209:
1208:
1206:
1205:
1200:
1195:
1190:
1185:
1180:
1174:
1172:
1168:
1167:
1165:
1164:
1159:
1154:
1149:
1144:
1139:
1134:
1129:
1124:
1118:
1116:
1112:
1111:
1109:
1108:
1103:
1098:
1093:
1088:
1083:
1078:
1076:Chromatography
1073:
1067:
1065:
1061:
1060:
1058:
1057:
1052:
1047:
1042:
1037:
1032:
1027:
1022:
1017:
1012:
1006:
1004:
998:
997:
992:
990:
989:
982:
975:
967:
960:
959:
920:
901:(3): 166–170.
881:
874:
851:
831:
816:
809:
788:
743:
741:
738:
737:
736:
731:
726:
724:Standard curve
719:
716:
715:
714:
700:
697:
683:
670:
666:
654:
640:
637:
623:
612:
601:
590:
579:
568:
557:
542:
539:
536:
529:
524:
521:
518:
513:
509:
505:
502:
497:
493:
489:
484:
477:
456:
452:
436:
435:
416:
412:
405:
402:
396:
391:
387:
383:
379:
374:
370:
363:
356:
353:
344:
339:
336:
325:
321:
317:
310:
306:
300:
295:
291:
265:
261:
238:
234:
211:
207:
186:
183:
133:
130:
95:
92:
81:Standardzugabe
64:
61:
52:
49:
26:
24:
14:
13:
10:
9:
6:
4:
3:
2:
1346:
1335:
1332:
1330:
1327:
1326:
1324:
1309:
1308:
1299:
1297:
1296:
1291:
1287:
1285:
1284:
1275:
1273:
1272:
1263:
1262:
1259:
1253:
1252:
1248:
1246:
1245:
1241:
1239:
1238:
1234:
1232:
1231:
1227:
1225:
1224:
1220:
1219:
1217:
1215:
1210:
1204:
1201:
1199:
1196:
1194:
1191:
1189:
1188:Matrix effect
1186:
1184:
1181:
1179:
1176:
1175:
1173:
1169:
1163:
1160:
1158:
1155:
1153:
1150:
1148:
1147:Pulverization
1145:
1143:
1140:
1138:
1135:
1133:
1130:
1128:
1125:
1123:
1120:
1119:
1117:
1113:
1107:
1104:
1102:
1099:
1097:
1094:
1092:
1089:
1087:
1084:
1082:
1079:
1077:
1074:
1072:
1069:
1068:
1066:
1062:
1056:
1053:
1051:
1048:
1046:
1043:
1041:
1038:
1036:
1033:
1031:
1028:
1026:
1023:
1021:
1018:
1016:
1013:
1011:
1008:
1007:
1005:
1003:
999:
995:
988:
983:
981:
976:
974:
969:
968:
965:
955:
951:
947:
943:
939:
935:
931:
924:
921:
916:
912:
908:
904:
900:
896:
892:
885:
882:
877:
875:0-203-99730-1
871:
867:
860:
858:
856:
852:
847:
840:
838:
836:
832:
827:
820:
817:
812:
806:
802:
795:
793:
789:
784:
780:
776:
772:
768:
764:
760:
753:
751:
749:
745:
739:
735:
732:
730:
727:
725:
722:
721:
717:
695:
684:
668:
664:
655:
635:
624:
610:
602:
588:
580:
566:
558:
540:
537:
534:
527:
519:
516:
511:
507:
503:
500:
495:
491:
482:
475:
454:
450:
441:
440:
439:
414:
400:
394:
389:
385:
377:
372:
368:
361:
351:
342:
337:
334:
319:
308:
304:
298:
293:
289:
281:
280:
279:
278:is given by
263:
259:
236:
232:
209:
205:
195:
192:
191:translational
184:
182:
179:
178:least squares
175:
169:
167:
157:
153:
151:
147:
143:
139:
131:
129:
127:
122:
120:
115:
113:
108:
104:
101:
93:
91:
88:
84:
82:
78:
74:
70:
62:
60:
58:
50:
48:
45:
41:
40:matrix effect
37:
33:
19:
1305:
1293:
1281:
1269:
1249:
1242:
1235:
1228:
1221:
1214:publications
1197:
1178:Chemometrics
1162:Sub-sampling
1101:Spectroscopy
940:(8): 992–7.
937:
933:
923:
898:
894:
884:
865:
845:
825:
819:
800:
766:
762:
437:
196:
190:
188:
170:
163:
146:spectrometer
135:
125:
123:
116:
109:
105:
99:
97:
89:
85:
80:
76:
72:
69:polarography
66:
56:
54:
31:
29:
1307:WikiProject
1171:Calibration
1132:Dissolution
1071:Calorimetry
934:The Analyst
769:(10): 703.
77:Eizhusatzes
67:In classic
1323:Categories
1212:Prominent
1137:Filtration
1064:Techniques
1045:Microscope
740:References
51:Variations
1106:Titration
783:0021-9584
699:¯
639:¯
538:−
517:−
501:−
483:∑
404:¯
395:−
378:∑
355:¯
1271:Category
1127:Dilution
1115:Sampling
954:18645637
915:34719967
718:See also
224:. Lower
1283:Commons
1223:Analyst
1142:Masking
132:Example
57:spiking
1295:Portal
952:
913:
872:
807:
781:
185:Error
950:PMID
911:PMID
870:ISBN
805:ISBN
779:ISSN
30:The
942:doi
938:133
903:doi
771:doi
140:by
117:In
1325::
948:.
936:.
932:.
909:.
899:59
897:.
893:.
854:^
834:^
791:^
777:.
767:57
765:.
761:.
747:^
128:.
986:e
979:t
972:v
956:.
944::
917:.
905::
878:.
813:.
785:.
773::
696:x
669:i
665:x
636:y
611:n
589:b
567:m
541:2
535:n
528:2
523:)
520:b
512:i
508:x
504:m
496:i
492:y
488:(
476:=
455:y
451:s
415:2
411:)
401:x
390:i
386:x
382:(
373:2
369:m
362:2
352:y
343:+
338:n
335:1
324:|
320:m
316:|
309:y
305:s
299:=
294:x
290:s
264:x
260:s
237:x
233:s
210:x
206:s
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.